Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Scope of the Study
2.3. Microscopic Studies
2.4. Microscopic and Morphometric Measurements
2.5. Statistical Analysis
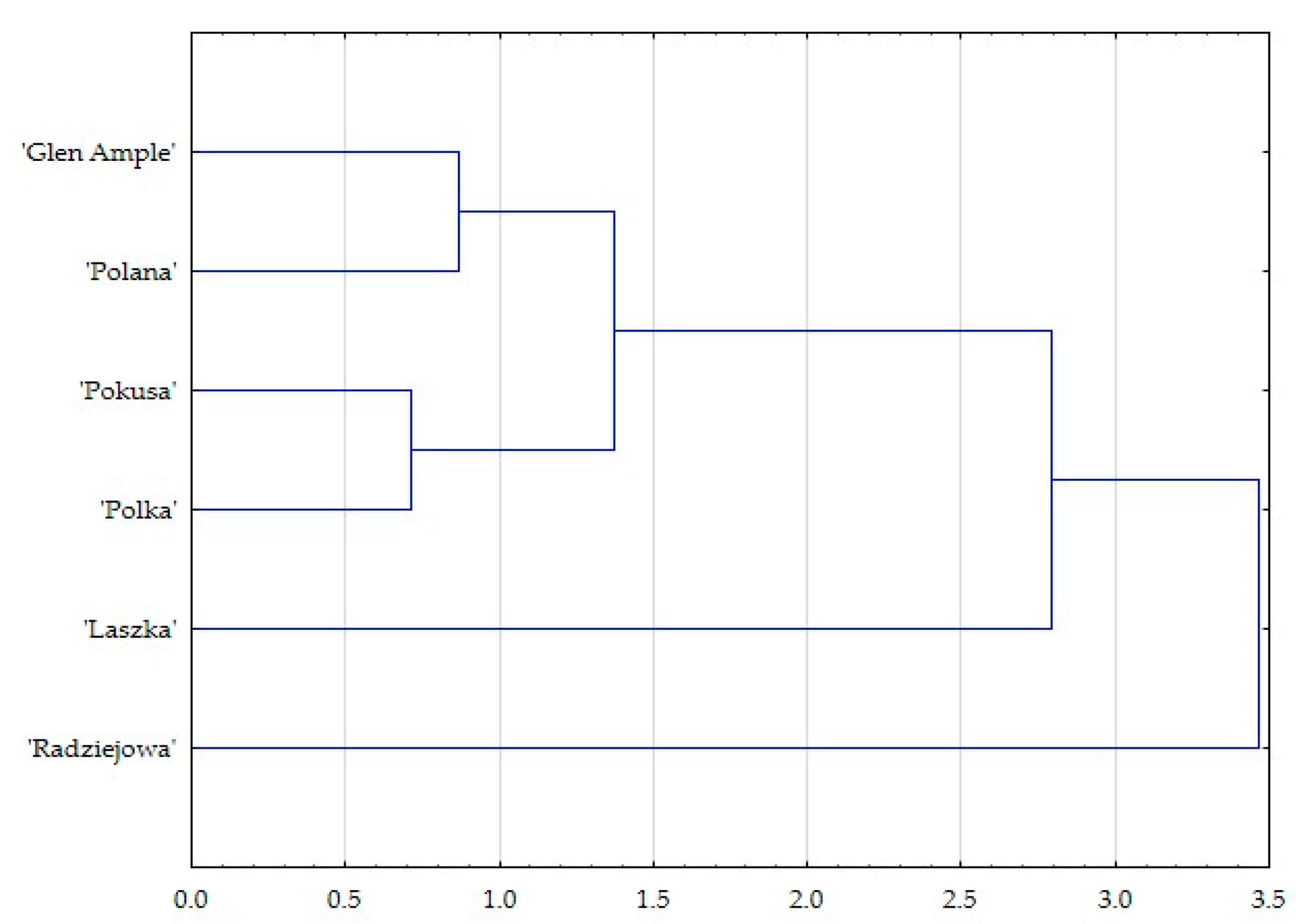
3. Results
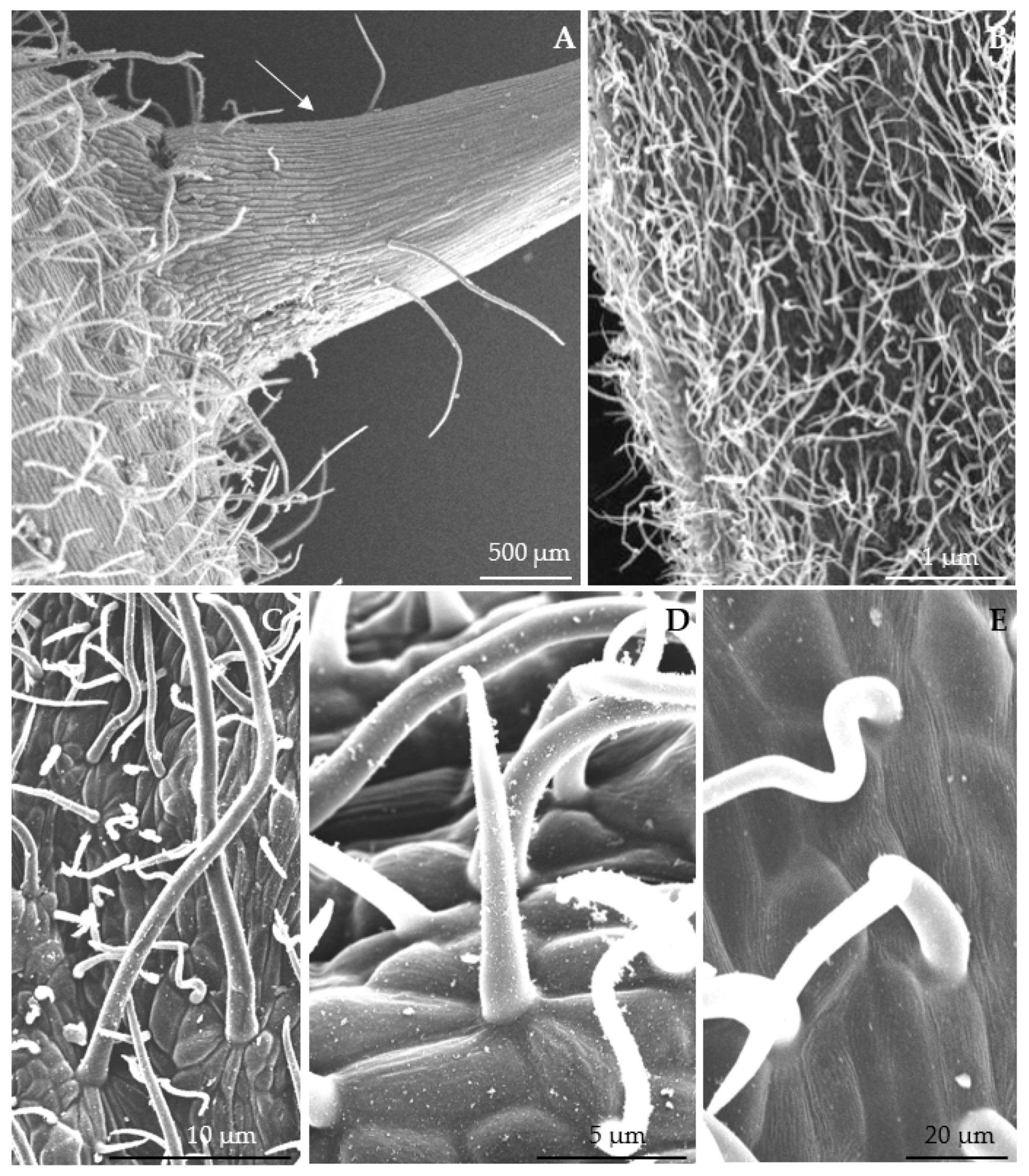
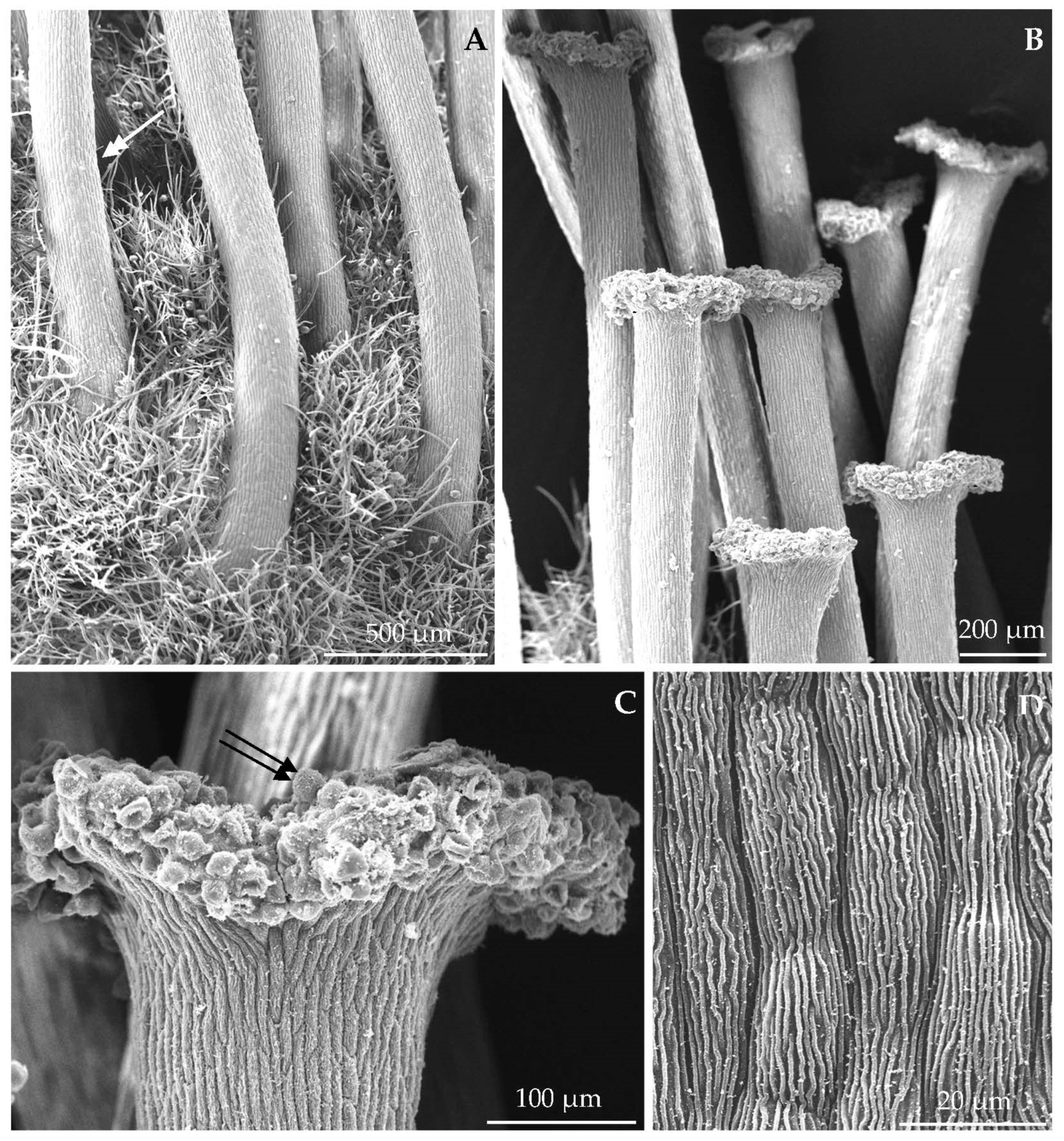
3.1. Micromorphological Traits of Some Flower Elements
3.2. Floral Nectaries
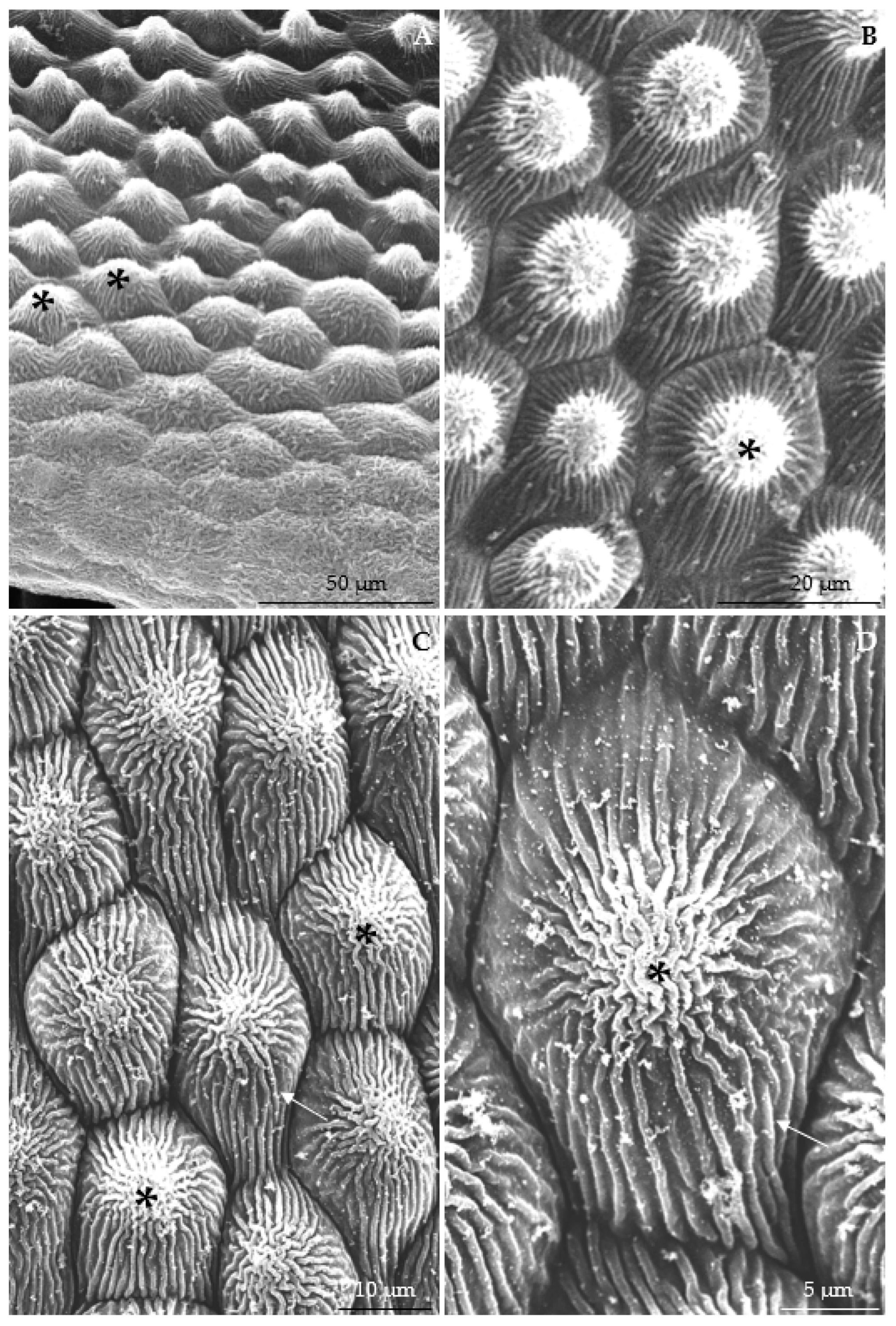
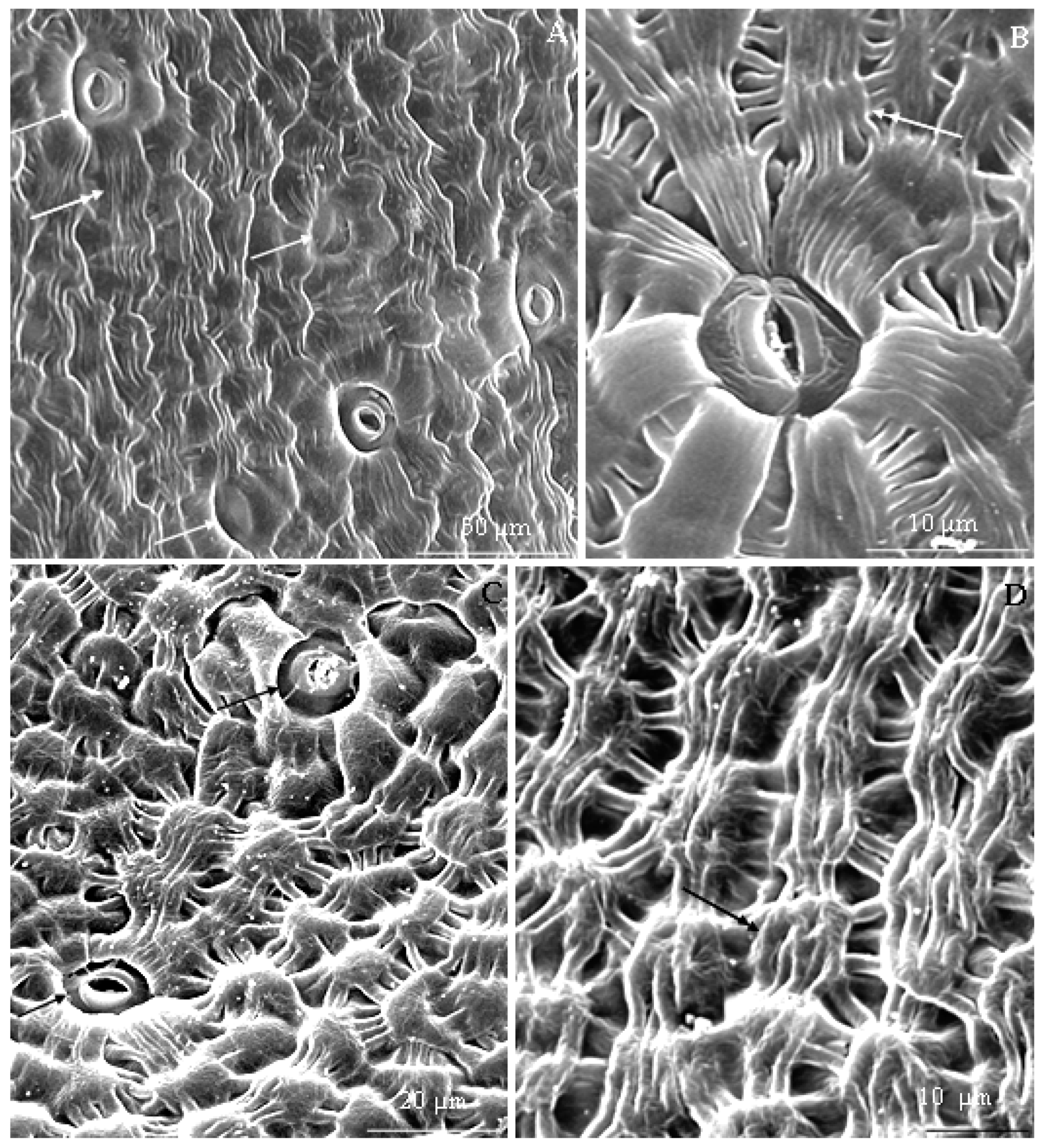
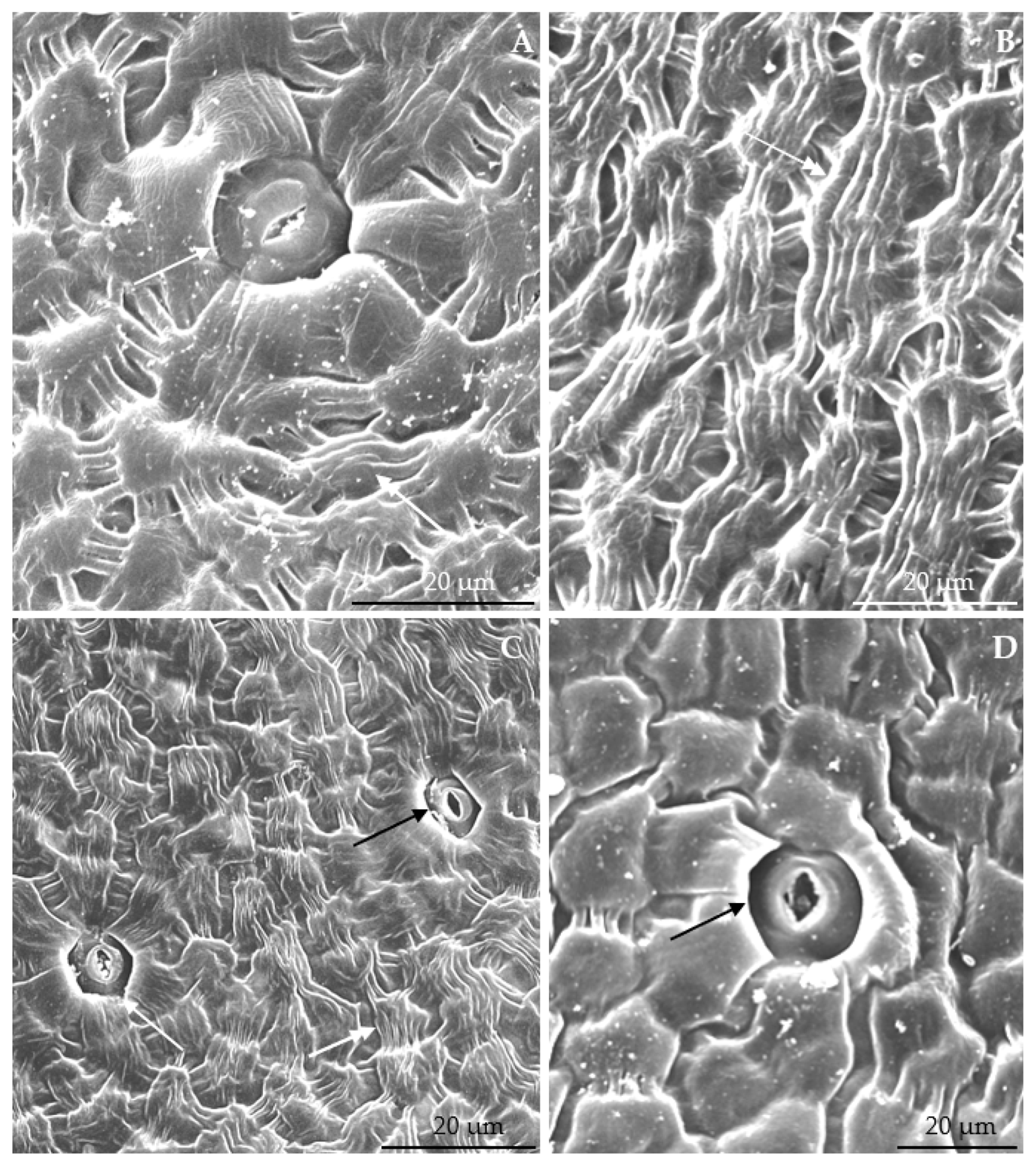
3.2.1. Micromorphology
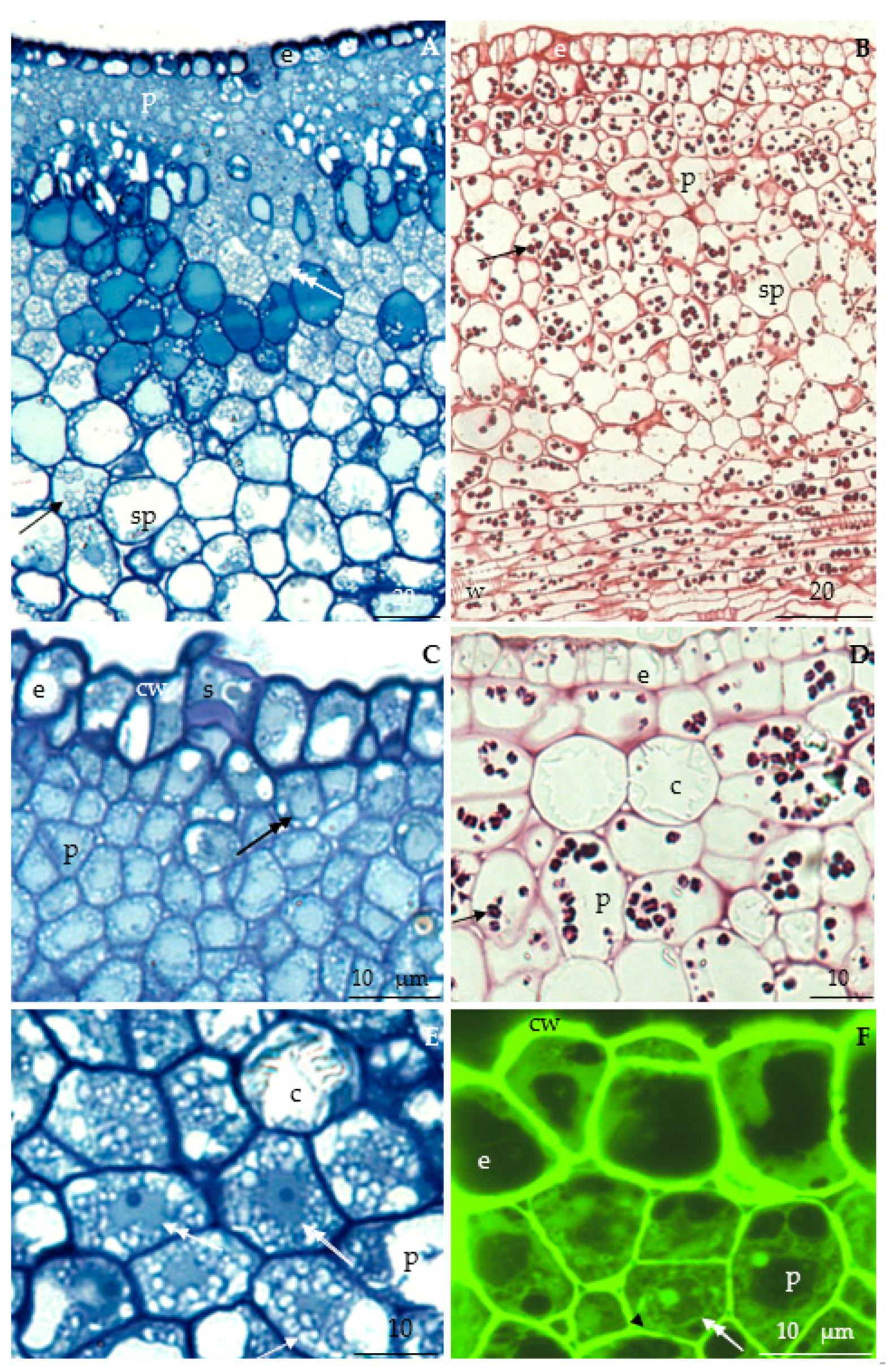
3.2.2. Anatomical Traits of Nectaries
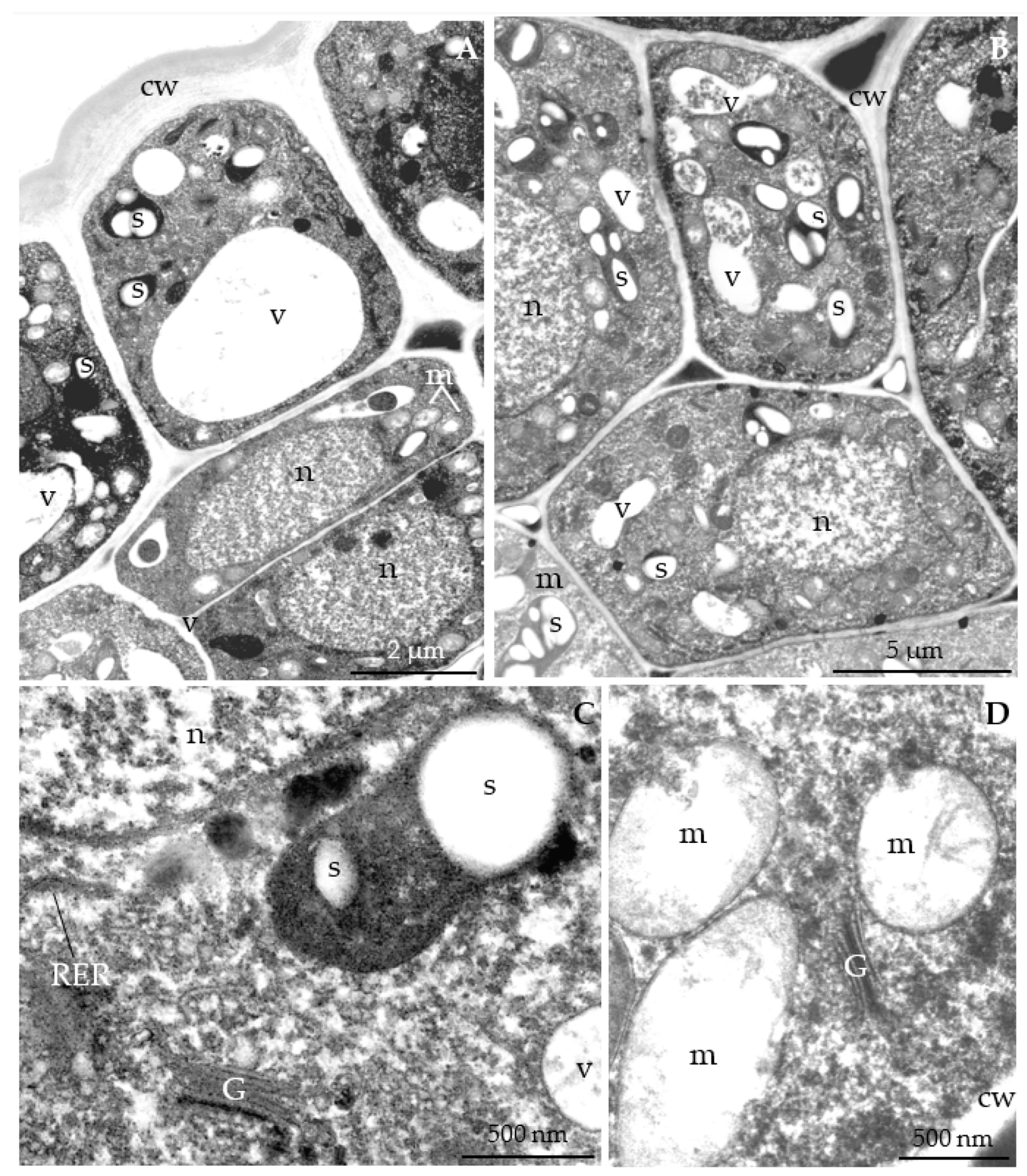
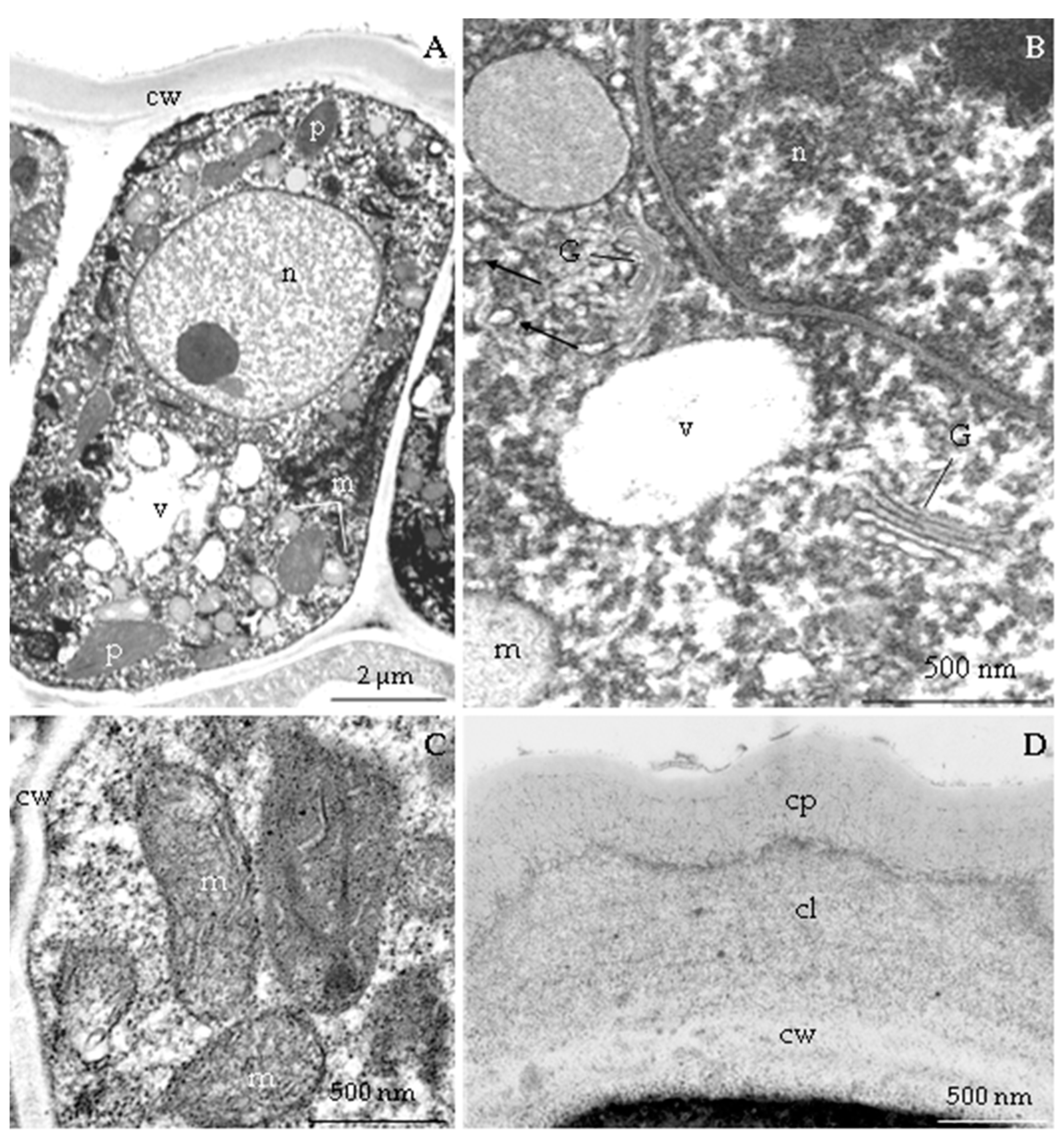
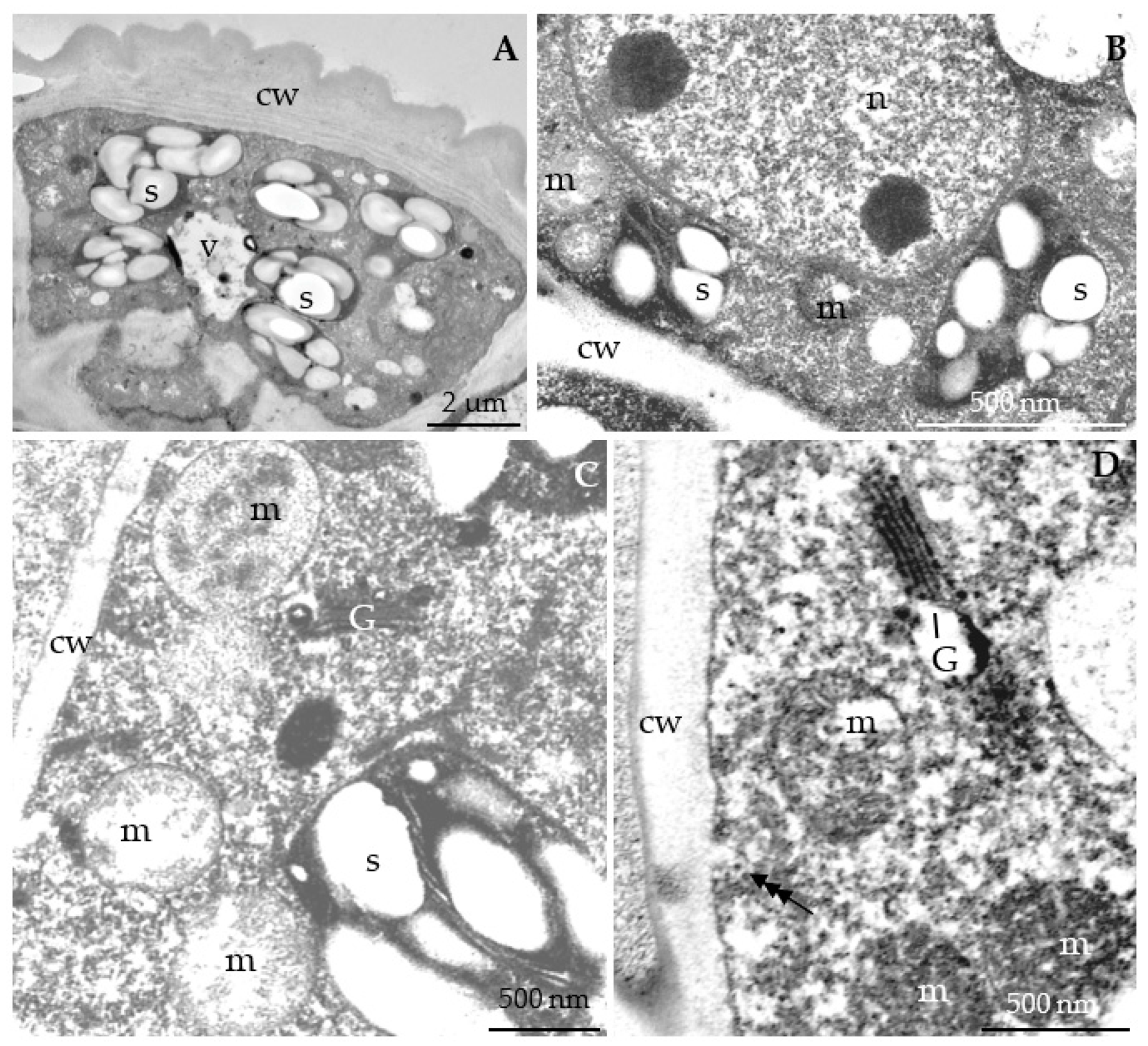
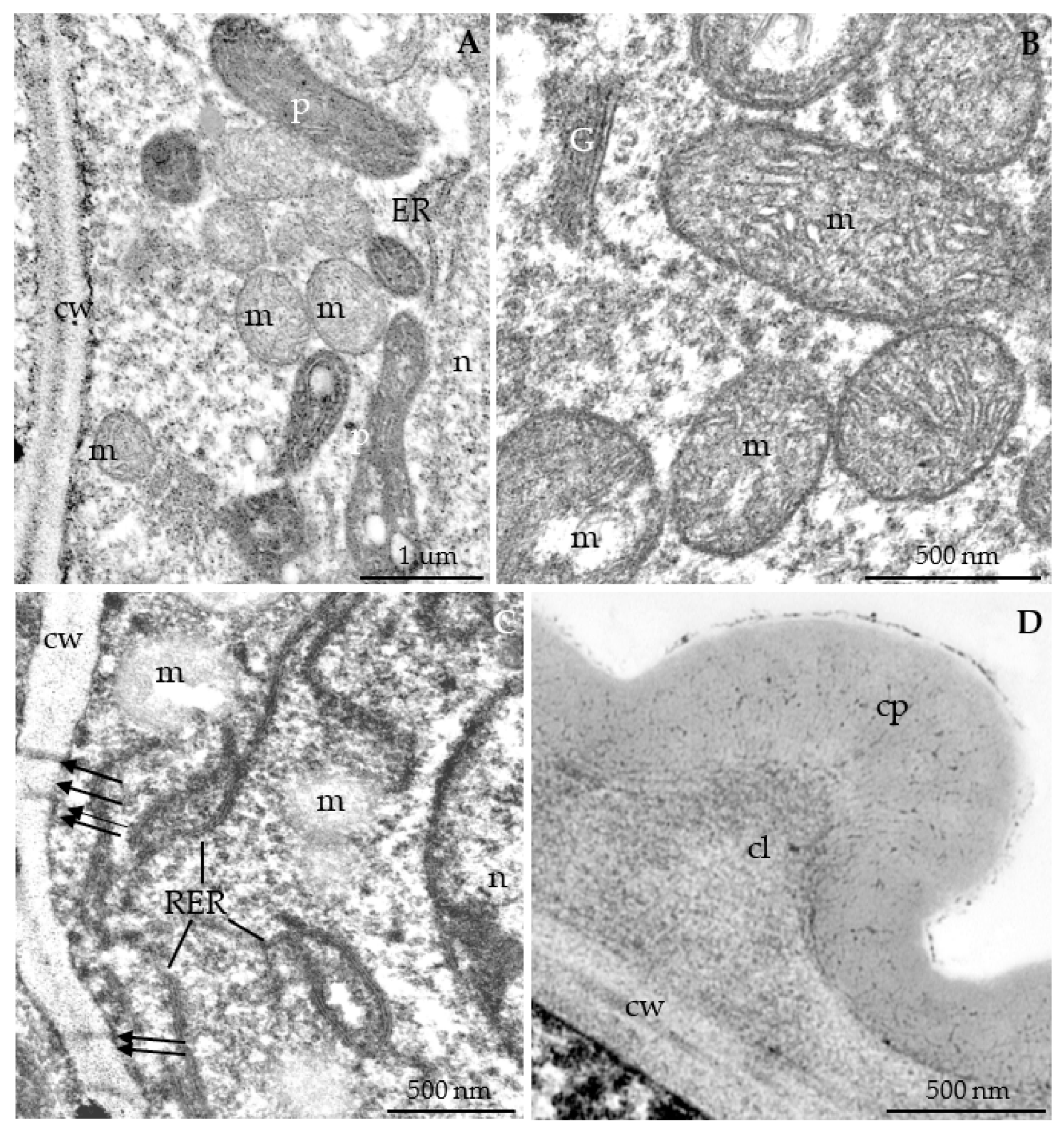
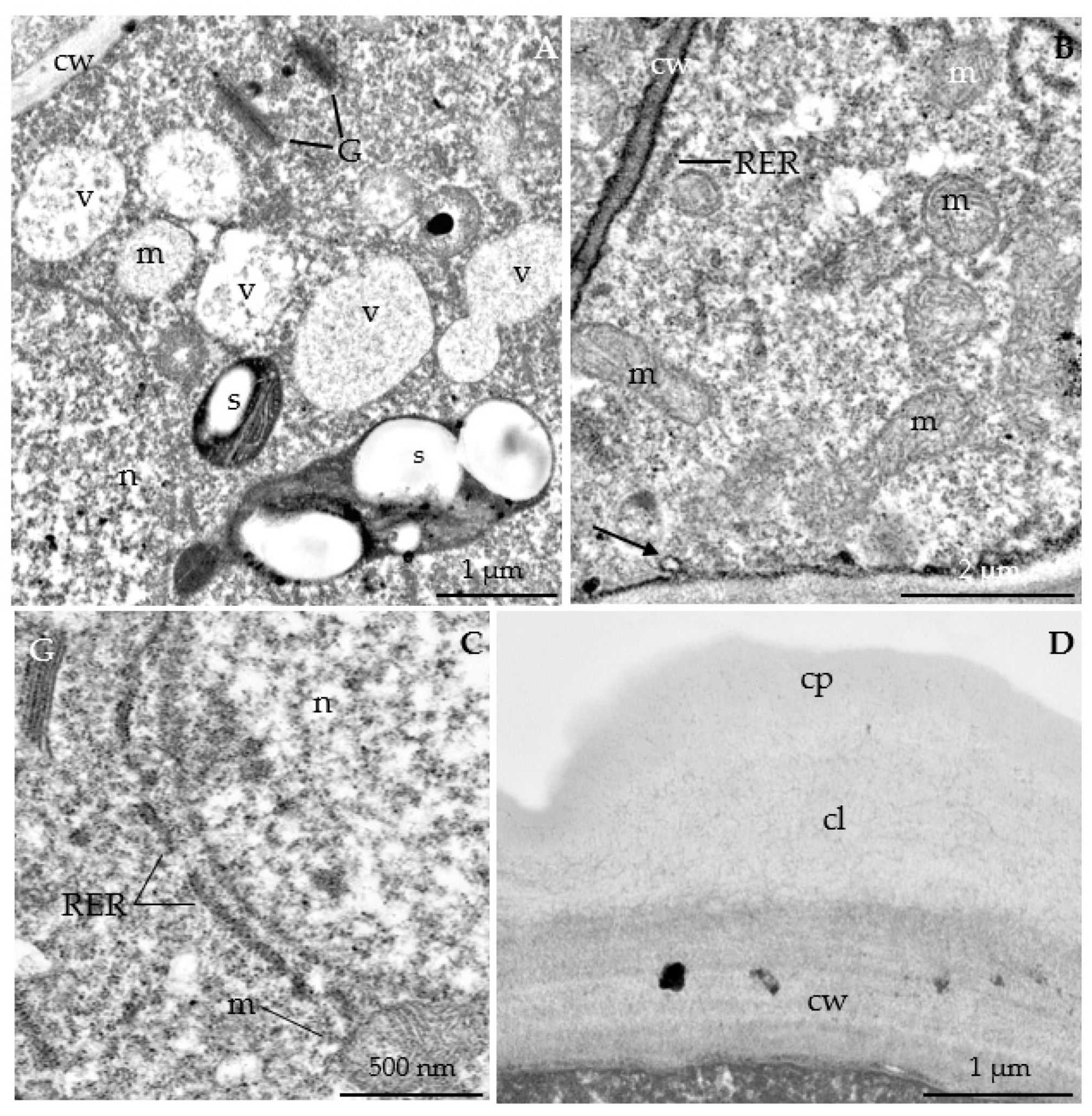
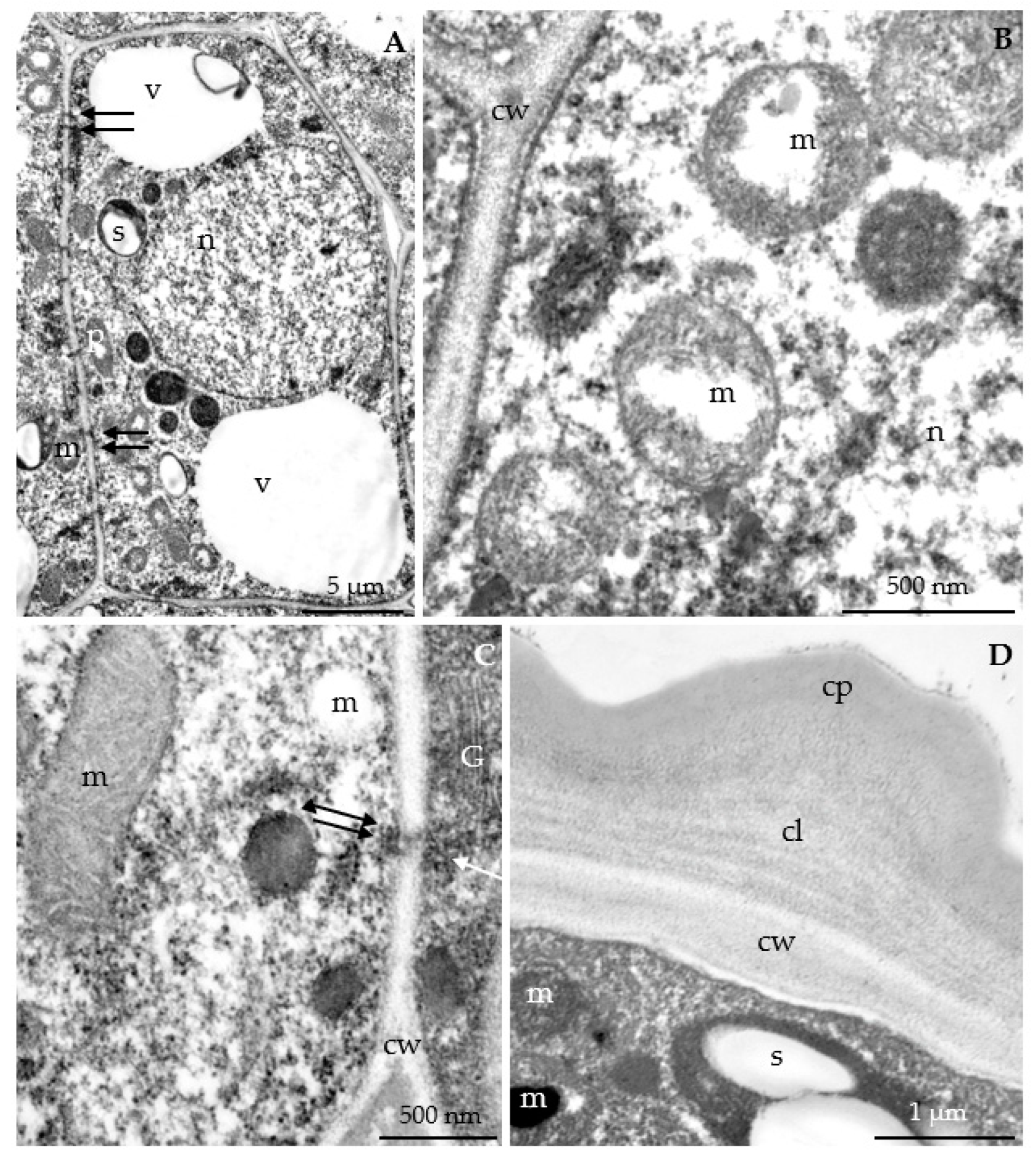
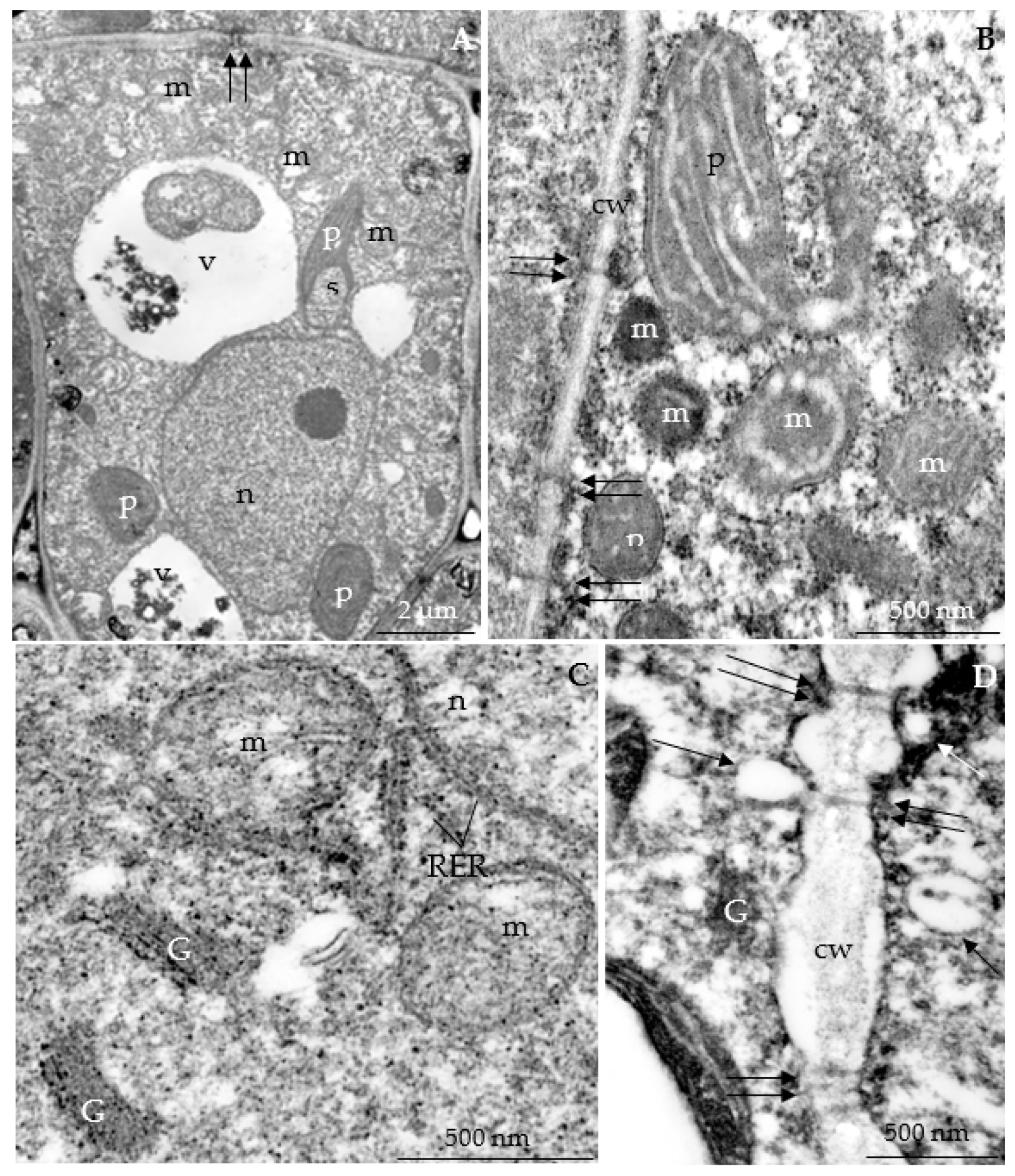
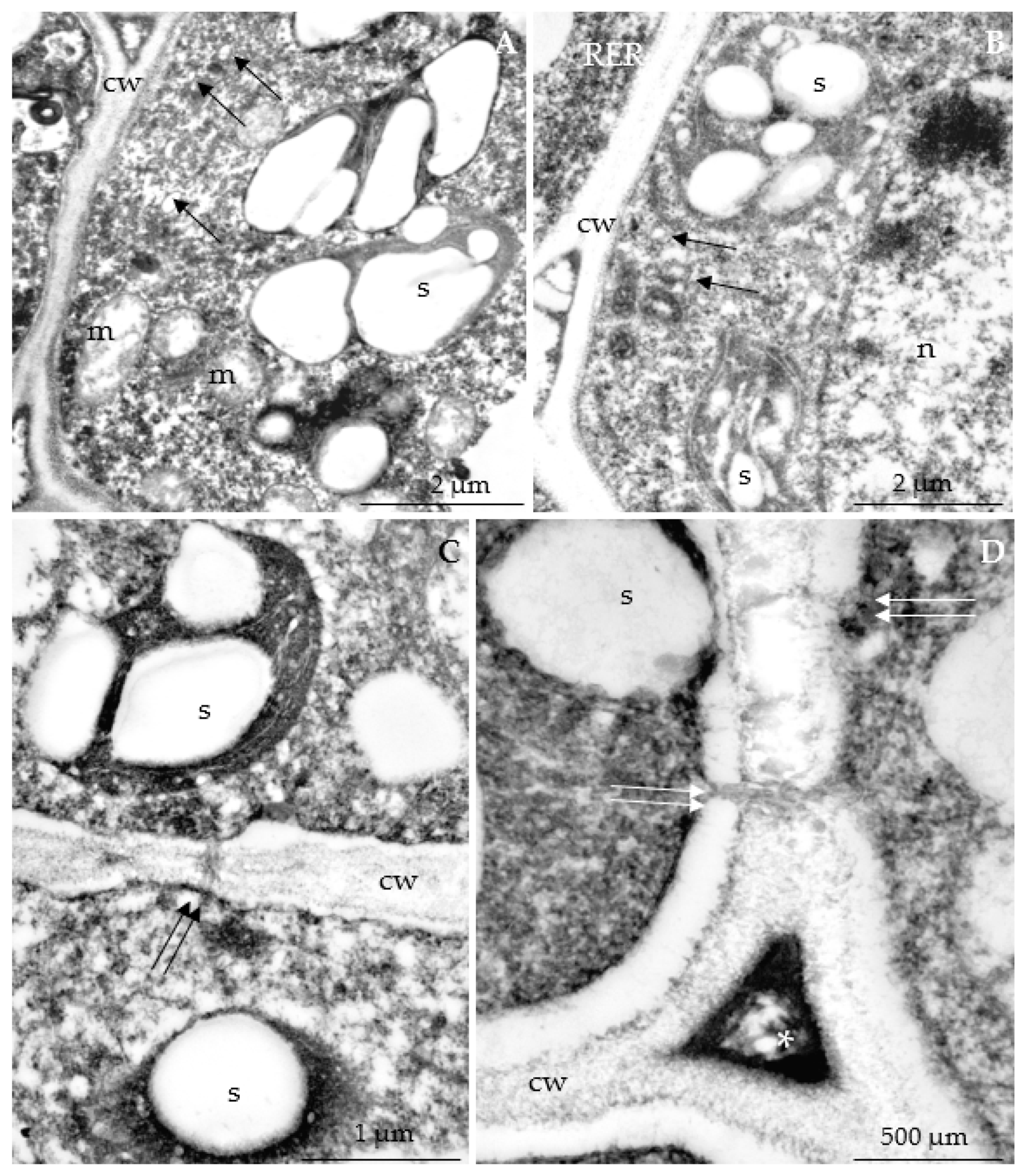
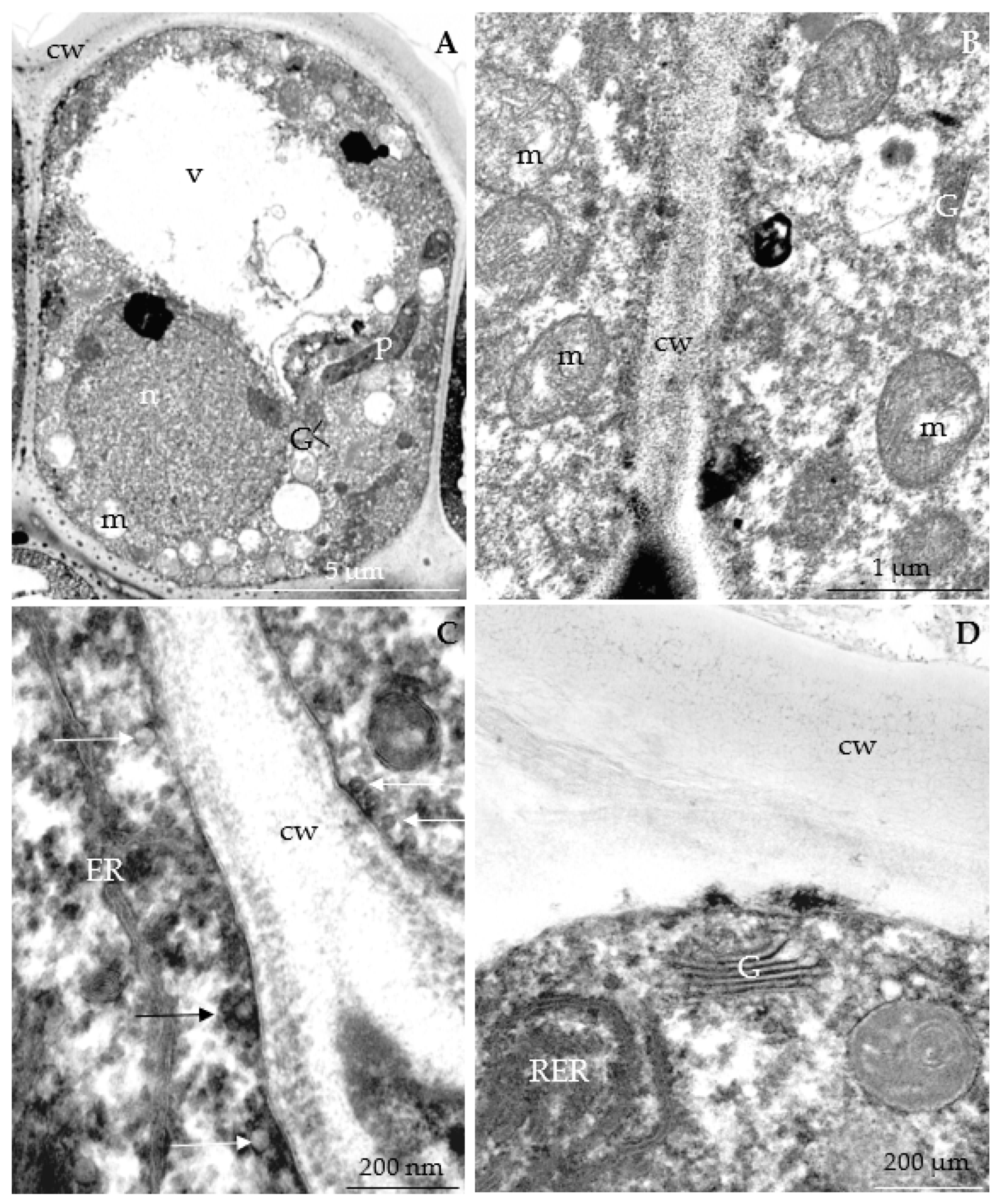
3.2.3. Nectary Ultrastructure
4. Discussion
4.1. Nectary Micromorphology
4.2. Nectary Anatomy
4.3. Nectary Ultrastructure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Fu, J.; Fang, Y.; Xiang, J.; Dong, H. Complete chloroplast genomes of Rubus species (Rosaceae) and comparative analysis within the genus. BMC Genom. 2022, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Manghwar, H.; Hu, W. Study on supergenus Rubus L.: Edible, medicinal, and phylogenetic characterization. Plants 2022, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Šarhanová, P.; Vašut, R.J.; Dančák, M.; Bureš, P.; Trávníček, B. New insights into the variability of reproduction modes in European populations of Rubus subgen. Rubus: How sexual are polyploid brambles? Sex. Plant Reprod. 2012, 25, 319–335. [Google Scholar] [CrossRef]
- Chase, M.W.; Reveal, J.L. A phylogenetic classification of the land plants to accompany APG III. Bot. J. Linn. 2009, 161, 122–127. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.H.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. Mol. Biol. Evol. 2017, 34, 262–281. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Siddiqui, M.F. Taxonomic reaffirmation of some members of family Cannabaceae, Moraceae, Rhamnaceae, Rosaceae and Urticaceae of order Rosales using DNA barcodings markers. Pak. J. Bot. 2022, 54, 231–241. [Google Scholar] [CrossRef]
- Thompson, M.M. Chromosome numbers of Rubus species at the national clonal germplasm repository. HortScience 1995, 30, 1447–1452. [Google Scholar] [CrossRef] [Green Version]
- Lechowicz, K.; Wrońska-Pilarek, D.; Bocianowski, J.; Maliński, T. Pollen morphology of Polish species from the genus Rubus L. (Rosaceae) and its systematic importance. PLoS ONE 2020, 15, e0221607. [Google Scholar] [CrossRef]
- Alice, L.A.; Campbell, C.S. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am. J. Bot. 1999, 86, 81–97. [Google Scholar] [CrossRef]
- Morden, C.W.; Gardner, D.E.; Weniger, D.A. Phylogeny and biogeography of Pacific Rubus subgenus Idaeobatus (Rosaceae) species: Investigating the origin of the endemic Hawaiian raspberry R. macraei. Pac. Sci. 2003, 57, 181–197. [Google Scholar] [CrossRef]
- Weber, H.E. Rubus. In Illustrierte Flora von Mitteleuropa; Hegi, G., Ed.; Blackwell: Berlin, Germany, 1995; pp. 284–595. [Google Scholar]
- Miyashita, T.; Kunitake, H.; Yotsukura, N.; Hoshino, Y. Assessment of genetic relationships among cultivated and wild Rubus accessions using AFLP markers. Sci. Hortic. 2015, 193, 165–173. [Google Scholar] [CrossRef]
- Lācis, G.; Kota-Dombrovska, I.; Strautiņa, S. Evaluation of red raspberry cultivars used for breeding and commercial growing in the Baltic region. Proc. Latv. Acad. Sci. B. 2017, 3, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Veljković, B.; Šoštarić, I.; Dajić-Stevanović, Z.; Liber, Z.; Šatović, Z. Genetic structure of wild raspberry populations in the Central Balkans depends on their location and on their relationship to commercial cultivars. Sci. Hortic. 2019, 256, 108606. [Google Scholar] [CrossRef]
- Kostryco, M.; Chwil, M.; Matraszek-Gawron, R. Comparison of the micromorphology and ultrastructure of pollen grains of selected Rubus idaeus L. cultivars grown in commercial plantation. Plants 2020, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Calabrese, D.; Guarnieri, M.; Giordano, E. Evolutionary and ecological considerations on nectar-mediated tripartite interactions in angiosperms and their relevance in the mediterranean basin. Plants 2021, 10, 507. [Google Scholar] [CrossRef]
- Slavković, F.; Dogimont, C.; Morin, H.; Boualem, A.; Bendahmane, A. The genetic control of nectary development. Trends Plant Sci. 2021, 26, 260–271. [Google Scholar] [CrossRef]
- Zambon, V.; Agostini, K.; Nepi, M.; Rossi, M.L.; Martinelli, A.P.; Sazima, M. The role of nectar traits and nectary morphoanatomy in the plant-pollinator interaction between Billbergia distachia (Bromeliaceae) and the hermit Phaethornis eurynome (Trochilidae). Bot. J. Linn. 2020, 192, 816–827. [Google Scholar] [CrossRef]
- Chwil, M.; Kostryco, M.; Matraszek-Gawron, R. Comparative studies on structure of the floral nectaries and the abundance of nectar production of Prunus laurocerasus L. Protoplasma 2019, 256, 1705–1726. [Google Scholar] [CrossRef] [Green Version]
- Farkas, A.; Orosz-Kovács, Z.S. Primary and secondary attractants of flowers in pear Pyrus betulifolia. Acta Hortic. 2004, 636, 317–324. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Konarska, A. Comparative studies of apple (Malus silvestris) and pear (Pyrus communis) nectaries. In Materials of the National Scientific Conference “Science in Horticultural Practice” Held on the 25th Anniversary of the Faculty of Horticulture, Agricultural Academy of Lublin; Agricultural Academy Publishing Office: Lublin, Poland, 1995; pp. 915–918. [Google Scholar]
- Weryszko-Chmielewska, E.; Konarska, A. Anatomy of the floral nectaries of 9 species from subf. Pomoideae (Rosaceae). Acta Agrobot. 1996, 49, 95–105. [Google Scholar] [CrossRef]
- Orosz-Kovács, Z.S.; Farkas, Á.; Horváth, A.; Takács-Róka, K. Structure of floral nectary in Maloideae and Prunoideae. In Magyar Botanikai Kutatások az Ezredfordulón. Tanulmányok Borhidi Attila 70. Születésnapja Tiszteletére; Salamon-Albert, É., Ed.; University of Pécs, Faculty of Natural Science, Department of Botany: Pécs, Hungary, 2002; pp. 213–226. [Google Scholar]
- Konarska, A. The comparison of nectaries structure of some varieties of ornamental apple. Acta Agrobot. 2007, 60, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Farkas, Á. Morphology and histology of the nectary in Hungarian local pear cultivars. Acta Hort. 2005, 671, 127–135. [Google Scholar] [CrossRef]
- Nagy-Deri, H.; Orosz-Kovács, Z.; Farkas, A. Morphological characterisation of the floral nectary in some apple-shaped and pear-shaped quince cultivars. Acta Bot. Hung. 2007, 49, 359–375. [Google Scholar] [CrossRef]
- Farkas, Á. Floral Attractivity of Pyrus Microtaxa. PhD. Dissertation, University of Pécs, Department of Botany, Pécs, Hungary, 2001; pp. 1–93. (In Hungarian). [Google Scholar]
- Chatt, E.C.; Mahalim, S.N.; Mohd-Fadzil, N.A.; Roy, R.; Klinkenberg, P.M.; Horner, H.T.; Hampton, H.T.; Carter, C.J.; Nikolau, B.J. Nectar biosynthesis is conserved among floral and extrafloral nectaries. Plant Physiol. 2021, 185, 1595–1616. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.C.; Jordano, P.; Valido, A. Functional consequences of plant-animal interactions along the mutualism-antagonism gradient. Ecology 2017, 98, 1266–1276. [Google Scholar] [CrossRef]
- Zhang, C.; Vereecken, N.J.; Wang, L.; Tian, B.; Dafni, A.; Yang, Y.; Duan, Y. Are nectar guide colour changes a reliable signal to pollinators that enhances reproductive success? Plant Ecol. Divers. 2017, 10, 89–96. [Google Scholar] [CrossRef]
- Grass, I.; Bohle, V.; Tscharntke, T.; Westphal, C. How plant reproductive success is determined by the interplay of antagonists and mutualists. Ecosphere 2018, 9, e02106. [Google Scholar] [CrossRef]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in plant-insect mutualistic relationships: From food reward to partner manipulation. Front. Plant. Sci. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Fahn, A. Structure and function of secretory cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar] [CrossRef]
- Pozo, M.I.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Peck, R.L., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 1–45. [Google Scholar]
- Kostryco, M.; Chwil, M. Structure of anther epidermis and endothecium, production of pollen, and content of selected nutrients in pollen grains from six Rubus idaeus L. cultivars. Agronomy 2021, 11, 1723. [Google Scholar] [CrossRef]
- Cole, R.M. Transmission electron microscopy. Basic techniques. In Methods in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press Inc.: New York, NY, USA, 2012; pp. 43–50. [Google Scholar]
- Hayat, M. Basic Techniques for Transmission Electron Microscopy; Academic Press: San Diego, CA, USA, 2012; p. 535. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure: Principles and Selected Methods; The Rmacarphi Pty Ltd: Melbourne, Australia, 1981; p. 357. [Google Scholar]
- Nevalainen, T.J.; Laitio, M.; Lindgren, I. Periodic acid Schiff (PAS) staining of epon-embedded tissues for light microscopy. Acta Histochem. 1972, 42, 230–233. [Google Scholar]
- Wędzony, M. Fluorescence Microscopy for Botanists; Dept. Plant Physiology Monographs: Krakow, Poland, 1996; p. 113. (In Polish) [Google Scholar]
- Harrison, C.J.; Jack, E.M.; Allen, T.D.; Harris, R. The fragile X: A scanning electron microscope study. J. Med. Genet. 1983, 20, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Chwil, M.; Konarska, A.; Weryszko-Chmielewska, E. Diversity of the floral nectaries surface of four Crataegus L. species. Acta Agrobot. 2006, 59, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Chwil, M.; Weryszko-Chmielewska, E. Comparison of features of the epidermis and the size of the floral nectary in four species of the genus Cotoneaster Med. Acta Agrobot. 2011, 64, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Sulborska-Różycka, A.; Sawidis, T. Structure of the nectary in Chaenomeles japonica (Thunb.) Lindl. ex Spach. in different stages of flowering with focus on nectar secretion. Protoplasma 2022, 1–10. [Google Scholar] [CrossRef]
- Konarska, A. Characteristics of the surface of the epidermis in floral nectaries and the receptacle of mountain ash (Sorbus aucuparia L.). Acta Agrobot. 2007, 60, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Orosz-Kovács, Z.; Gulyás, S.; Kaposavári, F. Cuticular pattern of the surface in some taxa of Prunoideae. Bot. Közl. 1990, 77, 133–138. [Google Scholar]
- Orosz-Kovacs, Z.; Róka, K.; Surányi, D.; Erdos, Z.; Kaposvari, F. Surface of intrafloral nectary in ‘Besztercei’ plum clones. Nova Ser. 1999, 1–4, 41–48. [Google Scholar]
- Orosz-Kovács, Z. Nectary structures in cherry cultivars. Acta Agron. Hung. 1993, 42, 239–253. [Google Scholar]
- Paiva, E.A.S. How does the nectar of stomata-free nectaries cross the cuticle? Acta Bot. Bras. 2017, 31, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sawhney, V.K.; Davis, A.R. Annular floral nectary with oil-producing trichomes in Salvia farinacea (Lamiaceae): Anatomy, histochemistry, ultrastructure, and significance. Am. J. Bot. 2014, 101, 1849–1867. [Google Scholar] [CrossRef] [Green Version]
- Nagy-Tóth, E.; Bubán, T.; Hevesi, M.; Orosz-Kovács, Z.S. Morphological characteristics of the nectary and composition of nectar in flowers of selected apple cultivars. Acta Hort. 2000, 538, 301–308. [Google Scholar] [CrossRef]
- Koteyeva, N.K. A novel structural type of plant cuticle. Dok. Biol. Sci. 2005, 403, 283–285. [Google Scholar] [CrossRef]
- Brehm, B.G.; Krell, D. Flavonoid localization in epidermal papillae of flower petals: A specialized adaptation for ultraviolet absorption. Science 1975, 190, 1221–1223. [Google Scholar] [CrossRef]
- Schulte, A.J.; Mail, M.; Hahn, L.A.; Barthlott, W. Ultraviolet patterns of flowers revealed in polymer replica–caused by surface architecture. Beilstein J. Nanotechnol. 2019, 10, 459–466. [Google Scholar] [CrossRef]
- Riederer, M.; Muller, C. Annual Plant Reviews, Biology of the Plant Cuticle; John Wiley & Sons: Würzburg, Germany, 2008; p. 443. [Google Scholar]
- Stpiczyńska, M.; Kamińska, M.; Zych, M. Nectary structure in dichogamous flowers of Polemonium caeruleum L. (Polemoniaceae). Acta Biol. Crac. Ser. Bot. 2012, 54, 61–68. [Google Scholar] [CrossRef]
- Chwil, M. Structure of Flower Nectaries and Beekeeping Value of Plant Factors from the Subfamily Prunoideae (Rosaceae). Habilitation Thesis, Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie, Lublin, Poland, 2013; p. 108. (In Polish). [Google Scholar]
- Weryszko-Chmielewska, E.; Masierowska, M.L.; Konarska, A. Characteristics of floral nectaries and nectar in two species of Crataegus (Rosaceae). Plant Syst. Evol. 2003, 238, 33–41. [Google Scholar] [CrossRef]
- Dmitruk, M.; Weryszko-Chmielewska, E. The morphology and ultrastructure of the nectaries of marrow (Cucurbita pepo L. convar. giromontiina). Acta Agrobot. 2013, 66, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Gaffal, K.P. How common is the ability of extrafloral nectaries to produce nectar droplets, to secrete nectar during the night and to store starch? Plant Biol. 2012, 14, 691–695. [Google Scholar] [CrossRef]
- Abedini, M.; Movafeghi, A.L.I.; Aliasgharpour, M.; Dadpour, M.R. Anatomy and ultrastructure of the floral nectary in Peganum harmala L. (Nitrariaceae). Plant Species Biol. 2013, 28, 185–192. [Google Scholar] [CrossRef]
- Davis, A.R.; Gunning, B.E.S. The modified stomata of the floral nectary of Vicia faba L. 1. Development, anatomy, and ultrastructure. Protoplasma 1992, 166, 134–152. [Google Scholar] [CrossRef]
- Wist, T.J.; Davis, A.R. Floral nectar production and nectary anatomy and ultrastructure of Echinacea purpurea (Asteraceae). Ann. Bot. 2006, 97, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Galetto, L. Nectary structure and nectar characteristics in some Bignoniaceae. Plant Syst. Evol. 1995, 196, 99–121. [Google Scholar] [CrossRef]
- Chwil, M.; Chwil, S. Micromorphology and ultrastructure of the floral nectaries of Polemonium caeruleum L. (Polemoniaceae). Protoplasma 2012, 249, 1059–1069. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Konarska, A. Micromorphology of Sorbus intermedia Pers. nectary surface in different phases of blooming (in Polish). Acta Agrobot. 2006, 59, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Hu, Z.; Muuml, I.M. Ultrastructural investigations on floral nectary of Arabidopsis thaliana prepared by high pressure freezing and freeze substitution. Biology Cell 1995, 84, 225. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Z.H. Cytological studies on the development of sieve element and floral nectary tissue in Arabidopsis thaliana. Acta Bot. Sin. 2002, 44, 9–14. [Google Scholar]
- Radice, S.; Galati, B.G. Floral nectary ultrastructure of Prunus persica (L.) Batch cv. Forastero (Newcomer), an Argentine peach. Plant Syst. Evol. 2003, 238, 23–32. [Google Scholar] [CrossRef]
- Nagy Tóth, E.; Filep, R.; Farkas, Á. Nectary structure of Cotoneaster roseus. Acta Biol. Szeged. 2011, 55, 243–246. Available online: http://160.114.156.156/index.php/abs/article/view/2754 (accessed on 1 June 2022).
- Bubán, T.; Orosz-Kovács, Z.; Farkas, Á. The nectary as the primary site of infection by Erwinia amylovora (Burr.) Winslow et al.: A mini review. Plant Syst. Evol. 2003, 238, 183–194. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Chwil, M. Micromorphology of the epidermis of the floral nectary of Rhododendron japonicum [A. Gray] JV Suringar ex EH Wilson. Acta Agrobot. 2007, 60, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Chwil, M. Flowering biology and structure of floral nectaries in Galanthus nivalis L. Acta Soc. Bot. Pol. Pol. 2016, 85, 3486. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Chwil, M. Structure of floral nectaries in Aesculus hippocastanum L. Acta Bot. Croat. 2017, 76, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Orosz-Kovács, Z.S.; Apostol, J. Structure of the nectary and yield in sweet cherry cultivars. Acta Hortic. 1993, 410, 467–470. [Google Scholar] [CrossRef]
- Orosz-Kovács, Z.S.; Kaposvari, F.; Gulyás, S. Structure of the floral nectary in sour cherry cultivars. Acta Hortic. 1993, 410, 463–466. [Google Scholar] [CrossRef]
- Paiva, E.A.S. How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann. Bot. 2016, 117, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Ascensão, L.; Pais, M.S. The leaf capitate trichomes of Leonotis leonurus: Histochemistry, ultrastructure and secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Tölke, E.D.; Bachelier, J.B.; Lima, E.A.; Galetto, L.; Demarco, D.; Carmello-Guerreiro, S.M. Diversity of floral nectary secretions and structure, and implications for their evolution in Anacardiaceae. Bot. J. Linn. Soc. 2018, 187, 209–231. [Google Scholar] [CrossRef]
- Tresmondi, F.; Nogueira, A.; Guimarães, E.; Machado, S.R. Morphology, secretion composition, and ecological aspects of stipular colleters in Rubiaceae species from tropical forest and savanna. Sci. Nat. 2015, 102, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mesquita-Neto, J.N.; Paiva, E.A.S.; Galetto, L.; Schlindwein, C. Nectar secretion of floral buds of Tococa guianensis mediates interactions with generalist ants that reduce florivory. Front Plant Sci. 2020, 11, 627. [Google Scholar] [CrossRef]
- Nepi, M. Nectary structure and ultrastructure. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 129–166. [Google Scholar]
- Stpiczyńska, M. Nectar resorption in the spur of Platanthera chlorantha Custer (Rchb.) Orchidaceae–structural and microautoradiographic study. Plant Syst. Evol. 2003, 238, 119–126. [Google Scholar] [CrossRef]
- Ning, X.P.; Tang, T.X.; Wu, H. Relationship between the morphological structure of floral nectaries and the formation, transport, and secretion of nectar in lychee. Trees 2017, 31, 1–14. [Google Scholar] [CrossRef]
- Nepi, M.; Cresti, L.; Guarnieri, M.; Pacini, E. Dynamics of nectar production and nectar homeostasis in male flowers of Cucurbita pepo L. Int. J. Plant Sci. 2011, 172, 183–190. [Google Scholar] [CrossRef]
- Paiva, E.A. Anatomy, ultrastructure, and secretory activity of the floral nectaries in Swietenia macrophylla (Meliaceae). Am. J. Bot. 2012, 99, 1910–1917. [Google Scholar] [CrossRef]
- Durkee, L.T.; Gaal, D.J.; Reisner, W.H. The floral and extra-floral nectaries of Passiflora. I. The floral nectary. Am. J. Bot. 1981, 68, 453–462. [Google Scholar] [CrossRef]
- Nepi, M.; Ciampolini, F.; Pacini, E. Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Ann. Bot. 1996, 78, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Gui, M.Y.; Liu, W.Z. Programmed cell death during floral nectary senescence in Ipomoea purpurea. Protoplasma 2014, 251, 677–685. [Google Scholar] [CrossRef]
- Mosti, S.; Friedman, R.C.; Pacini, E.; Brighigna, L.; Papini, A. Nectary ultrastructure and secretory modes in three species of Tillandsia (Bromeliaceae) that have different pollinators. Botany 2013, 91, 786–798. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Machado, S.R. The floral nectary of Hymenaea stigonocarpa (Fabaceae, Caesalpinioideae): Structural aspects during floral development. Ann. Bot. 2008, 101, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, S.R.; Gregório, E.A.; Rodrigues, T.M. Structural associations between organelle membranes in nectary parenchyma cells. Planta 2018, 247, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konarska, A.; Masierowska, M. Structure of floral nectaries and female-biased nectar production in protandrous species Geranium macrorrhizum and Geranium phaeum. Protoplasma 2020, 257, 501–523. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Galastri, N.A.; Oliveira, D.M.T. Nectary and elaiophore work together in flowers of Xylopia aromatica (Annonaceae): Structure indicates a role in pollination. Botany 2021, 99, 33–42. [Google Scholar] [CrossRef]
- Razem, F.A.; Davis, A.R. Anatomical and ultrastructural changes of the floral nectary of Pisum sativum L. during flower development. Protoplasma 1999, 206, 57–72. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Tobin, A.K. Compartmentation of metabolism within mitochondria and plastids. J. Exp. Bot. 2001, 52, 513–527. [Google Scholar] [CrossRef]
- Mercadante-Simões, M.O.; Paiva, E.A.S. Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): Ecological, morphological and structural aspects. C. R. Biol. 2013, 336, 400–406. [Google Scholar] [CrossRef]
- Horner, H.T.; Healy, R.A.; Cervantes-Martinez, T.; Palmer, R.G. Floral nectary fine structure and development in Glycine max L. (Fabaceae). Int. J. Plant Sci. 2003, 164, 675–690. [Google Scholar] [CrossRef] [Green Version]
- García, M.T.A.; Galati, B.G.; Hoc, P.S. Ultrastructure of the corona of scented and scentless flowers of Passiflora spp. (Passifloraceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 302–315. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae). Ann. Bot. 2009, 104, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.R.; Rodrigues, T.M. Autophagy and vacuolar biogenesis during the nectary development. Planta 2019, 250, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A. Microstructure of floral nectaries in Robinia viscosa var. hartwigii (Papilionoideae, Fabaceae)—A valuable but little-known melliferous plant. Protoplasma 2020, 257, 421–437. [Google Scholar] [CrossRef]
- Machado, S.R.; Rodrigues, T.M. Apoplasmic barrier in the extrafloral nectary of Citharexylum myrianthum (Verbenaceae). Planta 2021, 254, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Rodrigues, G.; de Barros, F.; Davis, A.R.; Cardoso-Gustavson, P. Floral glands in myophilous and sapromyophilous species of Pleurothallidinae (Epidendroideae, Orchidaceae)-osmophores, nectaries, and a unique sticky gland. Protoplasma 2021, 258, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.S.; Figueiredo, A.C.S. Floral nectaries from Limodorum abortivum (L.) Sw. and Epipactis atropurpurea Rafin. (Orchidaceae): Ultrastructural changes in plastids during the secretory process. Apidologie 1994, 25, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Vassilyev, A.E. On the mechanisms of nectar secretion: Revisited. Ann. Bot. 2010, 105, 349–354. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. [Google Scholar]
- Płachno, B.; Świątek, P.; Wistuba, A. The giant extra-floral nectaries of carnivorous Heliamphora folliculata: Architecture and ultrastructure. Acta Biol. Cracov. Series Botanica 2007, 4, 91–104. [Google Scholar]
- Young, R.E.; McFarlane, H.E.; Hahn, M.G.; Western, T.L.; Haughn, G.W.; Samuels, A.L. Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 2008, 20, 1623–1638. [Google Scholar] [CrossRef] [Green Version]
- Kram, B.W.; Xu, W.W.; Carter, C.J. Uncovering the Arabidopsis thaliana nectary transcriptome: Investigation of differential gene expression in floral nectariferous tissues. BMC Plant Biol. 2009, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhang, X. Floral nectaries and pseudonectaries in Eranthis (Ranunculaceae): Petal development, micromorphology, structure and ultrastructure. Protoplasma 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.A.; Lattar, E.C.; Galati, B.G.; Ferrucci, M.S. Nectary structure and ultrastructure in two floral morphs of Koelreuteria elegans subsp. formosana (Sapindaceae). Flora 2017, 226, 29–37. [Google Scholar] [CrossRef]
- Silva, S.C.D.M.; Machado, S.R.; Nepi, M.; Rodrigues, T.M. Structure and function of secretory glochids and nectar composition in two Opuntioideae (Cactaceae) species. Botany 2020, 98, 425–437. [Google Scholar] [CrossRef]
- Nawrath, C.; Schreiber, L.; Franke, R.B.; Geldner, N.; Reina-Pinto, J.J.; Kunst, L. Apoplastic diffusion barriers in Arabidopsis. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 11, p. e0167. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.F.; Machado, S.R. Anatomy, ultrastructure and secretion of Hibiscus pernambucensis Arruda (Malvaceae) extrafloral nectary. Rev. Bras. Bot. 2009, 32, 489–498. [Google Scholar] [CrossRef] [Green Version]





| Trait | Study Year | R. idaeus | |||||
|---|---|---|---|---|---|---|---|
| ‘Glen Ample’ | ‘Laszka’ | ‘Radziejowa’ | ‘Pokusa’ | ‘Polana’ | ‘Polka’ | ||
| Mean ± SD | |||||||
| Length of sepals | 2016 | 9.8 ± 1.09 aB | 8.87 ± 1.74 aB | 9.10 ± 0.80 aB | 10.64 ± 0.92 aAB | 12.00 ± 1.00 aA | 12.10 ± 1.16 aA |
| 2017 | 8.56 ± 0.86 aB | 8.48 ± 1.05 aB | 8.47 ± 0.94 aB | 13.58 ± 1.63 aA | 12.61 ± 0.87 aA | 11.73 ± 1.08 aA | |
| 2018 | 9.29 ± 1.22 aB | 9.44 ± 1.46 aB | 8.19 ± 0.90 aB | 13.64 ± 0.81 aA | 11.12 ± 1.73 aB | 11.73 ± 0.95 aB | |
| Mean | 9.22 ± 1.18 BC | 8.93 ± 1.51 C | 8.60 ± 0.95 C | 12.16 ± 1.92 A | 12.25 ± 1.15 A | 11.85 ± 1.08 AB | |
| Width of sepals | 2016 | 4.81 ± 0.59 aA | 4.68 ± 0.89 aA | 5.40 ± 0.70 aA | 4.55 ± 0.58 aA | 5.00 ± 1.00 aA | 4.63 ± 0.50 aA |
| 2017 | 4.87 ± 0.56 a | 4.75 ± 0.75 aA | 4.75 ± 0.43 aA | 5.20 ± 0.68 aA | 4.81 ± 0.47 aA | 4.48 ± 0.49 aA | |
| 2018 | 5.02 ± 0.67 a | 5.78 ± 0.77 aA | 5.96 ± 0.59 aA | 5.20 ± 0.41 aA | 4.53 ± 0.94 aA | 5.01 ± 1.78 aA | |
| Mean | 4.90 ± 0.62 A | 4.92 ± 1.01 A | 5.37 ± 0.63 A | 4.89 ± 0.68 A | 4.89 ± 0.71 A | 4.71 ± 1.13 A | |
| Length of corolla petals | 2016 | 6.98 ± 0.68 aA | 7.17 ± 0.59 aA | 6.23 ± 0.61 aA | 6.00 ± 0.86 aA | 7.32 ± 0.60 aA | 7.03 ± 0.56 aA |
| 2017 | 7.90 ± 4.9 aA | 7.50 ± 1.63 aA | 6.28 ± 0.64 aA | 8.86 ± 0.66 aA | 9.38 ± 2.33 aA | 7.46 ± 0.77 aA | |
| 2018 | 8.23 ± 0.50 aA | 8.50 ± 0.59 aA | 7.02 ± 0.45 aA | 9.40 ± 0.65 aA | 8.71 ± 0.77 aA | 7.60 ± 0.65 aA | |
| Mean | 7.71 ± 0.77 A | 7.68 ± 0.81 A | 6.51 ± 0.68 A | 7.64 ± 1.70 A | 8.21 ± 1.59 A | 7.37 ± 0.71 A | |
| Width of corolla petals | 2016 | 3.31 ± 0.55 aA | 3.71 ± 1.55 aA | 3.20 ± 0.50 aA | 2.70 ± 0.63 aA | 3.43 ± 0.51 aA | 3.15 ± 0.45 aA |
| 2017 | 3.86 ± 0.51 aA | 3.06 ± 0.50 aA | 3.10 ± 0.41 aA | 4.10 ± 0.51 aA | 3.70 ± 0.43 aA | 3.36 ± 0.47 aA | |
| 2018 | 4.04 ± 0.31 aA | 4.00 ± 0.34 aA | 3.58 ± 0.47 aA | 4.68 ± 0.45 aA | 4.29 ± 0.47 aA | 3.59 ± 0.46 aA | |
| Mean | 3.73 ± 0.56 A | 3.59 ± 1.08 A | 3.29 ± 0.51 A | 3.55 ± 0.98 A | 3.62 ± 0.55 A | 3.37 ± 0.49 A | |
| Trait | R. idaeus | |||||
|---|---|---|---|---|---|---|
| ‘Glen Ample’ | ‘Laszka’ | ‘Radziejowa’ | ‘Pokusa’ | ‘Polana’ | ‘Polka’ | |
| Mean ± SD | ||||||
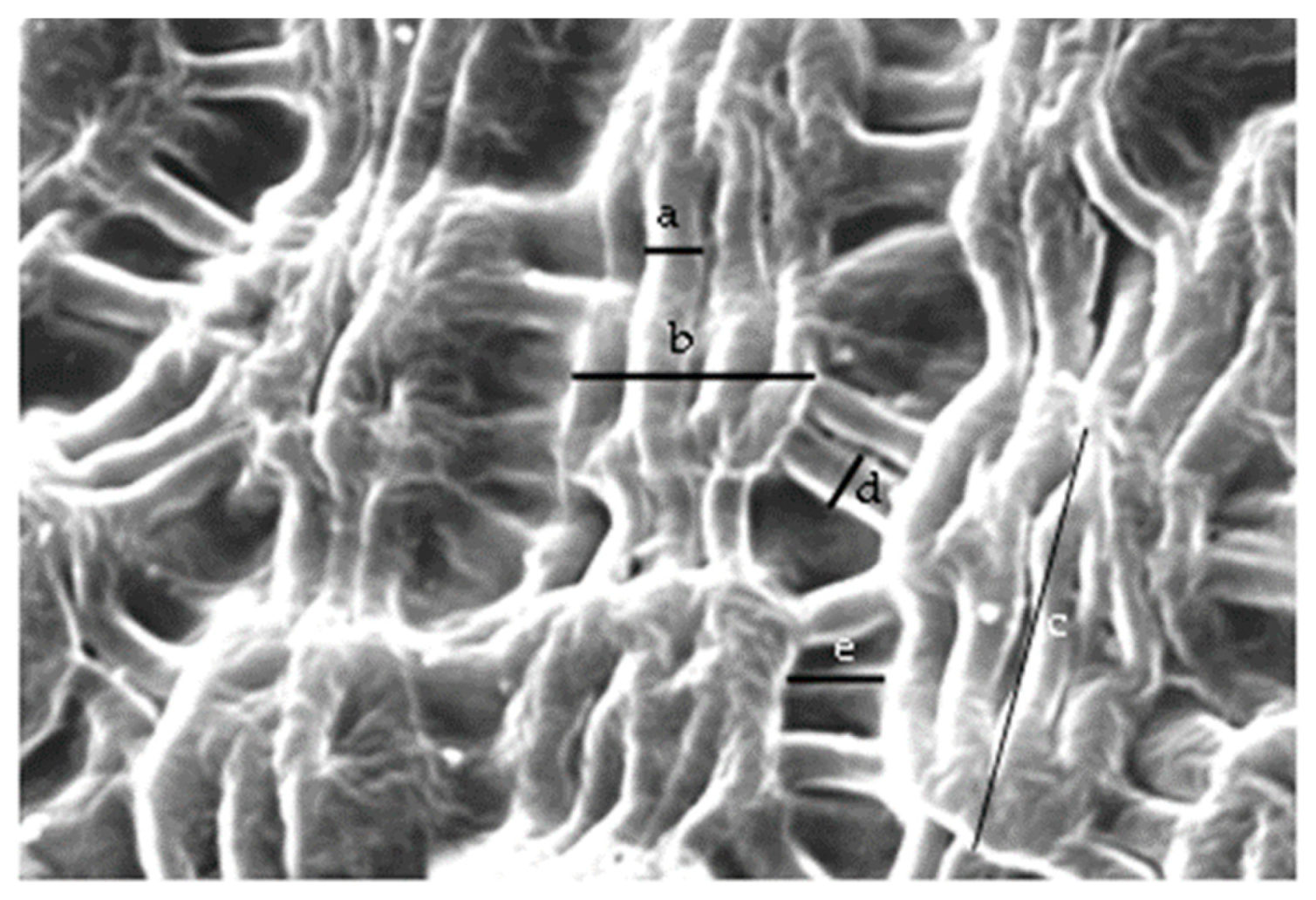
| Width of band-forming striae | 1.86 ± 0.16 A | 1.71 ± 0.24 A | 1.64 ± 0.20 A | 1.86 ± 0.27 A | 1.92 ± 0.36 A | 1.76 ± 0.18 A |
| Length of cuticle bands | 11.60 ± 1.31 A | 11.06 ± 1.95 A | 10.54 ± 1.95 A | 9.45 ± 2.10 A | 12.51 ± 2.07 A | 10.39 ± 1.19 A |
| Width of cuticle bands | 9.98 ± 1.58 AB | 8.31 ± 1.23 A-C | 8.15 ± 1.19 BC | 7.82 ± 1.62 C | 10.31 ± 1.24 A | 9.15 ± 0.91 A-C |
| Distance between cuticle bands | 4.01 ± 0.61 A | 3.69 ± 0.35 AB | 1.63 ± 0.26 C | 2.81 ± 0.71 BC | 4.81 ± 0.71 A | 1.87 ± 0.47 C |
| Width of striae connecting cuticle bands | 1.83 ± 0.19 A | 1.98 ± 0.22 A | 1.41 ± 0.21 A | 1.97 ± 0.26 A | 2.08 ± 0.69 A | 1.47 ± 0.21 A |
| Trait | R. idaeus | |||||||
|---|---|---|---|---|---|---|---|---|
| ‘Glen Ample’ | ‘Laszka’ | ‘Radziejowa’ | ‘Pokusa’ | ‘Polana’ | ‘Polka’ | |||
| Mean ± SD | ||||||||
| Length of stomata | µm | 12.37 ± 2.38 A | 14.96 ± 0.77 A | 11.91 ± 1.18 A | 10.82 ± 1.95 A | 13.41 ± 2.51 A | 11.88 ± 0.80 A | |
| Width of stomata | 10.84 ± 1.99 A | 11.54 ± 1.09 A | 8.74 ± 1.37 A | 8.32 ± 1.76 A | 11.25 ± 1.95 A | 10.34 ± 1.10 A | ||
| Surface area of stomata | µm2 | 95.18 ± 18.08 B | 130.28 ± 24.70 A | 70.84 ± 10.46 C | 87.24 ± 15.32 B | 101.32 ± 17.48 AB | 95.57 ± 14.26 B | |
| Width of aperture between cuticular ledges | µm | 1.93 ± 0.33 B | 2.78 ± 0.51 AB | 2.34 ± 0.60 B | 3.29 ± 0.56 A | 1.92 ± 0.28 B | 2.29 ± 0.46 AB | |
| Length of aperture between cuticular ledges | 4.37 ± 0.93 B | 7.65 ± 1.69 A | 3.26 ± 0.54 B | 4.12 ± 0.74 B | 6.25 ± 0.97 A | 4.37 ± 0.71 B | ||
| Number of stomata per 1 mm2 | 76.69 ± 15.74 B | 70.33 ± 20.15 BC | 107.21 ± 22.25 A | 55.64 ± 12.22 BC | 66.48 ± 16.94 BC | 48.21 ± 9.58 C | ||
| Diameter of stomatal complex | min. | µm | 26.53 ± 3.40 BC | 35.33 ± 3.38 A | 31.72 ± 2.80 AB | 25.84 ± 3.12 C | 24.36 ± 4.17 C | 29.90 ± 3.15 BC |
| max. | 34.52 ± 6.25 AB | 38.78 ± 3.96 A | 38.02 ± 4.28 A | 32.57 ± 3.57 B | 28.54 ± 5.43 B | 34.12 ± 3.19 AB | ||
| Trait | R. idaeus | ||||||
|---|---|---|---|---|---|---|---|
| ‘Glen Ample’ | ‘Laszka’ | ‘Radziejowa’ | ‘Pokusa’ | ‘Polana’ | ‘Polka’ | ||
| Mean ± SD | |||||||
| Height of nectary epidermis cells | μm | 11.24 ± 1.57 A | 11.15 ± 1.44 A | 9.32 ± 1.32 A | 11.40 ± 1.77 A | 9.24 ± 1.52 A | 12.24 ± 1.40 A |
| Width of nectary epidermis cells | 13.98 ± 1.16 A | 12.27 ± 1.12 A | 11.83 ± 1.34 A | 12.71 ± 0.81 A | 9.61 ± 0.73 A | 9.79 ± 1.59 A | |
| Thickness of nectary parenchyma layer | 173.72 ± 17.54 A | 89.98 ± 14.81 C | 168.11 ± 29.04 A | 111.20 ± 11.64 B | 115.69 ± 13.38 B | 81.88 ± 9.07 C | |
| Number of nectary parenchyma layers | 8.94 ± 1.39 A | 7.00 ± 1.03 A | 9.06 ± 1.95 A | 8.06 ± 1.06 A | 9.31 ± 1.14 A | 8.75 ± 0.68 A | |
| Diameter of nectary parenchyma cells | μm | 19.66 ± 1.98 A | 12.88 ± 1.20 B | 18.77 ± 1.74 A | 14.01 ± 2.41 B | 12.62 ± 2.19 B | 9.42 ± 2.99 B |
| Thickness of subnectary parenchyma layer | 199.25 ± 26.00 A | 178.97 ± 18.66 AB | 173.09 ± 19.45 A–C | 154.32 ± 21.75 BC | 144.84 ± 16.04 C | 176.39 ± 28.53 AB | |
| Number of subnectary parenchyma layers | 6.75 ± 0.93 AB | 6.75 ± 1.00 AB | 5.18 ± 1.52 B | 8.13 ± 0.89 A | 5.38 ± 0.62 B | 7.44 ± 2.16 AB | |
| Diameter of the subnectary parenchyma cells | μm | 30.06 ± 5.74 AB | 26.82 ± 2.95 A–C | 35.17 ± 7.33 A | 19.08 ± 2.97 C | 27.04 ± 2.29 A–C | 24.95 ± 5.93 BC |
| Trait | R. idaeus | |||||
|---|---|---|---|---|---|---|
| ‘Glen Ample’ | ‘Laszka’ | ‘Radziejowa’ | ‘Pokusa’ | ‘Polana’ | ‘Polka’ | |
| Mean ± SD | ||||||
| Thickness of the outer cell wall | 1.47 ± 0.37 B | 1.62 ± 0.35 B | 1.86 ± 0.26 B | 1.84 ± 0.36 B | 2.47 ± 0.20 A | 1.37 ± 0.26 B |
| Thickness of the cuticle lamellar layer | 0.65 ± 0.12 A | 0.76 ± 0.19 A | 0.73 ± 0.13 A | 0.85 ± 0.18 A | 0.82 ± 0.08 A | 0.65 ± 0.12 A |
| Thickness of the anticlinal cell wall | 0.53 ± 0.11 A | 0.46 ± 0.09 AB | 0.33 ± 0.06 BC | 0.50 ± 0.12 AB | 0.38 ± 0.08 BC | 0.58 ± 0.17 A |
| Thickness of the inner periclinal cell wall | 0.38 ± 0.09 B | 0.37 ± 0.13 B | 0.44 ± 0.10 AB | 0.46 ± 0.12 AB | 0.51 ± 0.12 AB | 0.63 ± 0.17 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostryco, M.; Chwil, M. Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties. Agriculture 2022, 12, 1017. https://doi.org/10.3390/agriculture12071017
Kostryco M, Chwil M. Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties. Agriculture. 2022; 12(7):1017. https://doi.org/10.3390/agriculture12071017
Chicago/Turabian StyleKostryco, Mikołaj, and Mirosława Chwil. 2022. "Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties" Agriculture 12, no. 7: 1017. https://doi.org/10.3390/agriculture12071017
APA StyleKostryco, M., & Chwil, M. (2022). Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties. Agriculture, 12(7), 1017. https://doi.org/10.3390/agriculture12071017





