Integrating Sowing Date with Chickpea Genotypes in Managing Fusarium Wilt in Morocco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Disease Parameters and Seed Yield
2.3. Data Analysis
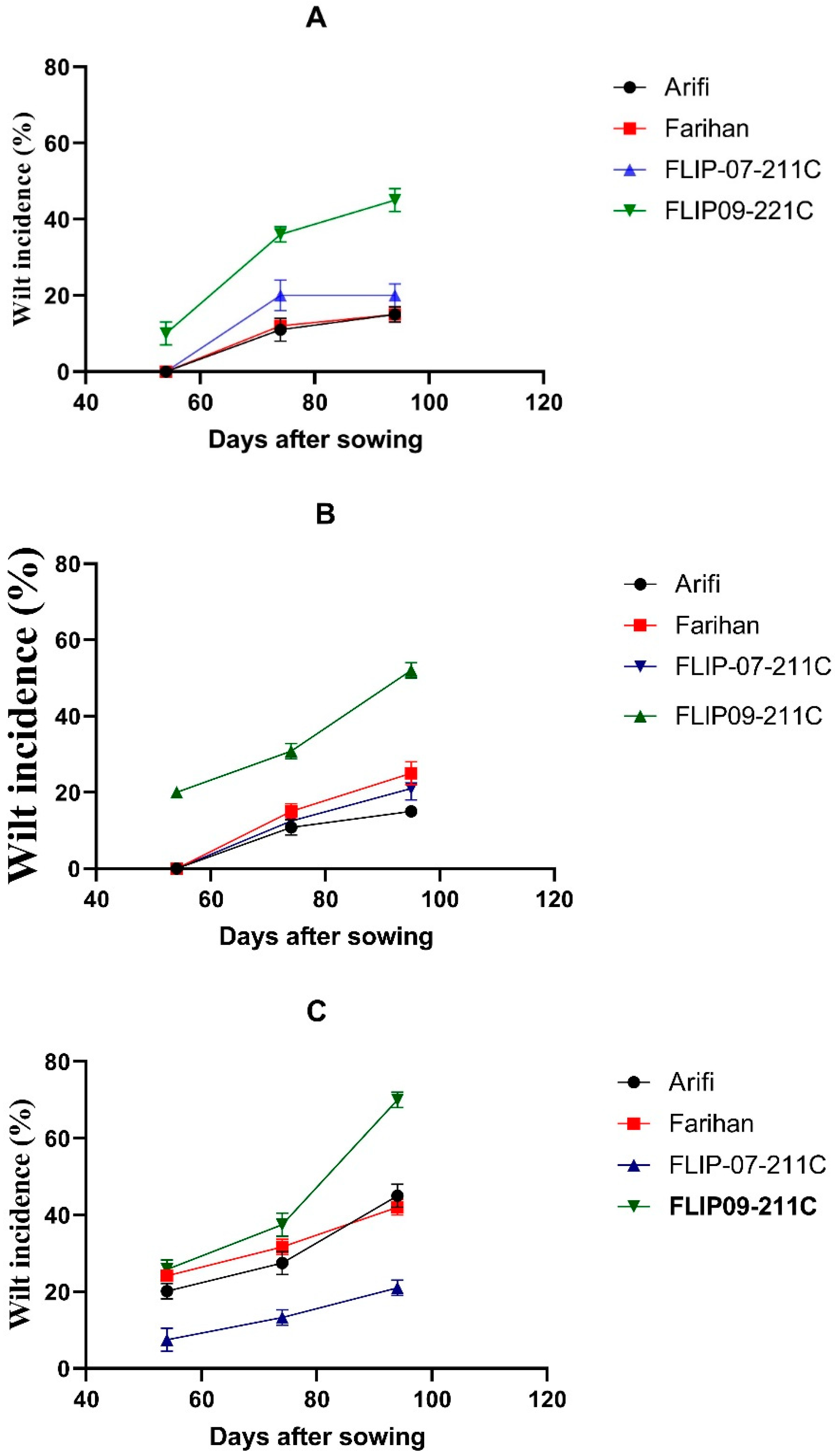
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mrabet, R.; Moussadek, R.; Fadlaoui, A.; Van Ranst, E. Conservation agriculture in dry areas of Morocco. Field Crops Res. 2012, 132, 84–94. [Google Scholar] [CrossRef]
- FOA. Food and Agriculture Organization. Available online: http://www.fao.org/faostat/en/#data (accessed on 11 March 2022).
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Shah, Z.; Mian, I.A.; Dawar, K.; Tariq, M.; Khan, B.; Mussarat, M.; Amin, H.; Ismail, M.; Ali, S.; et al. Soil fertility, N2 fixation and yield of chickpea as influenced by long-term biochar application under mung–chickpea cropping system. Sustainability 2020, 12, 9008. [Google Scholar] [CrossRef]
- Houasli, C.; Idrissi, O.; Nsarellah, N. Chickpea genetic improvement in Morocco: State of the art, progress, and prospects. Moroc. J. Agric. Sci. 2020, 1, 5–8. [Google Scholar]
- Siddique, K.H.M.; Krishnamurthy, L. Chickpea: Agronomy. Encycl. Food Grains 2016, 216–222. [Google Scholar] [CrossRef]
- Navas Cortés, J.; Hau, B.; Jiménez-Dıaz, R. Yield Loss in Chickpeas in Relation to Development of Fusarium Wilt Epidemics. Phytopathology 2000, 90, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Elbouazaoui, A.; Maafa, I.; Seid, A.; Douira, A.; Sripada, M.; Udupa. Status of wilt and root rot diseases of Kabuli chickpea in some regions of Morocco. In Proceedings of the Book of Abstract, 7th International Food Legumes Research Conference, Marrakesh, Morocco, 6–8 May 2018. [Google Scholar]
- Jiménez-Díaz, R.M.; Castillo, P.; del Mar Jiménez-Gasco, M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium mycotoxins, their metabolites (Free, emerging, and masked), food safety concerns, and health impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bruhl, C.A.; Imfeld, G.; Knabel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class. Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.C.; Singh, V.; Priyanka, K.; Upadhyay, B.K.; Singh, B. Combined application of fungal and bacterial bio-agents, together with fungicide and Mesorhizobium for integrated management of Fusarium wilt of chickpea. BioControl 2015, 60, 413–424. [Google Scholar] [CrossRef]
- Jamil, A.; Ashraf, S. Impacts of agronomic practices in the management of Fusarium wilt of chickpea. Australas. Plant Pathol. 2021, 50, 441–450. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younesi, H.; Chehri, K.; Sheikholeslami, M.; Safaee, D.; Naseri, B. Effects of sowing date and depth on Fusarium wilt development in chick pea cultivars. J. Plant Pathol. 2020, 102, 343–350. [Google Scholar] [CrossRef]
- Sharma, M.; Ghosh, R. An update on genetic resistance of chickpea to Ascochyta blight. Agronomy 2016, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Jendoubi, W.; Bouhadida, M.; Boukteb, A.; Béji, M.; Kharrat, M. Fusarium wilt affecting chickpea crop. Agriculture 2020, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, A.M.; Araújo, S.D.S.; Rubiales, D.; Vaz Patto, M.C. Fusarium wilt management in legume crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Kemal, S.A.; Abang, M.; Imtiaz, M.; Nader, A. Effect of integrated management on Fusarium wilt progression and grain yield of chickpea in Syria. Arch. Agron. Soil Sci. 2015, 61, 1551–1560. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Effects of iron and boron combinations on the suppression of Fusarium wilt in banana. Sci. Rep. 2016, 6, 38944. [Google Scholar] [CrossRef]
- Silva, L.L.; Duarte, I.; Lourenço, E.; Simões, N.; Chaves, M.M. Yield and water productivity of five chickpea varieties under supplemental irrigation in contrasting years. Irrig. Sci. 2014, 32, 393–403. [Google Scholar] [CrossRef]
- Landa, B.B.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M. Integrated management of Fusarium wilt of chickpea with sowing date, host resistance, and biological control. Phytopathology 2004, 94, 946–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazid, A.; Shideed, K.; El-Abdullah, M.; Zyadeh, G.; Juma’a, M. Impacts of crop improvement research on farmers livelihoods: The case of winter-sown chickpea in Syria. Exp. Agric. 2013, 49, 336–351. [Google Scholar] [CrossRef]
- Navas-Cortés, J.A.; Hau, B.; Jiménez-Díaz, R.M. Effect of sowing date, host cultivar, and race of Fusarium oxysporum f. sp. ciceris on development of Fusarium wilt of chickpea. Phytopathology 1998, 88, 1338–1346. [Google Scholar] [PubMed] [Green Version]
- Ahmed, S.; Akem, C.; Bayaa, B.; Erskine, W. Integrating host resistance with planting date and fungicide seed treatment to manage Fusarium wilt and so increase lentil yields. Int. J. Pest Manag. 2002, 48, 121–125. [Google Scholar] [CrossRef]
- Cruz, D.R.; Leandro, L.F.S.; Munkvold, G.P. Effects of Temperature and pH on Fusarium oxysporum and Soybean Seedling Disease. Plant Dis. 2019, 103, 3234–3243. [Google Scholar] [CrossRef]
- Landa, B.B.; Navas-Cortés, J.A.; del Mar Jimenez-Gasco, M.; Katan, J.; Retig, B.; Jiménez-Díaz, R.M. Temperature response of chickpea cultivars to races of Fusarium oxysporum f. sp. ciceris, causal agent of Fusarium wilt. Plant Dis. 2006, 90, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- López-Bellido, F.J.; López-Bellido, R.J.; Khalil, S.K.; López-Bellido, L. Effect of planting date on winter kabuli chickpea growth and yield under rainfed Mediterranean conditions. Agron. J. 2008, 100, 957–964. [Google Scholar] [CrossRef]



| Genotype | Type | Pedigree | Days to Flowering | Days to Maturity | Other Traits |
|---|---|---|---|---|---|
| ARIFI (FLIP98-50 C) | Kabuli | - | 99 | 150 | Erect plant, Aschochyta blight (AB) resistance, large seed size, |
| FARIHANE (FLIP84-78C) | Kabuli | - | 98 | 153 | Erect plant, (AB) resistance, large seed size |
| FLIP07-211C | Kabuli | X03TH132/FLIP 97-185CXFLIP99-47C | 92 | 144 | Erect plant, large seed size |
| FLIP09-221C | Kabuli | S00789(30 KR)-7/ | 84 | 136 | Erect plant, small seed size |
| Sources of Variation | Final Percent Wilt Incidence | AUDPC | Rate of Disease Development | Seed Yield |
|---|---|---|---|---|
| Season (S) | NS | NS | * | NS |
| Dates (D) | * | NS | NS | * |
| Genotypes (G) | *** | * | *** | NS |
| D*G | NS | NS | NS | NS |
| S*D | NS | NS | NS | NS |
| S*D*G | NS | NS | NS | NS |
| Sowing Date | Chickpea Genotypes | ||||
|---|---|---|---|---|---|
| Arifi | Farihan | FLIP-07-211C | FLIP-09-221C | Mean | |
| Mid December | 15.8 (±5.01) | 15.3 (±3.61) | 20.8 (±6.12) | 45.0 (±10.09) | 24.2 |
| Mid-January | 15.8 (±2.80) | 25.7 (±2.71) | 21.0 (±9.66) | 52.7 (±14.01) | 28.8 |
| Mid-February | 45.0 (±2.65) | 48.0 (±7.87) | 21.3 (±2.00) | 70.7 (±7.33) | 46.3 |
| Mean | 25.5 | 29.7 | 21.0 | 56.1 | |
| Sowing Date | Chickpea Genotypes | ||||
|---|---|---|---|---|---|
| Arifi | Farihan | FLIP-07-211C | FLIP-09-221C | Mean | |
| Mid-December | 277.5 (±96.21) | 311.0 (±133.56) | 319.0 (±32.90) | 860.0 (±60.86) | 441.9 |
| Mid-January | 505.0 (±31.16) | 900.3 (±66.30) | 323.3 (±35.44) | 807.5 (±39.17) | 634.0 |
| Mid-February | 649.3 (±38.79) | 840.0 (±46.77) | 352.8 (±31.34) | 966.7 (±53.65) | 702.2 |
| Mean | 477.3 | 683.8 | 331.7 | 878.1 | 592.7 |
| Sowing Date | Arifi | Farihan | FLIP-07-211C | FLIP09-221 C | Mean |
|---|---|---|---|---|---|
| Mid-December | 0.26 (±0.16) | 0.21 (±0.20) | 0.33 (±0.28) | 0.51 (±0.19) | 0.33 |
| Mid-January | 0.19 (±0.10) | 0.62 (±0.35) | 0.32 (±0.18) | 0.72 (±0.40) | 0.46 |
| Mid-February | 0.25 (±0.14) | 0.61 (±0.29) | 0.38 (±0.18) | 0.77 (±0.25) | 0.50 |
| Mean | 0.23 | 0.48 | 0.34 | 0.66 | 0.43 |
| Sowing Date | Chickpea Genotypes | ||||
|---|---|---|---|---|---|
| Arifi | Farihan | FLIP-07-211C | FLIP-09-221 C | Mean | |
| Mid-December | 1.8 (±0.93) | 2.2 (±0.27) | 1.8 (±0.25) | 1.8 (±0.63) | 1.9 |
| Mid-January | 1.7 (±0.29) | 1.6 (±0.48) | 1.6 (±0.10) | 1.5 (±0.45) | 1.6 |
| Mid-February | 1.9 (±0.27) | 1.5 (±1.1) | 1.5 (±0.18) | 1.1 (±0.13) | 1.5 |
| Mean | 1.8 | 1.7 | 1.6 | 1.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amine, E.; Douira, A.; Ilyass, M.; Ahmed, S. Integrating Sowing Date with Chickpea Genotypes in Managing Fusarium Wilt in Morocco. Agriculture 2022, 12, 773. https://doi.org/10.3390/agriculture12060773
Amine E, Douira A, Ilyass M, Ahmed S. Integrating Sowing Date with Chickpea Genotypes in Managing Fusarium Wilt in Morocco. Agriculture. 2022; 12(6):773. https://doi.org/10.3390/agriculture12060773
Chicago/Turabian StyleAmine, Elbouazaoui, Allal Douira, Maafa Ilyass, and Seid Ahmed. 2022. "Integrating Sowing Date with Chickpea Genotypes in Managing Fusarium Wilt in Morocco" Agriculture 12, no. 6: 773. https://doi.org/10.3390/agriculture12060773
APA StyleAmine, E., Douira, A., Ilyass, M., & Ahmed, S. (2022). Integrating Sowing Date with Chickpea Genotypes in Managing Fusarium Wilt in Morocco. Agriculture, 12(6), 773. https://doi.org/10.3390/agriculture12060773






