The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation
Abstract
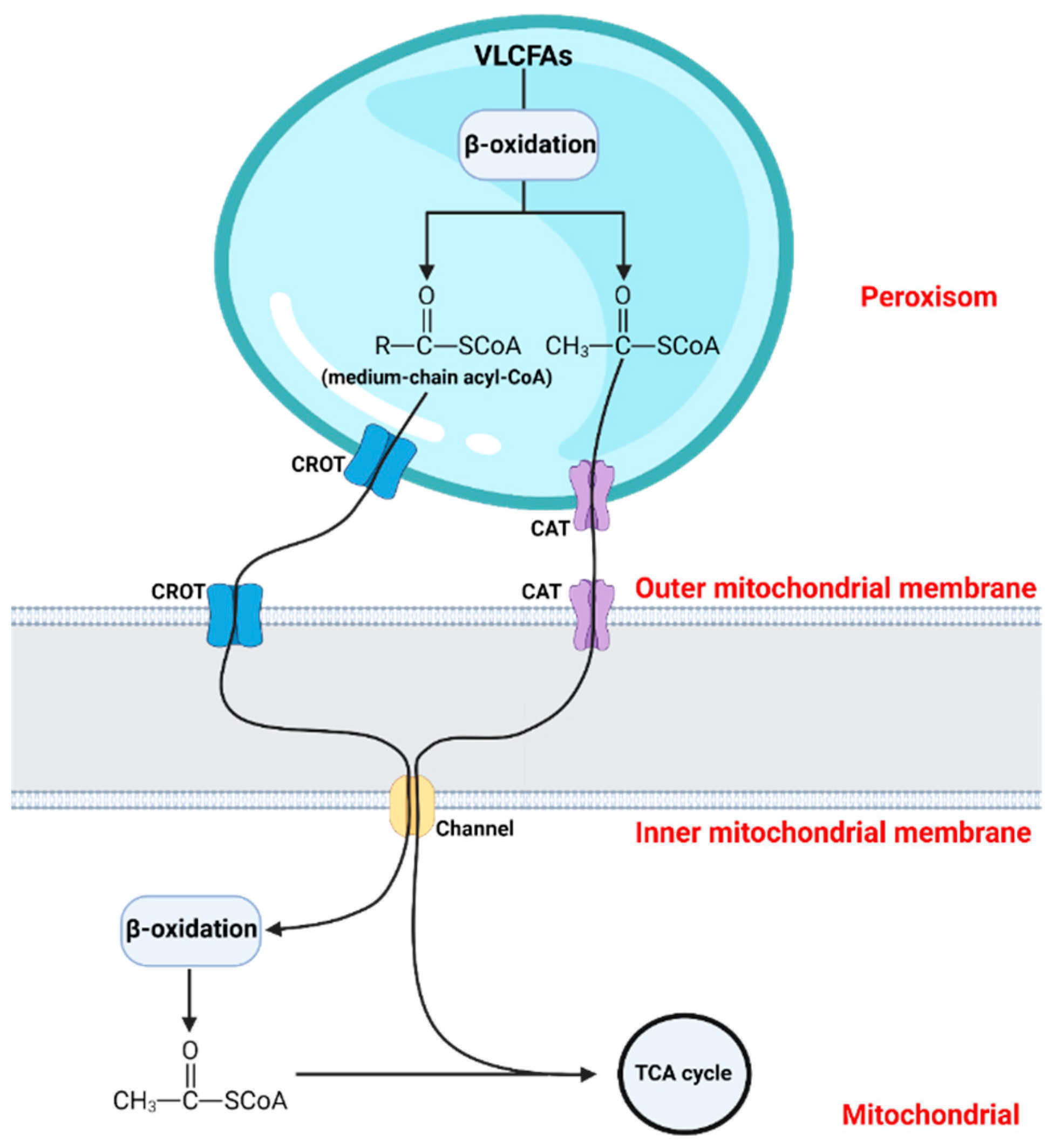
:1. Introduction
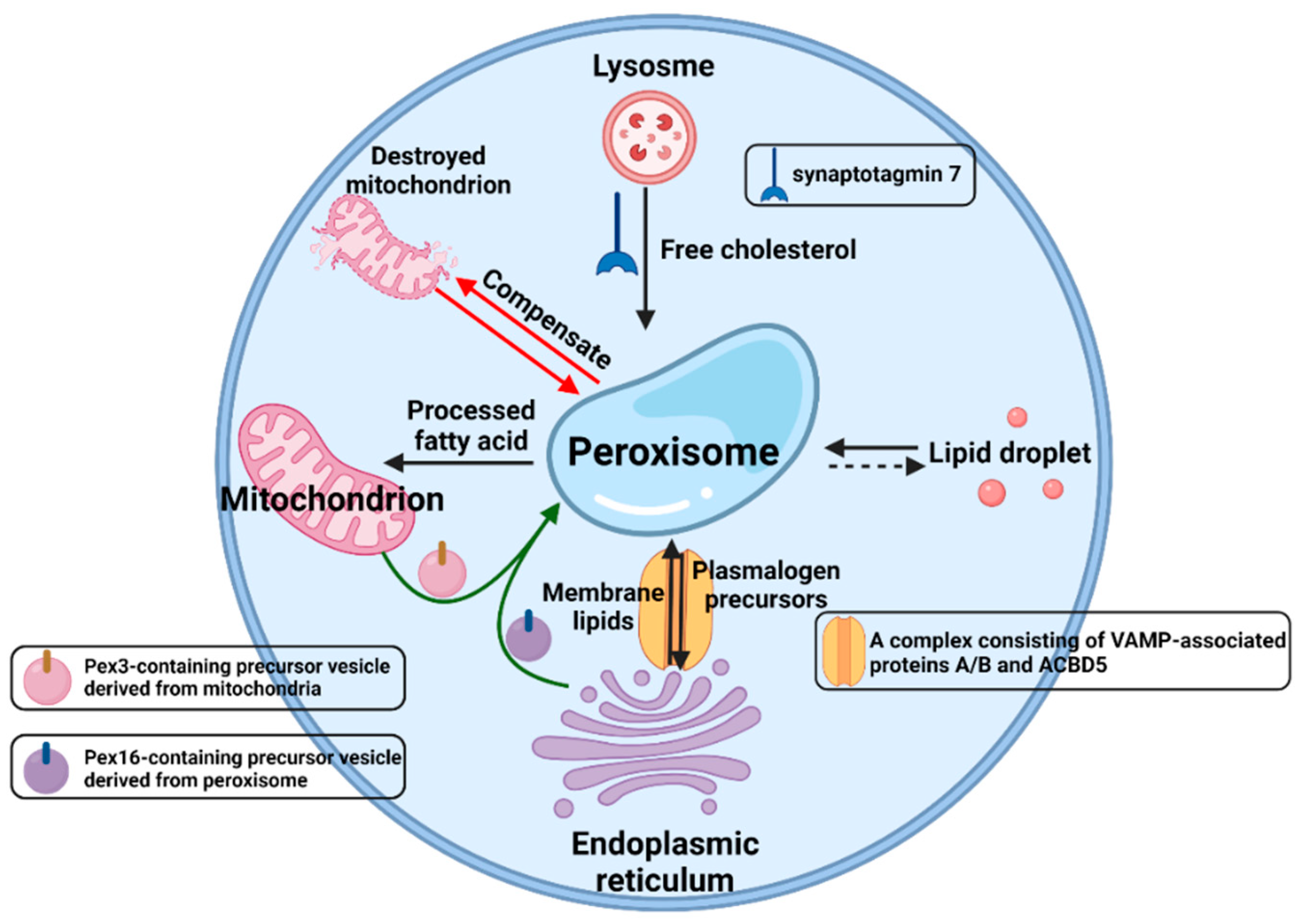
2. The Significance of Peroxisomal β-Oxidation
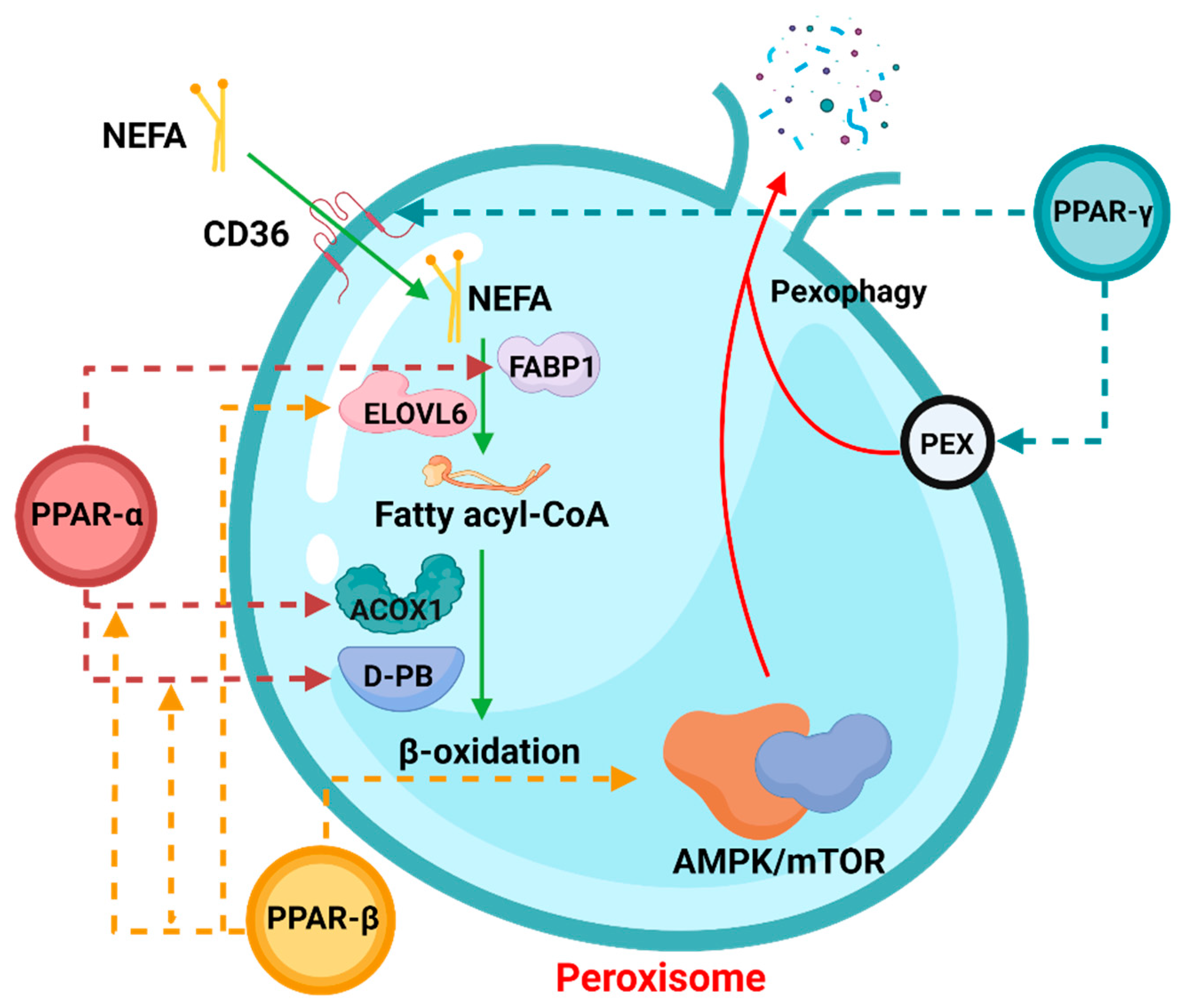
3. Factors Involved in the Regulation of the Peroxisomal β-Oxidation
3.1. PPAR
3.1.1. PPARα
3.1.2. PPARγ
3.1.3. PPARβ/δ
3.2. PGC-1α
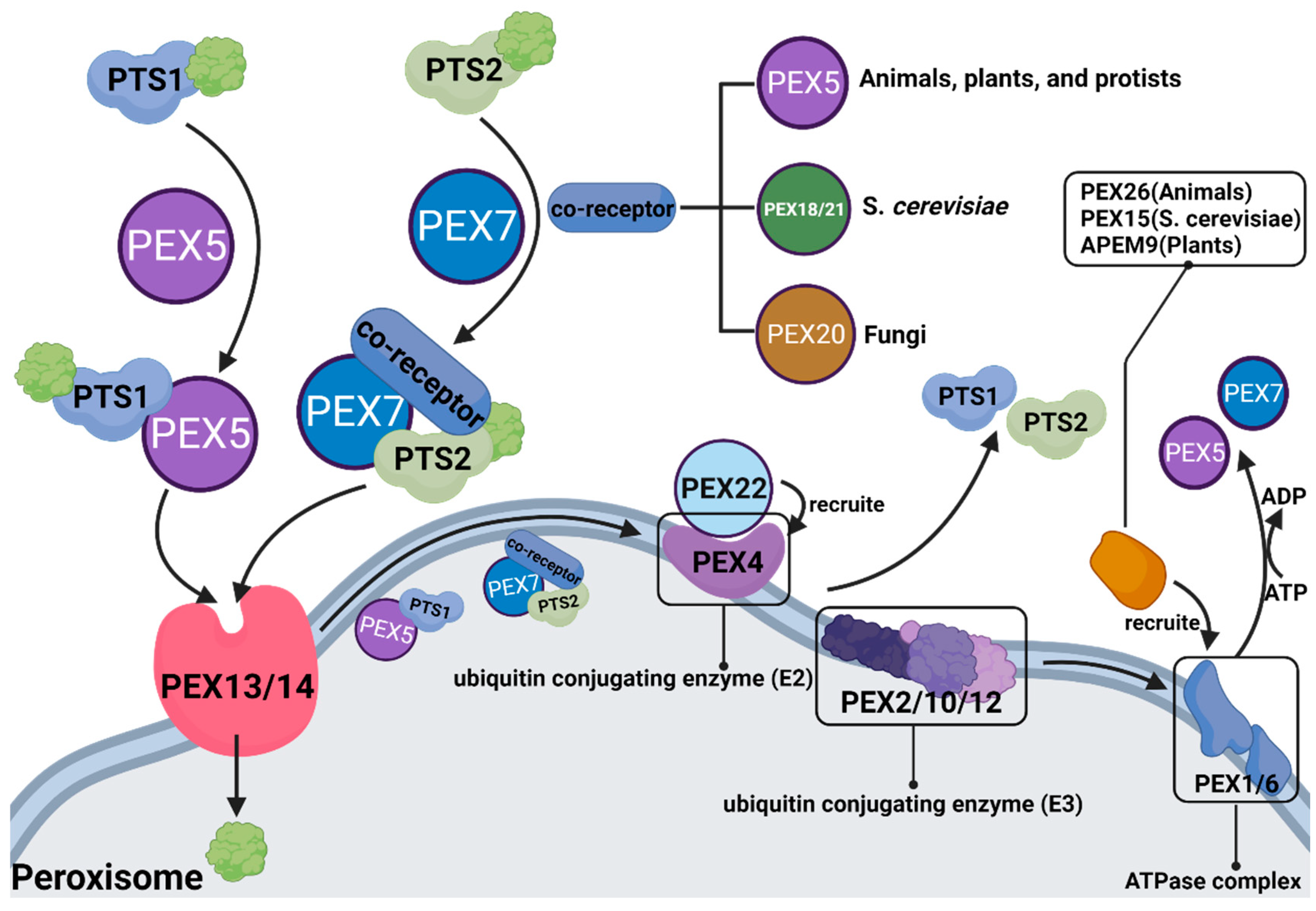
3.3. PEX
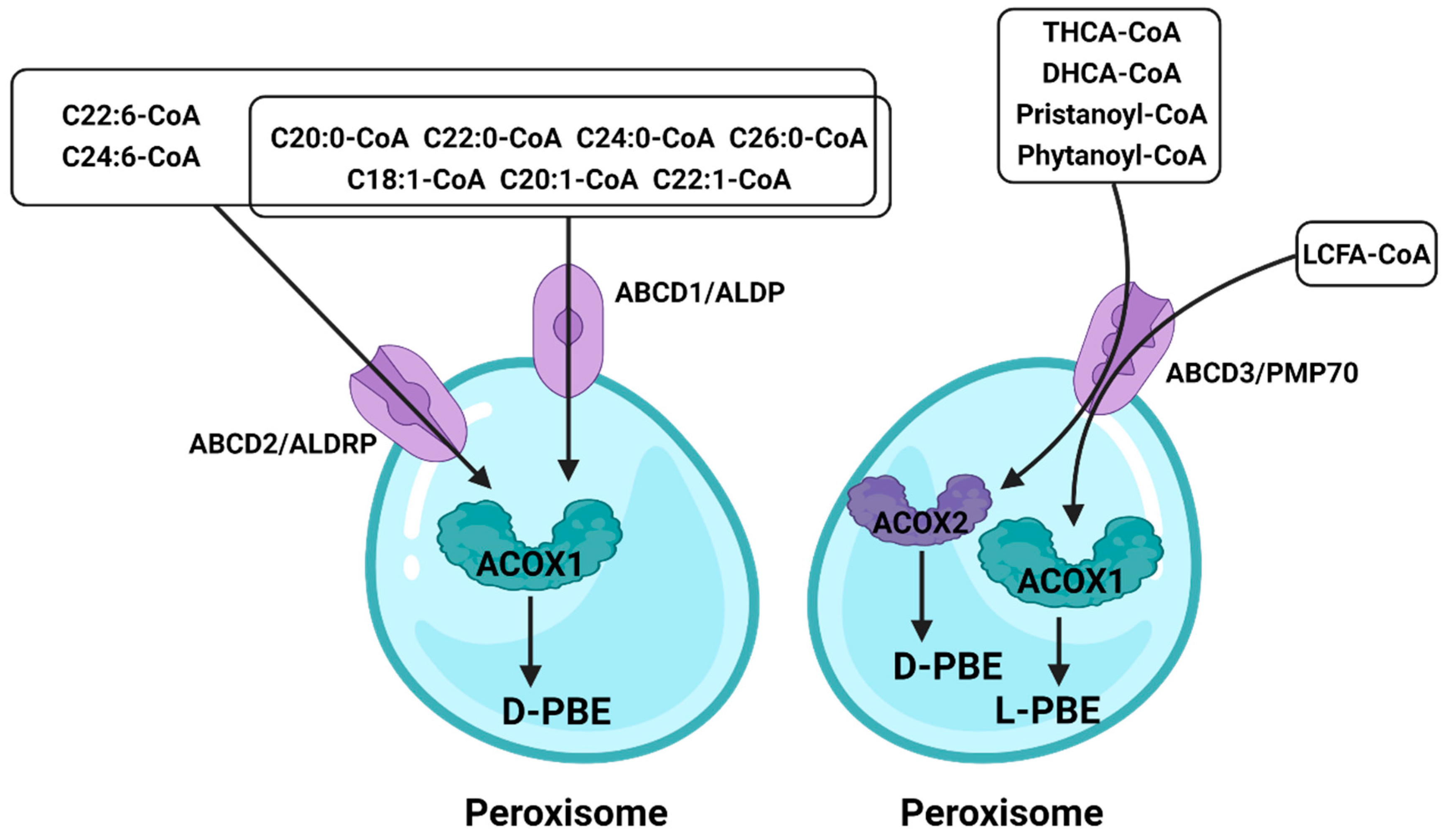
3.4. ATP-Binding Cassette (ABC)
3.5. Others
4. Discussion and Future Perspectives
| Peroxisome Biogenesis Disorders (PBDs) | Incentive | References |
|---|---|---|
| Zellweger spectrum disorders (ZSDs) | Genetic disorders caused by mutations in PEX genes | [134,135,136,137,138,139] |
| Zellweger syndrome (ZS) | Mutations in peroxisome biogenesis or mutations in PEX gene | [140,141,142,143,144,145,146,147] |
| Neonatal adrenoleukodystrophy (NALD) | Mutations in PEX gene | [148,149] |
| Infantile Refsum disease (IRD) | A medical condition within the ZSDs | [150,151,152,153] |
| Heimler syndrome (HS) | Biallelic mutations in PEX1 or PEX6 | [154,155,156] |
| Rhizomelic chondrodysplasia punctata (RCDP) | A peroxisome biogenesis disorder, may be related to PEX gene | [157,158,159,160,161] |
| X-linked adrenoleukodystrophy (X-ALD) | Mutations in ABCD1 gene | [130,162,163,164,165,166] |
| Acyl-CoA oxidase deficiency | Deletion or mutation of ACOX1 gene | [167,168,169] |
| D-Bifunctional protein deficiency | Deletion or mutation of D-BP protein | [170,171] |
| 3-Ketoacyl-CoA thiolase deficiency | Deletion or mutation of THIO enzyme | [172,173] |
| α-Methylacyl-CoA racemase deficiency | Biallelic mutations in AMACR gene | [174,175,176,177] |
| Mevalonate kinase deficiency | Mutations in peroxisome biogenesis or deletion or mutation of MK | [178,179,180,181,182] |
| Glutaric aciduria type 3 (glutaryl-CoA oxidase deficiency) | Deficiency of succinyl-CoA | [183,184,185] |
| Acatalasemia | Homozygous mutations in the catalase gene | [186,187,188] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Lu, Q.; Liang, Y.; Cui, X.; Wang, X.; Mao, Y.; Yang, Z. Circ11103 Interacts with miR-128/PPARGC1A to Regulate Milk Fat Metabolism in Dairy Cows. J. Agric. Food Chem. 2021, 69, 4490–4500. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Filppula, S.A.; Häyrinen, H.M.; Koivuranta, K.T.; Hakkola, E.H. Peroxisomal beta-oxidation of polyunsaturated fatty acids. Biochimie 1993, 75, 175–182. [Google Scholar] [CrossRef]
- Zhou, X.; Mei, H.; Agee, J.; Brown, T.; Mao, J. Racial differences in distribution of fatty acids in prostate cancer and benign prostatic tissues. Lipids Health Dis. 2019, 18, 189. [Google Scholar] [CrossRef] [Green Version]
- Valença, I.; Pértega-Gomes, N.; Vizcaino, J.R.; Henrique, R.M.; Lopes, C.; Baltazar, F.; Ribeiro, D. Localization of MCT2 at peroxisomes is associated with malignant transformation in prostate cancer. J. Cell. Mol. Med. 2015, 19, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Ha, X.; Wang, J.; Chen, K.; Deng, Y.; Zhang, X.; Feng, J.; Li, X.; Zhu, J.; Ma, Y.; Qiu, T.; et al. Free Fatty Acids Promote the Development of Prostate Cancer by Upregulating Peroxisome Proliferator-Activated Receptor Gamma. Cancer Manag. Res. 2020, 12, 1355–1369. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Erdbrügger, P.; Fröhlich, F. The role of very long chain fatty acids in yeast physiology and human diseases. Biol. Chem. 2020, 402, 25–38. [Google Scholar] [CrossRef]
- Mueller, N.; Sassa, T.; Morales-Gonzalez, S.; Schneider, J.; Salchow, D.J.; Seelow, D.; Knierim, E.; Stenzel, W.; Kihara, A.; Schuelke, M. De novo mutation in ELOVL1 causes ichthyosis, acanthosis nigricans, hypomyelination, spastic paraplegia, high frequency deafness and optic atrophy. J. Med. Genet. 2019, 56, 164–175. [Google Scholar] [CrossRef]
- Hama, K.; Fujiwara, Y.; Takashima, S.; Hayashi, Y.; Yamashita, A.; Shimozawa, N.; Yokoyama, K. Hexacosenoyl-CoA is the most abundant very long-chain acyl-CoA in ATP binding cassette transporter D1-deficient cells. J. Lipid Res. 2020, 61, 523–536. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cao, X.; Lu, Q.; Zhou, J.; Wang, Y.; Wu, Y.; Mao, Y.; Xu, H.; Yang, Z. circ01592 regulates unsaturated fatty acid metabolism through adsorbing miR-218 in bovine mammary epithelial cells. Food Funct. 2021, 12, 12047–12058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, J.; Wang, M.; Liu, J.; Zhang, L.; Loor, J.J.; Liang, Y.; Wu, H.; Yang, Z. Circ09863 Regulates Unsaturated Fatty Acid Metabolism by Adsorbing miR-27a-3p in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2020, 68, 8589–8601. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; He, A.; Tan, M.; Johnson, J.M.; Dean, J.M.; Pietka, T.A.; Chen, Y.; Zhang, X.; Hsu, F.F.; Razani, B.; et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2019, 129, 694–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defourny, J.; Aghaie, A.; Perfettini, I.; Avan, P.; Delmaghani, S.; Petit, C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl. Acad. Sci. USA 2019, 116, 8010–8017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Joshi, A.S.; Nebenfuehr, B.; Choudhary, V.; Satpute-Krishnan, P.; Levine, T.P.; Golden, A.; Prinz, W.A. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat. Commun. 2018, 9, 2940. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Ji, Y.; Jeon, Y.G.; Han, J.S.; Han, K.H.; Lee, J.H.; Lee, G.; Jang, H.; Choe, S.S.; Baes, M.; et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat. Commun. 2020, 11, 578. [Google Scholar] [CrossRef]
- Karabiyik, C.; Vicinanza, M.; Son, S.M.; Rubinsztein, D.C. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Dev. Cell 2021, 56, 1961–1975.e1965. [Google Scholar] [CrossRef]
- Angeli, E.; Trionfini, V.; Gareis, N.C.; Matiller, V.; Huber, E.; Rey, F.; Salvetti, N.R.; Ortega, H.H.; Hein, G.J. Protein and gene expression of relevant enzymes and nuclear receptor of hepatic lipid metabolism in grazing dairy cattle during the transition period. Res. Vet. Sci. 2019, 123, 223–231. [Google Scholar] [CrossRef]
- Harwood, W.S.; Drake, M.A. Validation of fluid milk consumer segments using qualitative multivariate analysis. J. Dairy Sci. 2020, 103, 10036–10047. [Google Scholar] [CrossRef]
- Flowers, S.; McFadden, B.R.; Carr, C.C.; Mateescu, R.G. Consumer preferences for beef with improved nutrient profile1. J. Anim. Sci. 2019, 97, 4699–4709. [Google Scholar] [CrossRef] [PubMed]
- Faria, O.A.C.; Kawamoto, T.S.; Dias, L.R.O.; Fidelis, A.A.G.; Leme, L.O.; Caixeta, F.M.C.; Gomes, A.; Sprícigo, J.F.W.; Dode, M.A.N. Maturation system affects lipid accumulation in bovine oocytes. Reprod. Fertil. Dev. 2021, 33, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Loor, J.J.; Li, C.; Liang, Y.; Li, N.; Shu, X.; Yang, Y.; Feng, X.; Du, X.; Wang, Z.; et al. Enhanced mitochondrial dysfunction and oxidative stress in the mammary gland of cows with clinical ketosis. J. Dairy Sci. 2021, 104, 6909–6918. [Google Scholar] [CrossRef] [PubMed]
- Cainzos, J.M.; Andreu-Vazquez, C.; Guadagnini, M.; Rijpert-Duvivier, A.; Duffield, T. A systematic review of the cost of ketosis in dairy cattle. J. Dairy Sci. 2022, 105, 6175–6195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, C.; Kong, Y.; Tan, P.; Liu, S.; Liu, Y.; Zeng, F.; Yuan, Y.; Zhao, B.; Wang, J. Elucidation of the mechanism of NEFA-induced PERK-eIF2α signaling pathway regulation of lipid metabolism in bovine hepatocytes. J. Steroid Biochem. Mol. Biol. 2021, 211, 105893. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Yu, H.; He, R.; Wang, P.; Gan, L.; Du, Y.; Huang, Y.; Abro, M.B.; Sohaib, S.; Pierzchala, M.; et al. Differentiation of Subclinical Ketosis and Liver Function Test Indices in Adipose Tissues Associated with Hyperketonemia in Postpartum Dairy Cattle. Front. Vet. Sci. 2021, 8, 796494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Sun, X.; Jia, H.; Zhao, C.; Xu, C. Adenosine 5’-monophosphate-activated protein kinase ameliorates bovine adipocyte oxidative stress by inducing antioxidant responses and autophagy. J. Dairy Sci. 2021, 104, 4516–4528. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, H.; Liu, L.; Song, Y.; Du, X.; Feng, S.; Wang, X.; Li, X.; Wang, Z.; Li, X.; et al. Non-esterified Fatty Acid Induce Dairy Cow Hepatocytes Apoptosis via the Mitochondria-Mediated ROS-JNK/ERK Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lu, X.; Arbab, A.A.I.; Wu, X.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin acts to suppress β-hydroxybutyric acid-mediated inflammatory responses through activation of AMPK signaling in bovine hepatocytes. J. Anim. Sci. 2021, 99, skab153. [Google Scholar] [CrossRef]
- Wu, Z.L.; Chen, S.Y.; Qin, C.; Jia, X.; Deng, F.; Wang, J.; Lai, S.J. Clinical Ketosis-Associated Alteration of Gene Expression in Holstein Cows. Genes 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenet. Chromatin 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaee, N.; Heidarpour, M.; Khoramian, B. Milk metabolites, proteins and oxidative stress markers in dairy cows suffering from Staphylococcus aureus subclinical mastitis with or without spontaneous cure. J. Dairy Res. 2021, 88, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Li, L.; Han, Z.; Mao, S.; Wang, G. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones 2019, 24, 453–460. [Google Scholar] [CrossRef]
- Ciampi, F.; Sordillo, L.M.; Gandy, J.C.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947. [Google Scholar] [CrossRef]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338. [Google Scholar] [CrossRef]
- Cai, D.; Li, Y.; Zhang, K.; Zhou, B.; Guo, F.; Holm, L.; Liu, H.Y. Co-option of PPARα in the regulation of lipogenesis and fatty acid oxidation in CLA-induced hepatic steatosis. J. Cell. Physiol. 2021, 236, 4387–4402. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, N.; Viswakarma, N.; Sun, R.; Schipma, M.J.; Shang, M.; Thorp, E.B.; Kanwar, Y.S.; Thimmapaya, B.; Reddy, J.K. PIMT/NCOA6IP Deletion in the Mouse Heart Causes Delayed Cardiomyopathy Attributable to Perturbation in Energy Metabolism. Int. J. Mol. Sci. 2018, 19, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.Y.; Gu, H.; Li, Y.; Hu, P.; Yang, Y.; Li, K.; Li, H.; Zhang, K.; Zhou, B.; Wu, H.; et al. Dietary Conjugated Linoleic Acid Modulates the Hepatic Circadian Clock Program via PPARα/REV-ERBα-Mediated Chromatin Modification in Mice. Front. Nutr. 2021, 8, 711398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Ma, L.; Jiang, E.; Xu, H.; Chen, R.; Yang, Q.; Chen, H.; Li, Z.; Lan, X. Reduced representation bisulfite sequencing (RRBS) of dairy goat mammary glands reveals DNA methylation profiles of integrated genome-wide and critical milk-related genes. Oncotarget 2017, 8, 115326–115344. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.; Loor, J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y.; Ding, H.; Dong, J.; Zhang, R.; Huang, D.; Lei, L.; Wang, Z.; Liu, G.; Li, X. Insulin suppresses the AMPK signaling pathway to regulate lipid metabolism in primary cultured hepatocytes of dairy cows. J. Dairy Res. 2018, 85, 157–162. [Google Scholar] [CrossRef]
- Zou, D.; Liu, R.; Shi, S.; Du, J.; Tian, M.; Wang, X.; Hou, M.; Duan, Z.; Ma, Y. BHBA regulates the expressions of lipid synthesis and oxidation genes in sheep hepatocytes through the AMPK pathway. Res. Vet. Sci. 2021, 140, 153–163. [Google Scholar] [CrossRef]
- Socha, B.M.; Łada, P.; Szczepańska, A.A.; Łupicka, M.; Korzekwa, A.J. The influence of experimentally induced endometritis on the PPAR expression profile in the bovine endometrium. Theriogenology 2018, 122, 74–83. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; Cajas, Y.N.; Peréz-Cerezales, S.; Leal, C.L.V.; Agirregoitia, E.; Gutierrez-Adán, A.; González, E.M.; Rizos, D. Nobiletin enhances the development and quality of bovine embryos in vitro during two key periods of embryonic genome activation. Sci. Rep. 2021, 11, 11796. [Google Scholar] [CrossRef]
- Cai, D.; Li, H.; Zhou, B.; Han, L.; Zhang, X.; Yang, G.; Yang, G. Conjugated linoleic acid supplementation caused reduction of perilipin1 and aberrant lipolysis in epididymal adipose tissue. Biochem. Biophys. Res. Commun. 2012, 422, 621–626. [Google Scholar] [CrossRef]
- El Ouarrat, D.; Isaac, R.; Lee, Y.S.; Oh, D.Y.; Wollam, J.; Lackey, D.; Riopel, M.; Bandyopadhyay, G.; Seo, J.B.; Sampath-Kumar, R.; et al. TAZ Is a Negative Regulator of PPARγ Activity in Adipocytes and TAZ Deletion Improves Insulin Sensitivity and Glucose Tolerance. Cell Metab. 2020, 31, 162–173.e165. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, H.; Zhao, R. Nuclear Receptors in Hepatic Glucose and Lipid Metabolism During Neonatal and Adult Life. Curr. Protein Pept. Sci. 2017, 18, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.M.; He, A.; Tan, M.; Wang, J.; Lu, D.; Razani, B.; Lodhi, I.J. MED19 Regulates Adipogenesis and Maintenance of White Adipose Tissue Mass by Mediating PPARγ-Dependent Gene Expression. Cell Rep. 2020, 33, 108228. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Nadeem, A.; Li, Z.; Javed, M.; Liu, Q.; Azhar, J.; Rehman, M.S.; Cui, K.; Rehman, S.U. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. Int. J. Mol. Sci. 2021, 22, 2463. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.T.; Girma, D.D.; Bionaz, M.; Ma, L.; Bu, D.P. Hepatic transcriptomic adaptation from prepartum to postpartum in dairy cows. J. Dairy Sci. 2021, 104, 1053–1072. [Google Scholar] [CrossRef]
- Schmitt, E.; Ballou, M.A.; Correa, M.N.; DePeters, E.J.; Drackley, J.K.; Loor, J.J. Dietary lipid during the transition period to manipulate subcutaneous adipose tissue peroxisome proliferator-activated receptor-γ co-regulator and target gene expression. J. Dairy Sci. 2011, 94, 5913–5925. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Han, Z.; Lu, X.; Zhang, H.; Arbab, A.A.I.; Loor, J.J.; Yang, Y.; Yang, Z. Identification of Milk Fat Metabolism-Related Pathways of the Bovine Mammary Gland during Mid and Late Lactation and Functional Verification of the ACSL4 Gene. Genes 2020, 11, 1357. [Google Scholar] [CrossRef]
- Sandri, E.C.; Camêra, M.; Sandri, E.M.; Harvatine, K.J.; De Oliveira, D.E. Peroxisome proliferator-activated receptor gamma (PPARγ) agonist fails to overcome trans-10, cis-12 conjugated linoleic acid (CLA) inhibition of milk fat in dairy sheep. Anim. Int. J. Anim. Biosci. 2018, 12, 1405–1412. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, X.; Ji, Y.; Cheng, S.; Wang, M.; Zhang, C.; Yu, X.; Zhao, R.; Zhang, W.; et al. Acetyl-coenzyme A acyltransferase 2 promote the differentiation of sheep precursor adipocytes into adipocytes. J. Cell. Biochem. 2019, 20, 8021–8031. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, R.; Ma, W.; Zhao, Q.; Zhang, G. Comparison of lipid deposition of intramuscular preadipocytes in Tan sheep co-cultured with satellite cells or alone. J. Anim. Physiol. Anim. Nutr. 2021, 10, 1111. [Google Scholar] [CrossRef]
- Wallace, J.M.; Milne, J.S.; Aitken, B.W.; Aitken, R.P.; Adam, C.L. Ovine prenatal growth-restriction and sex influence fetal adipose tissue phenotype and impact postnatal lipid metabolism and adiposity in vivo from birth until adulthood. PLoS ONE 2020, 15, e0228732. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ren, C.; Meng, F.; Deng, K.; Zhang, G.; Wang, F. Effects of algae supplementation in high-energy dietary on fatty acid composition and the expression of genes involved in lipid metabolism in Hu sheep managed under intensive finishing system. Meat Sci. 2019, 157, 107872. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fensterseifer, S.R.; Austin, K.J.; Ford, S.P.; Alexander, B.M. Effects of maternal obesity on maternal and fetal plasma concentrations of adiponectin and expression of adiponectin and its receptor genes in cotyledonary and adipose tissues at mid- and late-gestation in sheep. Anim. Reprod. Sci. 2018, 197, 231–239. [Google Scholar] [CrossRef]
- Yao, Y.C.; Song, X.T.; Zhai, Y.F.; Liu, S.; Lu, J.; Xu, X.; Qi, M.Y.; Zhang, J.N.; Huang, H.; Liu, Y.F.; et al. Transcriptome analysis of sheep follicular development during prerecruitment, dominant, and mature stages after FSH superstimulation. Domest. Anim. Endocrinol. 2021, 74, 106563. [Google Scholar] [CrossRef]
- Hassanpour, H.; Khalaji-Pirbalouty, V.; Adibi, M.; Nazari, H. Involvement of peroxisome proliferator-activated receptors in the estradiol production of ovine Sertoli cells. Vet. Res. Forum Int. Q. J. 2017, 8, 251–257. [Google Scholar]
- Penna-de-Carvalho, A.; Graus-Nunes, F.; Rabelo-Andrade, J.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Enhanced pan-peroxisome proliferator-activated receptor gene and protein expression in adipose tissue of diet-induced obese mice treated with telmisartan. Exp. Physiol. 2014, 99, 1663–1678. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Wang, L.; Yao, S.; Jin, L.; Yang, J.; Zhang, Y.; Ning, G.; Zhang, Z. PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation. Cell Death Dis. 2019, 10, 197. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate, a New Selective PPARα Modulator: Drug Concept and Its Clinical Applications for Dyslipidemia and Metabolic Diseases. Curr. Atheroscler. Rep. 2020, 22, 5. [Google Scholar] [CrossRef] [Green Version]
- De Vries, E.; Bolier, R.; Goet, J.; Parés, A.; Verbeek, J.; de Vree, M.; Drenth, J.; van Erpecum, K.; van Nieuwkerk, K.; van der Heide, F.; et al. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology 2021, 160, 734–743.e736. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Mora, C.; Pintado, C.; Mazuecos, L.; Fernández, A.; López, V.; Andrés, A.; Gallardo, N. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARβ/δ. Implications in cardiac remodeling. Metab. Clin. Exp. 2021, 115, 154453. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pais, A.; Ferreira, R.; Neves, J.S.; Vitorino, R.; Moreira-Gonçalves, D.; Nogueira-Ferreira, R. Sex differences on adipose tissue remodeling: From molecular mechanisms to therapeutic interventions. J. Mol. Med. 2020, 98, 483–493. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, J.M. Sex-specific regulation of immune responses by PPARs. Exp. Mol. Med. 2017, 49, e364. [Google Scholar] [CrossRef]
- Hara, S.; Furukawa, F.; Mukai, K.; Yazawa, T.; Kitano, T. Peroxisome proliferator-activated receptor alpha is involved in the temperature-induced sex differentiation of a vertebrate. Sci. Rep. 2020, 10, 11672. [Google Scholar] [CrossRef]
- Pierrot, N.; Ris, L.; Stancu, I.C.; Doshina, A.; Ribeiro, F.; Tyteca, D.; Baugé, E.; Lalloyer, F.; Malong, L.; Schakman, O.; et al. Sex-regulated gene dosage effect of PPARα on synaptic plasticity. Life Sci. Alliance 2019, 2, e201800262. [Google Scholar] [CrossRef]
- De Felice, M.; Melis, M.; Aroni, S.; Muntoni, A.L.; Fanni, S.; Frau, R.; Devoto, P.; Pistis, M. The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci. Ther. 2019, 25, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Luft, C.; Levices, I.P.; Pedrazza, L.; de Oliveira, J.R.; Donadio, M.V.F. Sex-dependent metabolic effects of pregestational exercise on prenatally stressed mice. J. Dev. Orig. Health Dis. 2021, 12, 271–279. [Google Scholar] [CrossRef]
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef]
- Xin, G.; Chen, Y.; Topchyan, P.; Kasmani, M.Y.; Burns, R.; Volberding, P.J.; Wu, X.; Cohn, A.; Chen, Y.; Lin, C.W.; et al. Targeting PIM1-Mediated Metabolism in Myeloid Suppressor Cells to Treat Cancer. Cancer Immunol. Res. 2021, 9, 454–469. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.; Sivasami, P.; Ramirez, R.N.; Zhang, Y.; Benoist, C.; Mathis, D. Interferon-α-producing plasmacytoid dendritic cells drive the loss of adipose tissue regulatory T cells during obesity. Cell Metab. 2021, 33, 1610–1623.e1615. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, N.; Park, E.S.; Yun, H.; Ha, T.U.; Jeon, H.; Yu, J.; Choi, S.; Shin, B.; Yu, J.; et al. T-Cell Death Associated Gene 51 Is a Novel Negative Regulator of PPARγ That Inhibits PPARγ-RXRα Heterodimer Formation in Adipogenesis. Mol. Cells 2021, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.A.; Vonghia, L.; Kwanten, W.J.; Vanwolleghem, T.; Ebo, D.G.; Michielsen, P.P.; De Man, J.G.; Gama, L.; De Winter, B.Y.; Francque, S.M. Adoptive Cell Transfer of Regulatory T Cells Exacerbates Hepatic Steatosis in High-Fat High-Fructose Diet-Fed Mice. Front. Immunol. 2020, 11, 1711. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontecha-Barriuso, M.; Martín-Sánchez, D.; Martinez-Moreno, J.M.; Carrasco, S.; Ruiz-Andrés, O.; Monsalve, M.; Sanchez-Ramos, C.; Gómez, M.J.; Ruiz-Ortega, M.; Sánchez-Niño, M.D.; et al. PGC-1α deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J. Pathol. 2019, 249, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagattin, A.; Hugendubler, L.; Mueller, E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 20376–20381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.Y.; Zheng, D.; Houmard, J.A.; Brault, J.J.; Hickner, R.C.; Cortright, R.N. Overexpression of PGC-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, e253–e263. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2021, 320, e1053–e1067. [Google Scholar] [CrossRef]
- Summermatter, S.; Baum, O.; Santos, G.; Hoppeler, H.; Handschin, C. Peroxisome proliferator-activated receptor {gamma} coactivator 1{alpha} (PGC-1{alpha}) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J. Biol. Chem. 2010, 285, 32793–32800. [Google Scholar] [CrossRef] [Green Version]
- Held-Hoelker, E.; Klein, S.L.; Rings, F.; Salilew-Wondim, D.; Saeed-Zidane, M.; Neuhoff, C.; Tesfaye, D.; Schellander, K.; Hoelker, M. Cryosurvival of in vitro produced bovine embryos supplemented with l-Carnitine and concurrent reduction of fatty acids. Theriogenology 2017, 96, 145–152. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, T.; Zhang, L.; Fu, L.; Hu, Y.; Li, H.; Li, C.; Zhang, J.; Liang, B.; Liu, J. miR-669a-5p promotes adipogenic differentiation and induces browning in preadipocytes. Adipocyte 2022, 11, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yan, Q.; Yang, H.; Ren, A.; He, Z.; Tan, Z. Maternal intake restriction programs the energy metabolism, clock circadian regulator and mTOR signals in the skeletal muscles of goat offspring probably via the protein kinase A-cAMP-responsive element-binding proteins pathway. Anim. Nutr. 2021, 7, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Distel, B.; Erdmann, R.; Gould, S.J.; Blobel, G.; Crane, D.I.; Cregg, J.M.; Dodt, G.; Fujiki, Y.; Goodman, J.M.; Just, W.W.; et al. A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 1996, 135, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Barros-Barbosa, A.; Rodrigues, T.A.; Ferreira, M.J.; Pedrosa, A.G.; Teixeira, N.R.; Francisco, T.; Azevedo, J.E. The intrinsically disordered nature of the peroxisomal protein translocation machinery. FEBS J. 2019, 286, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Xia, Z.J.; Farré, J.C.; Subramani, S. TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J. Cell Biol. 2017, 216, 2843–2858. [Google Scholar] [CrossRef]
- Klouwer, F.C.C.; Falkenberg, K.D.; Ofman, R.; Koster, J.; van Gent, D.; Ferdinandusse, S.; Wanders, R.J.A.; Waterham, H.R. Autophagy Inhibitors Do Not Restore Peroxisomal Functions in Cells with the Most Common Peroxisome Biogenesis Defect. Front. Cell Dev. Biol. 2021, 9, 661298. [Google Scholar] [CrossRef]
- Lotz-Havla, A.S.; Woidy, M.; Guder, P.; Friedel, C.C.; Klingbeil, J.M.; Bulau, A.M.; Schultze, A.; Dahmen, I.; Noll-Puchta, H.; Kemp, S.; et al. iBRET Screen of the ABCD1 Peroxisomal Network and Mutation-Induced Network Perturbations. J. Proteome Res. 2021, 20, 4366–4380. [Google Scholar] [CrossRef]
- Coppa, A.; Guha, S.; Fourcade, S.; Parameswaran, J.; Ruiz, M.; Moser, A.B.; Schlüter, A.; Murphy, M.P.; Lizcano, J.M.; Miranda-Vizuete, A.; et al. The peroxisomal fatty acid transporter ABCD1/PMP-4 is required in the C. elegans hypodermis for axonal maintenance: A worm model for adrenoleukodystrophy. Free Radic. Biol. Med. 2020, 152, 797–809. [Google Scholar] [CrossRef]
- Zierfuss, B.; Weinhofer, I.; Kühl, J.S.; Köhler, W.; Bley, A.; Zauner, K.; Binder, J.; Martinović, K.; Seiser, C.; Hertzberg, C.; et al. Vorinostat in the acute neuroinflammatory form of X-linked adrenoleukodystrophy. Ann. Clin. Transl. Neurol. 2020, 7, 639–652. [Google Scholar] [CrossRef]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Zhao, S.; Huang, J.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Functional redundancy of two ABC transporter proteins in mediating toxicity of Bacillus thuringiensis to cotton bollworm. PLoS Pathog. 2020, 16, e1008427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jiang, D.; Wang, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Independent and Synergistic Effects of Knocking out Two ABC Transporter Genes on Resistance to Bacillus thuringiensis Toxins Cry1Ac and Cry1Fa in Diamondback Moth. Toxins 2020, 13, 9. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Bhat, B.; Bhat, S.A.; Yaseen, M.; Mir, S.; Raza, M.; Iquebal, M.A.; Shah, R.A.; Ganai, N.A. SNPs in Mammary Gland Epithelial Cells Unraveling Potential Difference in Milk Production Between Jersey and Kashmiri Cattle Using RNA Sequencing. Front. Genet. 2021, 12, 666015. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.I.; Santiago, K.G.; Lee, D.; Ha, S.; Seo, K. RNA Sequencing (RNA-Seq) Based Transcriptome Analysis in Immune Response of Holstein Cattle to Killed Vaccine against Bovine Viral Diarrhea Virus Type I. Animals 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhou, Y.; Yang, Y.; Lv, C.; Liu, X.; Wang, Y. Involvement of ABC-transporters and acyltransferase 1 in intracellular cholesterol-mediated autophagy in bovine alveolar macrophages in response to the Bacillus Calmette-Guerin (BCG) infection. BMC Immunol. 2020, 21, 26. [Google Scholar] [CrossRef]
- Sales, A.D.; Duarte, A.B.G.; Rocha, R.M.P.; Brito, I.R.; Locatelli, Y.; Alves, B.G.; Alves, K.A.; Figueiredo, J.R.; Rodrigues, A.P.R. Transcriptional downregulation of ABC transporters is related to follicular degeneration after vitrification and in vitro culture of ovine ovarian tissue. Theriogenology 2022, 177, 127–132. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, M.; Gu, Y.; Fan, G.; Liu, C.; Li, Y.; Sun, R.; Wu, J.; Li, J.; Xue, X.; et al. Aurantio-Obtusin Attenuates Non-Alcoholic Fatty Liver Disease Through AMPK-Mediated Autophagy and Fatty Acid Oxidation Pathways. Front. Pharmacol. 2021, 12, 826628. [Google Scholar] [CrossRef]
- Xie, Y.H.; Xiao, Y.; Huang, Q.; Hu, X.F.; Gong, Z.C.; Du, J. Role of the CTRP6/AMPK pathway in kidney fibrosis through the promotion of fatty acid oxidation. Eur. J. Pharmacol. 2021, 892, 173755. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Li, Y.; Li, Z.; Zhai, W.; Sun, Q.; Yang, X.; Roth, M.; Lu, S. mTOR regulates PRMT1 expression and mitochondrial mass through STAT1 phosphorylation in hepatic cell. Biochim. Biophys. Acta. Mol. Cell Res. 2021, 1868, 119017. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, W.; Yin, X.; Zheng, X.; Li, Q.; Zhang, H.; Zheng, L.; Feng, X. Resveratrol-induced brown fat-like phenotype in 3T3-L1 adipocytes partly via mTOR pathway. Food Nutr. Res. 2020, 64, 3656. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hu, W. Asprosin promotes β-cell apoptosis by inhibiting the autophagy of β-cell via AMPK-mTOR pathway. J. Cell. Physiol. 2021, 236, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 752117. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Tian, M.X.; Sun, R.Q.; Zhang, M.L.; Zhou, L.S.; Jin, L.; Chen, L.L.; Zhou, W.J.; Duan, K.L.; Chen, Y.J.; et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018, 19, e45124. [Google Scholar] [CrossRef]
- Shumar, S.A.; Kerr, E.W.; Geldenhuys, W.J.; Montgomery, G.E.; Fagone, P.; Thirawatananond, P.; Saavedra, H.; Gabelli, S.B.; Leonardi, R. Nudt19 is a renal CoA diphosphohydrolase with biochemical and regulatory properties that are distinct from the hepatic Nudt7 isoform. J. Biol. Chem. 2018, 293, 4134–4148. [Google Scholar] [CrossRef] [Green Version]
- Shumar, S.A.; Kerr, E.W.; Fagone, P.; Infante, A.M.; Leonardi, R. Overexpression of Nudt7 decreases bile acid levels and peroxisomal fatty acid oxidation in the liver. J. Lipid Res. 2019, 60, 1005–1019. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Jin, Y.; Zhang, D.; Chen, J.; Qi, Y.; Fan, R.; Luo, J.; Xu, L.; Ma, W.; et al. Lipid metabolism disorders effects of 6:2 chlorinated polyfluorinated ether sulfonate through Hsa-miRNA-532-3p/Acyl-CoA oxidase 1(ACOX1) pathway. Ecotoxicol. Environ. Saf. 2021, 228, 113011. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, Q.; Tao, H.; Liu, Y.; Zhang, N.; Li, X.F.; Suo, X.J.; Yang, Q.P.; Chen, M.X. ACOX1, regulated by C/EBPα and miR-25-3p, promotes bovine preadipocyte adipogenesis. J. Mol. Endocrinol. 2021, 66, 195–205. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, Y.T.; Tseng, Y.J.; Zhang, J. miR-222 targets ACOX1, promotes triglyceride accumulation in hepatocytes. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2019, 18, 360–365. [Google Scholar] [CrossRef]
- Lai, Y.H.; Liu, H.; Chiang, W.F.; Chen, T.W.; Chu, L.J.; Yu, J.S.; Chen, S.J.; Chen, H.C.; Tan, B.C. MiR-31-5p-ACOX1 Axis Enhances Tumorigenic Fitness in Oral Squamous Cell Carcinoma Via the Promigratory Prostaglandin E2. Theranostics 2018, 8, 486–504. [Google Scholar] [CrossRef]
- Li, G.; Fu, S.; Chen, Y.; Jin, W.; Zhai, B.; Li, Y.; Sun, G.; Han, R.; Wang, Y.; Tian, Y.; et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int. J. Mol. Sci. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.X.; Wang, L.Y.; Chen, S.; Wang, D.D.; Fang, Z. circ_0005379 inhibits the progression of oral squamous cell carcinoma by regulating the miR-17-5p/acyl-CoA oxidase 1 axis. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2021, 39, 425–433. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, J.; Kim, K.; Moon, H.; Min, C.; Moon, B.; Kim, D.; Yang, S.; Park, H.; Lee, G.; et al. The Peroxisomal Localization of Hsd17b4 Is Regulated by Its Interaction with Phosphatidylserine. Mol. Cells 2021, 44, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.Y.; Yao, C.B.; Li, J.T.; Zhao, X.N.; Yang, H.B.; Zhang, M.; Yin, M.; Chen, J.; Lei, Q.Y. Acetylation targets HSD17B4 for degradation via the CMA pathway in response to estrone. Autophagy 2017, 13, 538–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, R.; Huang, Y.; Feng, Y.; Fu, Y.; Chen, L.; Chen, Z.; Cai, Y.; Zhang, Y.; Chen, Y. Acetylation-mediated degradation of HSD17B4 regulates the progression of prostate cancer. Aging 2020, 12, 14699–14717. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Cao, Y.; Xiao, C.; Liu, Y.; Jin, H.; Cao, Y. Effect of the ACAA1 Gene on Preadipocyte Differentiation in Sheep. Front. Genet. 2021, 12, 649140. [Google Scholar] [CrossRef]
- Ranea-Robles, P.; Violante, S.; Argmann, C.; Dodatko, T.; Bhattacharya, D.; Chen, H.; Yu, C.; Friedman, S.L.; Puchowicz, M.; Houten, S.M. Murine deficiency of peroxisomal L-bifunctional protein (EHHADH) causes medium-chain 3-hydroxydicarboxylic aciduria and perturbs hepatic cholesterol homeostasis. Cell. Mol. Life Sci. CMLS 2021, 78, 5631–5646. [Google Scholar] [CrossRef]
- Landau, Y.E.; Heimer, G.; Barel, O.; Shalva, N.; Marek-Yagel, D.; Veber, A.; Javasky, E.; Shilon, A.; Nissenkorn, A.; Ben-Zeev, B.; et al. Four patients with D-bifunctional protein (DBP) deficiency: Expanding the phenotypic spectrum of a highly variable disease. Mol. Genet. Metab. Rep. 2020, 25, 100631. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Hogenhout, E.M.; Koster, J.; van Roermund, C.W.T.; Ijlst, L.; Moser, A.B.; Wanders, R.J.; Waterham, H.R. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum. Mutat. 2007, 28, 904–912. [Google Scholar] [CrossRef]
- Turk, B.R.; Theda, C.; Fatemi, A.; Moser, A.B. X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int. J. Dev. Neurosci. 2020, 80, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, A.; Mattie, S.; Prudent, J.; McBride, H.M. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 2017, 542, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Sun, W.; Balaz, M.; He, A.; Klug, M.; Wieland, S.; Caiazzo, R.; Raverdy, V.; Pattou, F.; Lefebvre, P.; et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat. Metab. 2021, 3, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- MacLean, G.E.; Argyriou, C.; Di Pietro, E.; Sun, X.; Birjandian, S.; Saberian, P.; Hacia, J.G.; Braverman, N.E. Zellweger spectrum disorder patient-derived fibroblasts with the PEX1-Gly843Asp allele recover peroxisome functions in response to flavonoids. J. Cell. Biochem. 2019, 120, 3243–3258. [Google Scholar] [CrossRef]
- Enns, G.M.; Ammous, Z.; Himes, R.W.; Nogueira, J.; Palle, S.; Sullivan, M.; Ramirez, C. Diagnostic challenges and disease management in patients with a mild Zellweger spectrum disorder phenotype. Mol. Genet. Metab. 2021, 134, 217–222. [Google Scholar] [CrossRef]
- Wangler, M.F.; Hubert, L.; Donti, T.R.; Ventura, M.J.; Miller, M.J.; Braverman, N.; Gawron, K.; Bose, M.; Moser, A.B.; Jones, R.O.; et al. A metabolomic map of Zellweger spectrum disorders reveals novel disease biomarkers. Genet. Med. 2018, 20, 1274–1283. [Google Scholar] [CrossRef] [Green Version]
- Klouwer, F.C.C.; Koot, B.G.P.; Berendse, K.; Kemper, E.M.; Ferdinandusse, S.; Koelfat, K.V.K.; Lenicek, M.; Vaz, F.M.; Engelen, M.; Jansen, P.L.M.; et al. The cholic acid extension study in Zellweger spectrum disorders: Results and implications for therapy. J. Inherit. Metab. Dis. 2019, 42, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Zeynelabidin, S.; Klouwer, F.C.C.; Meijers, J.C.M.; Suijker, M.H.; Engelen, M.; Poll-The, B.T.; van Ommen, C.H. Coagulopathy in Zellweger spectrum disorders: A role for vitamin K. J. Inherit. Metab. Dis. 2018, 41, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.N.; Ammous, Z.; Eroglu, Y.; Hernandez, E.; Heubi, J.; Himes, R.; Palle, S. Cholbam® and Zellweger spectrum disorders: Treatment implementation and management. Orphanet J. Rare Dis. 2021, 16, 388. [Google Scholar] [CrossRef]
- Semenova, N.A.; Kurkina, M.V.; Marakhonov, A.V.; Dadali, E.L.; Taran, N.N.; Strokova, T.V. A novel mutation in the PEX26 gene in a family from Dagestan with members affected by Zellweger spectrum disorder. Mol. Genet. Metab. Rep. 2021, 27, 100754. [Google Scholar] [CrossRef]
- Havali, C.; Dorum, S.; Akbaş, Y.; Görükmez, O.; Hirfanoglu, T. Two different missense mutations of PEX genes in two similar patients with severe Zellweger syndrome: An argument on the genotype-phenotype correlation. J. Pediatric Endocrinol. Metab. JPEM 2020, 33, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Lucaccioni, L.; Righi, B.; Cingolani, G.M.; Lugli, L.; Della Casa, E.; Torcetta, F.; Iughetti, L.; Berardi, A. Overwhelming sepsis in a neonate affected by Zellweger syndrome due to a compound heterozygosis in PEX 6 gene: A case report. BMC Med. Genet. 2020, 21, 229. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Ruiz, A.; Galindo-Ferreiro, A.; Alkatan, H. A clinical case of Zellweger syndrome in a patient with a previous history of ocular medulloepithelioma. Saudi J. Ophthalmol. 2018, 32, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Hosoba, K.; Itabashi, T.; Iwane, A.H.; Akutsu, S.N.; Ochiai, H.; Saito, Y.; Yamamoto, T.; Matsuura, S. Insufficiency of ciliary cholesterol in hereditary Zellweger syndrome. EMBO J. 2020, 39, e103499. [Google Scholar] [CrossRef]
- Lipiński, P.; Stawiński, P.; Rydzanicz, M.; Wypchło, M.; Płoski, R.; Stradomska, T.J.; Jurkiewicz, E.; Ferdinandusse, S.; Wanders, R.J.A.; Vaz, F.M.; et al. Mild Zellweger syndrome due to functionally confirmed novel PEX1 variants. J. Appl. Genet. 2020, 61, 87–91. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Lin, S.B.; Li, W.X.; Yang, L.; Zhang, R.; Chen, C.; Yuan, L. PEX26 gene genotype-phenotype correlation in neonates with Zellweger syndrome. Transl. Pediatrics 2021, 10, 1825–1833. [Google Scholar] [CrossRef]
- Cheillan, D. Zellweger Syndrome Disorders: From Severe Neonatal Disease to Atypical Adult Presentation. Adv. Exp. Med. Biol. 2020, 1299, 71–80. [Google Scholar] [CrossRef]
- Al-Essa, M.; Dhaunsi, G.S. Selective receptor-mediated impairment of growth factor activity in neonatal- and X-linked adrenoleukodystrophy patients. J. Pediatric Endocrinol. Metab. JPEM 2019, 32, 733–738. [Google Scholar] [CrossRef]
- Farrell, D.F. Neonatal adrenoleukodystrophy: A clinical, pathologic, and biochemical study. Pediatric Neurol. 2012, 47, 330–336. [Google Scholar] [CrossRef]
- Elghawy, O.; Zhang, A.Y.; Duong, R.; Wilson, W.G.; Shildkrot, E.Y. Ophthalmic Diagnosis and Novel Management of Infantile Refsum Disease with Combination Docosahexaenoic Acid and Cholic Acid. Case Rep. Ophthalmol. Med. 2021, 2021, 1345937. [Google Scholar] [CrossRef]
- Dhaunsi, G.S.; Alsaeid, M.; Akhtar, S. Phytanic acid attenuates insulin-like growth factor-1 activity via nitric oxide-mediated γ-secretase activation in rat aortic smooth muscle cells: Possible implications for pathogenesis of infantile Refsum disease. Pediatr. Res. 2017, 81, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Mierau, G.; Wartchow, E.P.; Shimada, H.; Yano, S. Histologic and ultrastructural features in early and advanced phases of Zellweger spectrum disorder (infantile Refsum disease). Ultrastruct. Pathol. 2018, 42, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, N.; Shao, J.B.; Li, H.; Li, J.; Xi, J.M.; Xu, W.H.; Jiang, H. Allogeneic Hematopoietic Stem Cell Transplantation for PEX1-Related Zellweger Spectrum Disorder: A Case Report and Literature Review. Front. Pediatrics 2021, 9, 672187. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.J.; Hu, F.Y.; Xu, P.; Qi, Y.H.; Li, J.K.; Zhang, Y.J.; Chen, F.; Chang, Q.; Song, F.; Shen, S.M.; et al. Expanding the clinical and genetic spectrum of Heimler syndrome. Orphanet J. Rare Dis. 2019, 14, 290. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Abe, Y.; Kim, Y.J.; Fujiki, Y.; Kim, J.W. Identification of a Homozygous PEX26 Mutation in a Heimler Syndrome Patient. Genes 2021, 12, 646. [Google Scholar] [CrossRef]
- Daich Varela, M.; Jani, P.; Zein, W.M.; D’Souza, P.; Wolfe, L.; Chisholm, J.; Zalewski, C.; Adams, D.; Warner, B.M.; Huryn, L.A.; et al. The peroxisomal disorder spectrum and Heimler syndrome: Deep phenotyping and review of the literature. Am. J. Med Genet. Part C Semin. Med. Genet. 2020, 184, 618–630. [Google Scholar] [CrossRef]
- Cordisco, A.; Pelo, E.; Di Tommaso, M.; Biagiotti, R. A new GNPAT variant of foetal rhizomelic chondrodysplasia punctata. Mol. Genet. Genom. Med. 2021, 9, e1733. [Google Scholar] [CrossRef]
- Fallatah, W.; Schouten, M.; Yergeau, C.; Di Pietro, E.; Engelen, M.; Waterham, H.R.; Poll-The, B.T.; Braverman, N. Clinical, biochemical, and molecular characterization of mild (nonclassic) rhizomelic chondrodysplasia punctata. J. Inherit. Metab. Dis. 2021, 44, 1021–1038. [Google Scholar] [CrossRef]
- Abousamra, O.; Kandula, V.; Duker, A.L.; Rogers, K.J.; Bober, M.B.; Mackenzie, W.G. Cervical Spine Deformities in Children With Rhizomelic Chondrodysplasia Punctata. J. Pediatric Orthop. 2019, 39, e680–e686. [Google Scholar] [CrossRef]
- Duker, A.L.; Niiler, T.; Kinderman, D.; Schouten, M.; Poll-The, B.T.; Braverman, N.; Bober, M.B. Rhizomelic chondrodysplasia punctata morbidity and mortality, an update. Am. J. Med. Genet. Part A 2020, 182, 579–583. [Google Scholar] [CrossRef]
- Braverman, N.E.; Steinberg, S.J.; Fallatah, W.; Duker, A.; Bober, M. Rhizomelic Chondrodysplasia Punctata Type 1. In GeneReviews(®); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Lee, D.K.; Long, N.P.; Jung, J.; Kim, T.J.; Na, E.; Kang, Y.P.; Kwon, S.W.; Jang, J. Integrative lipidomic and transcriptomic analysis of X-linked adrenoleukodystrophy reveals distinct lipidome signatures between adrenomyeloneuropathy and childhood cerebral adrenoleukodystrophy. Biochem. Biophys. Res. Commun. 2019, 508, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.; Kaltsas, G. Adrenal Insufficiency Due to X-Linked Adrenoleukodystrophy. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Manor, J.; Chung, H.; Bhagwat, P.K.; Wangler, M.F. ABCD1 and X-linked adrenoleukodystrophy: A disease with a markedly variable phenotype showing conserved neurobiology in animal models. J. Neurosci. Res. 2021, 99, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Palakuzhiyil, S.V.; Christopher, R.; Chandra, S.R. Deciphering the modifiers for phenotypic variability of X-linked adrenoleukodystrophy. World J. Biol. Chem. 2020, 11, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Van de Stadt, S.I.W.; Huffnagel, I.C.; Turk, B.R.; van der Knaap, M.S.; Engelen, M. Imaging in X-Linked Adrenoleukodystrophy. Neuropediatrics 2021, 52, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Enokizono, T.; Ohto, T.; Tanaka, M.; Watanabe, S.; Takada, Y.; Iwama, K.; Mizuguchi, T.; Matsumoto, N.; Morita, M.; et al. Novel ACOX1 mutations in two siblings with peroxisomal acyl-CoA oxidase deficiency. Brain Dev. 2021, 43, 475–481. [Google Scholar] [CrossRef]
- Chung, H.L.; Wangler, M.F.; Marcogliese, P.C.; Jo, J.; Ravenscroft, T.A.; Zuo, Z.; Duraine, L.; Sadeghzadeh, S.; Li-Kroeger, D.; Schmidt, R.E.; et al. Loss- or Gain-of-Function Mutations in ACOX1 Cause Axonal Loss via Different Mechanisms. Neuron 2020, 106, 589–606.e586. [Google Scholar] [CrossRef]
- Raas, Q.; Saih, F.E.; Gondcaille, C.; Trompier, D.; Hamon, Y.; Leoni, V.; Caccia, C.; Nasser, B.; Jadot, M.; Ménétrier, F.; et al. A microglial cell model for acyl-CoA oxidase 1 deficiency. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2019, 1864, 567–576. [Google Scholar] [CrossRef]
- Yang, S.M.; Cao, C.D.; Ding, Y.; Wang, M.J.; Yue, S.J. D-bifunctional protein deficiency caused by HSD17B4 gene mutation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi = Chin. J. Contemp. Pediatrics 2021, 23, 1058–1063. [Google Scholar] [CrossRef]
- Werner, K.M.; Cox, A.J.; Qian, E.; Jain, P.; Ji, W.; Tikhonova, I.; Castaldi, C.; Bilguvar, K.; Knight, J.; Ferdinandusse, S.; et al. D-bifunctional protein deficiency caused by splicing variants in a neonate with severe peroxisomal dysfunction and persistent hypoglycemia. Am. J. Med. Genet. Part A 2022, 188, 357–363. [Google Scholar] [CrossRef]
- Veenvliet, A.R.J.; Garrelfs, M.R.; Udink ten Cate, F.E.A.; Ferdinandusse, S.; Denis, S.; Fuchs, S.A.; Schwantje, M.; Geurtzen, R.; van Wegberg, A.M.J.; Huigen, M.C.D.G.; et al. Neonatal Long-Chain 3-Ketoacyl-CoA Thiolase deficiency: Clinical-biochemical phenotype, sodium-D,L-3-hydroxybutyrate treatment experience and cardiac evaluation using speckle echocardiography. Mol. Genet. Metab. Rep. 2022, 31, 100873. [Google Scholar] [CrossRef]
- Martines, A.M.F.; van Eunen, K.; Reijngoud, D.J.; Bakker, B.M. The promiscuous enzyme medium-chain 3-keto-acyl-CoA thiolase triggers a vicious cycle in fatty-acid beta-oxidation. PLoS Comput. Biol. 2017, 13, e1005461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsalamah, A.K.; Khan, A.O. Asymptomatic retinal dysfunction in alpha-methylacyl-CoA racemase deficiency. Mol. Vis. 2021, 27, 396–402. [Google Scholar] [PubMed]
- Gündüz, M.; Ünal, Ö.; Küçükçongar-Yavaş, A.; Kasapkara, Ç. Alpha methyl acyl CoA racemase deficiency: Diagnosis with isolated elevated liver enzymes. Turk. J. Pediatrics 2019, 61, 289–291. [Google Scholar] [CrossRef]
- Herzog, K.; van Lenthe, H.; Wanders, R.J.A.; Vaz, F.M.; Waterham, H.R.; Ferdinandusse, S. Identification and diagnostic value of phytanoyl- and pristanoyl-carnitine in plasma from patients with peroxisomal disorders. Mol. Genet. Metab. 2017, 121, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Tanti, M.J.; Maguire, M.J.; Warren, D.J.; Bamford, J. Late onset AMACR deficiency with metabolic stroke-like episodes and seizures. BMJ Case Rep. 2022, 15, e247964. [Google Scholar] [CrossRef]
- Boursier, G.; Rittore, C.; Milhavet, F.; Cuisset, L.; Touitou, I. Mevalonate Kinase-Associated Diseases: Hunting for Phenotype-Genotype Correlation. J. Clin. Med. 2021, 10, 1552. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e139. [Google Scholar] [CrossRef] [Green Version]
- Bader-Meunier, B.; Martins, A.L.; Charbit-Henrion, F.; Meinzer, U.; Belot, A.; Cuisset, L.; Faye, A.; Georgin-Lavialle, S.; Quartier, P.; Remy-Piccolo, V.; et al. Mevalonate Kinase Deficiency: A Cause of Severe Very-Early-Onset Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1853–1857. [Google Scholar] [CrossRef]
- Politiek, F.A.; Waterham, H.R. Compromised Protein Prenylation as Pathogenic Mechanism in Mevalonate Kinase Deficiency. Front. Immunol. 2021, 12, 724991. [Google Scholar] [CrossRef]
- Jeyaratnam, J.; Frenkel, J. Management of Mevalonate Kinase Deficiency: A Pediatric Perspective. Front. Immunol. 2020, 11, 1150. [Google Scholar] [CrossRef]
- Leandro, J.; Bender, A.; Dodatko, T.; Argmann, C.; Yu, C.; Houten, S.M. Glutaric aciduria type 3 is a naturally occurring biochemical trait in inbred mice of 129 substrains. Mol. Genet. Metab. 2021, 132, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dorum, S.; Havalı, C.; Görükmez, Ö.; Görükmez, O. Two patients with glutaric aciduria type 3: A novel mutation and brain magnetic resonance imaging findings. Turk. J. Pediatrics 2020, 62, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.J.; Kitzler, T.M.; Feigenbaum, A.; Geraghty, M.T.; Al-Dirbashi, O.; Bherer, P.; Auray-Blais, C.; Gravel, S.; McIntosh, N.; Siriwardena, K.; et al. Glutaric Aciduria Type 3: Three Unrelated Canadian Cases, with Different Routes of Ascertainment. JIMD Rep. 2018, 39, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Singh, S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surf. B Biointerfaces 2019, 175, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Ogino, N.; Nagaoka, K.; Tomizuka, K.; Matsuura-Harada, Y.; Eitoku, M.; Suganuma, N.; Ogino, K. Compromised glutathione synthesis results in high susceptibility to acetaminophen hepatotoxicity in acatalasemic mice. Food Chem. Toxicol. 2021, 156, 112509. [Google Scholar] [CrossRef]
- Zarrouk, A.; Nury, T.; El Hajj, H.I.; Gondcaille, C.; Andreoletti, P.; Moreau, T.; Cherkaoui-Malki, M.; Berger, J.; Hammami, M.; Lizard, G.; et al. Potential Involvement of Peroxisome in Multiple Sclerosis and Alzheimer’s Disease: Peroxisome and Neurodegeneration. Adv. Exp. Med. Biol. 2020, 1299, 91–104. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zong, W.; Zhang, M.; Chen, Z.; Yang, Z. The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation. Agriculture 2022, 12, 947. https://doi.org/10.3390/agriculture12070947
Lu Q, Zong W, Zhang M, Chen Z, Yang Z. The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation. Agriculture. 2022; 12(7):947. https://doi.org/10.3390/agriculture12070947
Chicago/Turabian StyleLu, Qinyue, Weicheng Zong, Mingyixing Zhang, Zhi Chen, and Zhangping Yang. 2022. "The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation" Agriculture 12, no. 7: 947. https://doi.org/10.3390/agriculture12070947
APA StyleLu, Q., Zong, W., Zhang, M., Chen, Z., & Yang, Z. (2022). The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation. Agriculture, 12(7), 947. https://doi.org/10.3390/agriculture12070947







