Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Plant Sample Collection and Measurements
2.4. Statistical Analysis
3. Results
3.1. Effects of Grazing Intensity on L. chinensis Rust Incidence and Growth
3.2. Effects of Grazing Intensity on Stoichiometry of Non-Infected and Rust-Infected L. chinensis
3.3. The Relationship between Relative Growth Rate and C:N:P Stoichiometry
3.4. The Responses Ratios of L. chinensis to Rust
3.5. The Interactions of Grazing and Rust to L. chinensis
3.6. Factors Influencing DM of L. chinensis
4. Discussion
4.1. Effects of Grazing on Rust
4.2. Effects of Grazing and Disease on DM
4.3. Effects of Grazing and Disease on Relative Growth Rate and Ecological Stoichiometry
4.4. Grazing Alleviated the Loss of DM Caused by Disease
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Wang, D.; Wu, G.; Zhu, Y.; Shi, Z. Grazing exclusion effects on above- and below-ground C and N pools of typical grassland on the Loess Plateau (China). Catena 2014, 123, 113–120. [Google Scholar] [CrossRef]
- Yan, R.; Xin, X.; Yan, Y.; Wang, X.; Zhang, B.; Yang, G.; Liu, S.; Deng, Y.; Li, L. Impacts of differing grazing rates on canopy structure and species composition in Hulunber Meadow Steppe. Rangel. Ecol. Manag. 2015, 68, 54–64. [Google Scholar] [CrossRef]
- Hou, F.J.; Yang, Z.Y. Effects of grazing of livestock on grassland. Acta Ecol. Sin. 2006, 26, 244–264. [Google Scholar]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.R.; Tang, H.J.; Lv, S.H.; Jin, D.Y.; Xin, X.P.; Chen, B.R.; Zhang, B.H.; Yan, Y.C.; Wang, X.; Murray, P.J.; et al. Response of ecosystem CO2 fluxes to grazing intensities-a five-year experiment in the Hulunber meadow steppe of China. Sci. Rep. 2017, 7, 9491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.W.; Huang, X.H.; Wang, Y.R. Hormonal and temperature regulation of seed dormancy and germination in Leymus chinensis. Plant Growth Regul. 2012, 67, 199–207. [Google Scholar] [CrossRef]
- Reeder, J.D.; Schuman, G.E.; Morgan, J.A.; Lecain, D.R. Response of organic and inorganic carbon and nitrogen to long-term Grazing of the shortgrass steppe. Environ. Manag. 2004, 33, 485–495. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Wardle, D.A. Trophic downgrading of planet earth. Science 2011, 6040, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wu, J.; Clark, C.M.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Sitters, J.; Bakker, E.S.; Veldhuis, M.P.; Veen, G.F.; Olde Venterink, H.; Vanni, M.J. The Stoichiometry of Nutrient Release by Terrestrial Herbivores and Its Ecosystem Consequences. Front. Earth Sci. 2017, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Sitters, J.; Cherif, M.; Egelkraut, D.; Giesler, R.; Olofsson, J. Long-term heavy reindeer grazing promotes plant phosphorus limitation in arctic tundra. Funct. Ecol. 2019, 33, 1233–1242. [Google Scholar] [CrossRef]
- Sasaki, T.; Okayasu, T.; Jamsran, U.; Takeuchi, K. Threshold changes in vegetation along a grazing gradient in Mongolian rangelands. J. Ecol. 2007, 96, 145–154. [Google Scholar] [CrossRef]
- Hilker, T.; Natsagdorj, E.; Waring, R.H.; Lyapustin, A.; Wang, Y. Satellite observed widespread decline in Mongolian grasslands largely due to overgrazing. Glob. Change Biol. 2014, 20, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldridge, D.J.; Delgado-Baquerizo, M. Continental-scale impacts of livestock grazing on ecosystem supporting and regulating services. Land Degrad. Dev. 2016, 28, 1473–1481. [Google Scholar] [CrossRef]
- Lu, X.; Kelsey, K.C.; Yan, Y.; Sun, J.; Wang, X.; Cheng, G.; Neff, J.C. Effects of grazing on ecosystem structure and function of alpine grasslands in Qinghai- Tibetan Plateau: A synthesis. Ecosphere 2017, 8, e1656. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Nan, Z.; Christensen, M.J. Cattle grazing alters the interaction of seed-borne fungi and two foliar pathogens of Leymus chinensis in a meadow steppe. Eur. J. Plant Pathol. 2019, 155, 207–218. [Google Scholar] [CrossRef]
- Nan, Z.B.; Li, C.J.; Wang, Y.W.; Wang, Y.R. Lucerne common leaf spot forage quality, photosynthesis rate and field resistance. Acta Prataculturae Sin. 2001, 10, 26–34. [Google Scholar]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; Mccraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Cadotte, M.W.; Chen, F.; He, J.; Zhou, S. Warming affects foliar fungal diseases more than precipitation in a Tibetan alpine meadow. New Phytol. 2019, 221, 1574–1584. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.T.; Wang, X.Y.; Duan, T.Y. Effect of disease on photosynthesis, nutrition, and nodulation of leguminous forage. Pratacultural Sci. 2019, 36, 152–160. [Google Scholar]
- Nan, Z.B.; Li, C.J. Forage and turfgrass disease in China and their control. In Forage and Turfgrass Pathological Research in China; Nan, Z.B., Li, C.J., Eds.; Ocean Press: Beijing, China, 2003; pp. 3–10. [Google Scholar]
- Gray, F.A.; Koch, D.W. Influence of late season harvesting, fall grazing, and fungicide treatment on Verticillium Wilt incidence, plant density, and forage yield of alfalfa. Plant Dis. 2004, 88, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalto, S.L.; Decaestecker, E.; Pulkkinen, K. A three-way perspective of stoichiometric changes on host–parasite interactions. Trends Parasitol. 2015, 31, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.W.; Christensen, M.; Li, C.H.; Hou, F.J.; Nan, Z.B. Grazing intensity affects communities of culturable root-associated fungi in a semiarid grassland of northwest China. Land Degrad. Dev. 2017, 29, 361–373. [Google Scholar] [CrossRef]
- Wennström, A.; Ericson, L. Variation in disease incidence in grazed and ungrazed sites for the system Pulsatilla pratensis-Puccinia pulsatillae. Oikos 1991, 60, 35–39. [Google Scholar] [CrossRef]
- Lu, X.; Nan, Z.B. Perspectives in effects of grazing on diversity of grassland plant community and forage diseases. Pratacultural Sci. 2015, 32, 1423–1431. [Google Scholar]
- Liu, X.; Lyu, S.; Zhou, S.; Bradshaw, C.J.A. Warming and fertilization alter the dilution effect of host diversity on disease severity. Ecology 2016, 97, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Nan, Z.; Kardol, P.; Duan, T.; Song, H.; Wang, J.; Li, C.; Hou, F. Effects of interspecific competition on plant-soil feedbacks generated by long-term grazing. Soil Biol. Biochem. 2018, 126, 133–143. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Tilman, D.; Haddad, N.M.; Naeem, S.; Mitchell, C.E.; Haarstad, J.; Ritchie, M.E.; Howe, K.M.; Reich, P.B.; Siemann, E.; et al. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol. Lett. 2002, 2, 286–293. [Google Scholar] [CrossRef]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Rottstock, T.; Joshi, J.; Kummer, V.; Fischer, M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology 2014, 95, 1907–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bever, J.D.; Mangan, S.A.; Alexander, H.M. Maintenance of plant species diversity by pathogens. Annu. Rev. Ecol. Evol. 2015, 46, 305–325. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecular to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Hessen, D.O.; Jensen, T.C.; Kyle, M.; Elser, J.J. RNA responses to N-and P-limitation; reciprocal regulation of stoichiometry and growth rate in Brachionus. Funct. Ecol. 2007, 21, 956–962. [Google Scholar] [CrossRef]
- Allen, A.P.; Gillooly, J.F. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 2009, 12, 369–384. [Google Scholar] [CrossRef]
- Van der Wal, R.; Brooker, R.W. Mosses mediate grazer impacts on grass abundance in Arctic. Funct. Ecol. 2004, 18, 77–86. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Holland, J.N.; Cheng, W.; Crossley, D.A. Herbivore-induced changes in plant carbon allocation: Assessment of below-ground C fluxes using carbon-14. Oecologia 1996, 107, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, W.E.; Frank, D.A. Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 2001, 82, 2397–2402. [Google Scholar] [CrossRef]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Tilman, D.; Knops, J.M.H. Herbivore effects on plant and nitrogen dynamics in Oak Savanna. Ecology 1998, 79, 165–177. [Google Scholar] [CrossRef]
- Elser, J.J.; Dobberfuhl, D.R.; Mackay, N.A.; Schampel, J.H. Organism size, life history, and N:P stoichiometry. Bioscience 1996, 46, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.; Munk, L. Nitrogen-induced changes in colony density and spore production of Erysiphe graminis f.sp. hordei on seedlings of six spring barley cultivars. Plant Pathol. 1997, 46, 191–202. [Google Scholar] [CrossRef]
- Frenken, T.; Wierenga, J.; Gsell, A.S.; van Donk, E.; Rohrlack, T.; Van de Waal, D.B. Changes in N:P supply ratios affect the ecological stoichiometry of a toxic Cyanobacterium and its fungal parasite. Front. Microbiol. 2017, 8, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, A.J.; Taylor, B.W. Using ecological stoichiometry to understand and predict infectious diseases. Oikos 2018, 127, 1399–1409. [Google Scholar] [CrossRef]
- Daleo, P.; Silliman, B.; Alberti, J.; Escapa, M.; Canepuccia, A.; Peña, N.; Iribarne, O. Grazer facilitation of fungal infection and the control of plant growth in south-western Atlantic salt marshes. J. Ecol. 2009, 97, 781–787. [Google Scholar] [CrossRef]
- Li, Y.Z.; Nan, Z.B. The Methods of Diagnose, Investigation and Loss Evaluation for Forage Diseases; Phenix Press: Nanjing, China, 2015. [Google Scholar]
- Wang, Q.; Li, F.; Zhao, L.; Zhang, E.; Shi, S.; Zhao, W.; Song, W.; Vance, M.M. Effects of irrigation and nitrogen application rates on nitrate nitrogen distribution and fertilizer nitrogen loss, wheat yield and nitrogen uptake on a recently reclaimed sandy farmland. Plant Soil 2010, 337, 325–339. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science Society of American: Madison, WI, USA, 1982; pp. 1–129. [Google Scholar]
- Lajeunesse, M.J. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 2011, 92, 2049–2055. [Google Scholar] [CrossRef]
- Lepš, J.; Petr, Š. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Li, Z.; Wang, X.; Wang, D.L. First report of rust disease caused by Puccinia elymi on Leymus chinensis in China. Plant Pathol. 2008, 57, 376. [Google Scholar] [CrossRef]
- Zhang, Y.; Nan, Z.; Xin, X. Response of plant fungal diseases to beef cattle grazing intensity in Hulunber grassland. Plant Dis. 2020, 104, 2905–2913. [Google Scholar] [CrossRef]
- Silva, L.S.; Silva, V.J.; Yasuoka, J.I.; Sollenberger, L.E.; Pedreira, C.G.S. Tillering dynamics of ‘Mulato II’ brachiariagrass under continuous stocking. Crop Sci. 2020, 60, 1105–1112. [Google Scholar] [CrossRef]
- Develey-Rivière, M.P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A. Population dynamics of pathogens with multiple host species. Am. Nat. 2004, 164, S64–S78. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Keesing, F. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. 2012, 43, 157–182. [Google Scholar] [CrossRef]
- Rudolf, V.H.W.; Antonovics, J. Species coexistence and pathogens with frequency-dependent transmission. Am. Nat. 2005, 166, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, C.J.; Norton, D.A.; Hobbs, R.J. Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: Implications for restoration. Austral Ecol. 2000, 25, 36–47. [Google Scholar] [CrossRef]
- Altesor, A.; Oesterheld, M.; Leoni, E.; Lezama, F.; Rodríguez, C. Effect of grazing on community structure and productivity of a Uruguayan grassland. Plant Ecol. 2005, 179, 83–91. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, D.; Ba, L.; Bai, Y.; Liu, B. Interactions between herbivory and resource availability on grazing tolerance of Leymus chinensis. Environ. Exp. Bot. 2008, 63, 113–122. [Google Scholar] [CrossRef]
- Yan, R.R.; Tang, H.J.; Xin, X.P.; Chen, B.R.; Murray, P.J.; Yan, Y.C.; Wang, X.; Yang, G.X. Grazing intensity and driving factors affect soil nitrous oxide fluxes during the growing seasons in the Hulunber meadow steppe of China. Environ. Res. Lett. 2016, 11, 54004. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Yan, R.; Chen, X.; Wang, X.; Yu, Q.; Zhang, Y.; Ning, C.; Hou, L.; Zhang, Y.; Xin, X. Grazing affects the ecological stoichiometry of the plant–soil–microbe system on the Hulunber Steppe, China. Sustainability 2019, 11, 5226. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.; Davies, W.J. Keeping in touch: Responses of the whole plant to deficits. Adv. Bot. Res. 1996, 22, 229. [Google Scholar]
- Loper, G.M.; Hanson, C.H.; Graham, J.H. Coumestrol content of alfalfa as affected by selection for resistance to foliar disease. Crop Sci. 1967, 7, 189–192. [Google Scholar] [CrossRef]
- Bernot, R.J. Parasite-host elemental content and the effects of a parasite on host-consumer-driven nutrient recycling. Freshw. Sci. 2013, 32, 299–308. [Google Scholar] [CrossRef]
- Jin, Q.; Zhou, G.Y.; Liu, J.A.; He, W.H. The role of cell wall degrading enzymes in the pathogenic process of Camellia oleifera disease caused by Colletotrichum gloeosporioides. Plant Prot. 2016, 43, 97–102. [Google Scholar]
- Ågren, G.I. The C: N: P stoichiometry of autotrophs-theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; de Ruiter, P. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Makino, W.; Cotner, J.B.; Sterner, R.W.; Elser, J.J. Are bacteria more like plants or animals Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct. Ecol. 2003, 17, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Halvorson, H.M.; Small, G.E. Observational field studies are not appropriate tests of consumer stoichiometric homeostasis. Freshw. Sci. 2016, 35, 1103–1116. [Google Scholar] [CrossRef]
- Zhang, T.; Weng, Y.; Yao, F.J.; Shi, Y.T.; Cui, G.W.; Hu, G.F. Effect of grazing intensity on ecological stoichiometry of Deyeuxia angustifolia and meadow soil. Acta Prataculturae Sin. 2014, 23, 20–28. [Google Scholar]

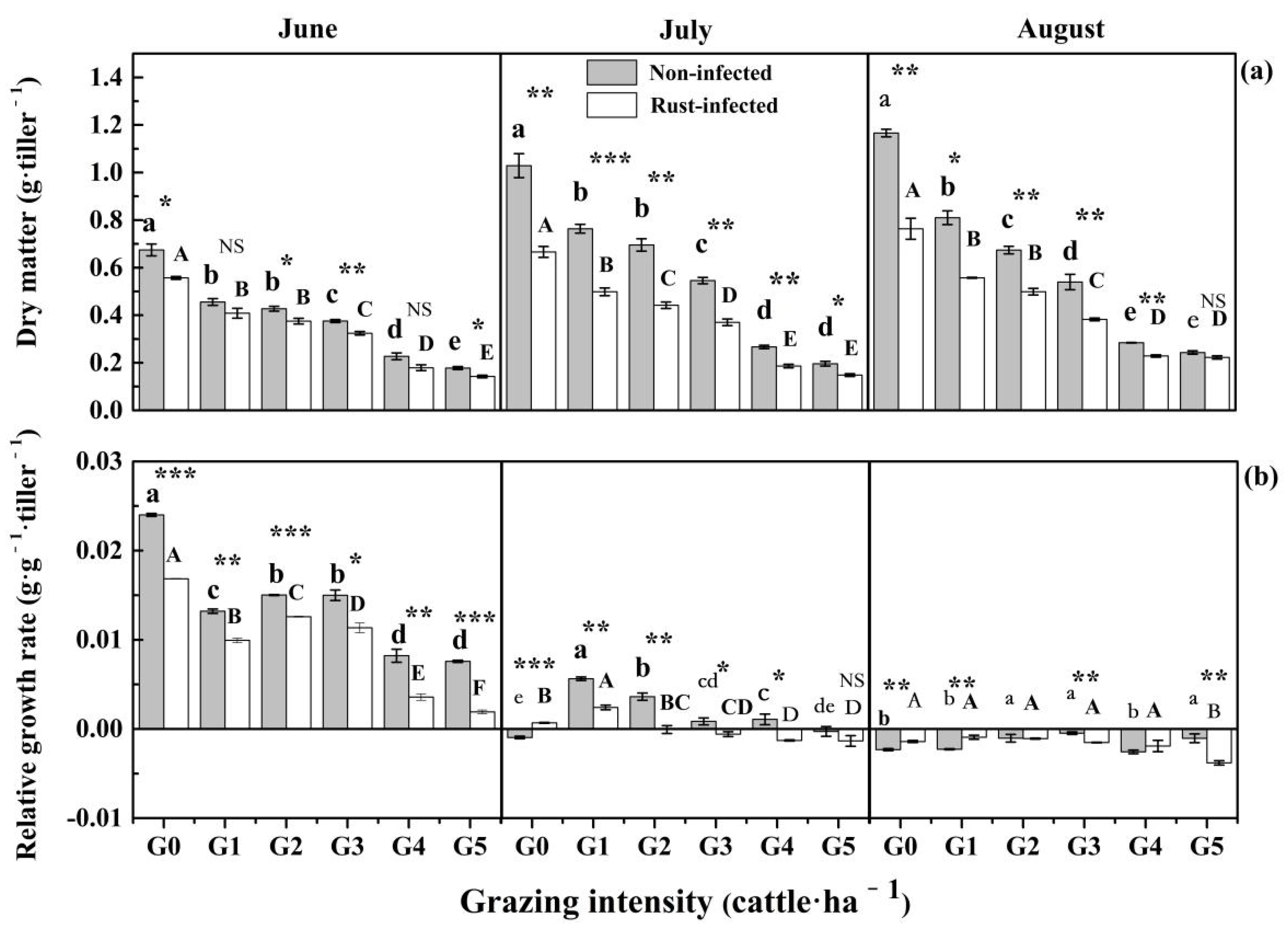


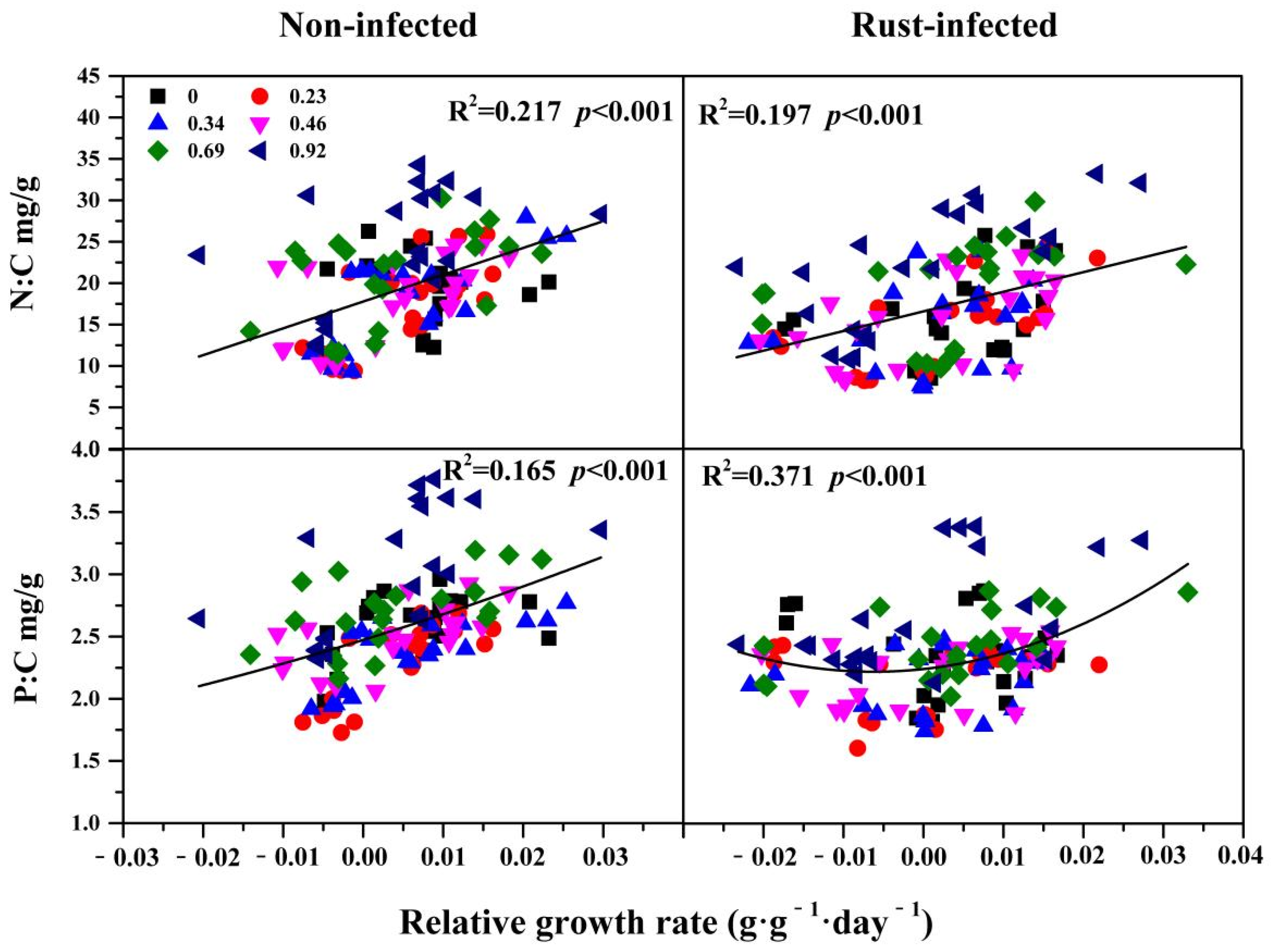
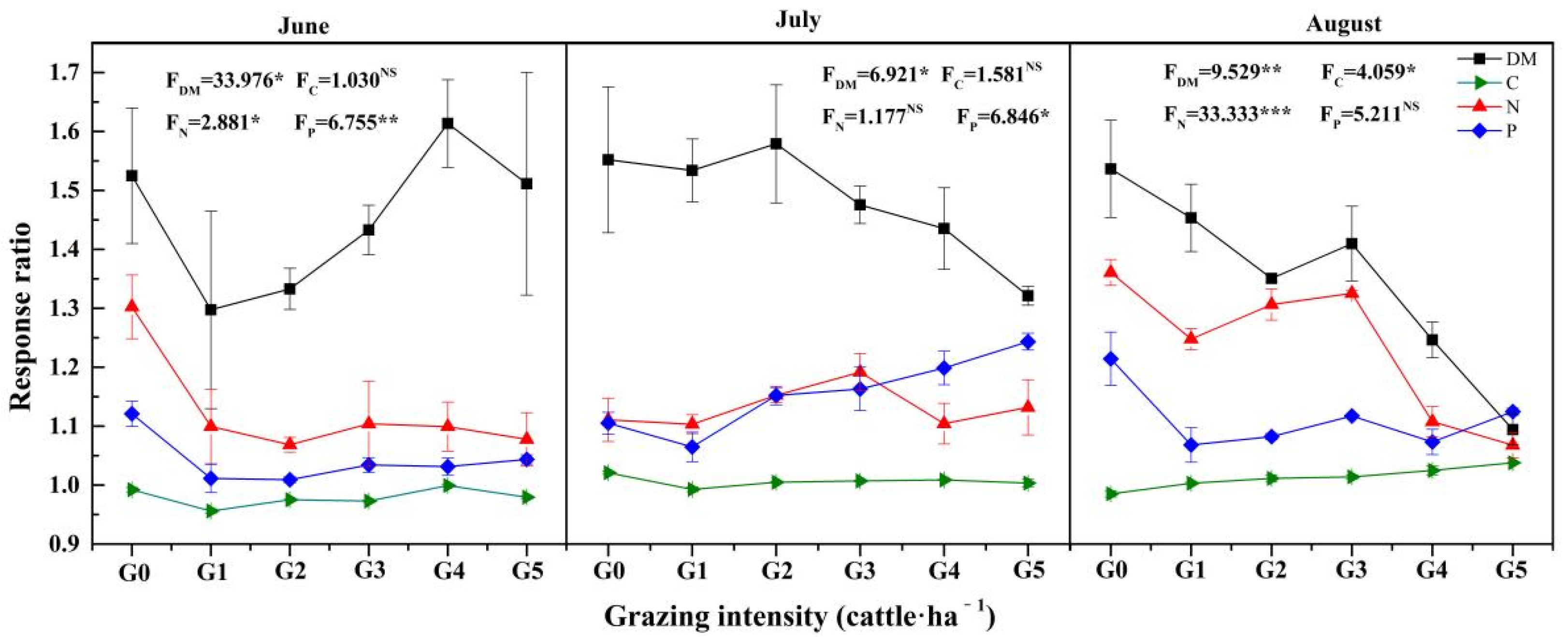
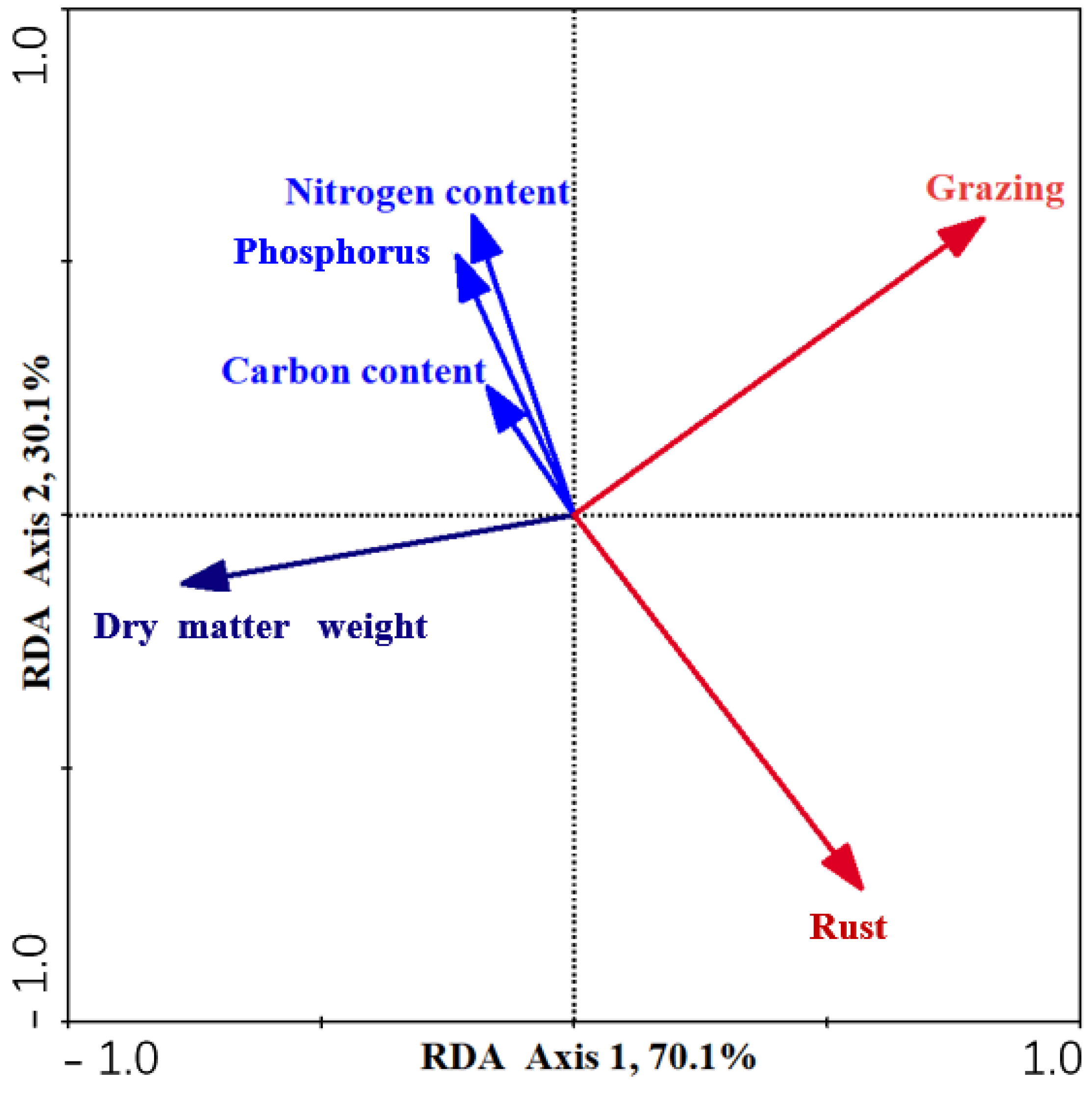

| Source | df | DM | RW | Carbon | Nitrogen | Phosphorus | C:N | C:P | N:P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | ||
| SD | 5 | 5.52 | 0.015 | 41.73 | 0.023 | 31.07 | 0.031 | 126.57 | 0.008 | 17.89 | 0.053 | 40.50 | 0.024 | 28.61 | 0.034 | 27.43 | 0.035 |
| RU | 1 | 6.71 | 0.045 | 3.11 | 0.251 | 0.17 | 0.718 | 24.71 | 0.025 | 6.38 | 0.111 | 5.05 | 0.138 | 7.60 | 0.133 | 0.75 | 0.449 |
| GI | 5 | 24.54 | 0.002 | 61.78 | <0.001 | 4.41 | 0.065 | 35.41 | 0.001 | 15.54 | 0.005 | 15.33 | 0.005 | 32.33 | 0.001 | 3.60 | 0.093 |
| SD × RU | 4 | 8.35 | 0.007 | 7.15 | 0.012 | 5.50 | 0.024 | 3.93 | 0.055 | 12.64 | 0.002 | 17.72 | 0.001 | 6.78 | 0.014 | 10.74 | 0.043 |
| SD × GI | 25 | 3.82 | 0.023 | 10.76 | <0.001 | 4.21 | 0.016 | 7.40 | 0.020 | 10.11 | 0.001 | 7.61 | 0.002 | 6.62 | 0.003 | 1.90 | 0.164 |
| RU × GI | 5 | 6.52 | 0.006 | 0.24 | 0.936 | 1.02 | 0.457 | 1.56 | 0.258 | 2.01 | 0.162 | 2.23 | 0.132 | 0.46 | 0.800 | 4.15 | 0.027 |
| SD × RU × GI | 20 | 3.05 | 0.001 | 0.71 | 0.714 | 0.53 | 0.865 | 0.10 | 1.000 | 0.53 | 0.867 | 0.10 | 1.000 | 0.82 | 0.612 | 0.11 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Nan, Z.; Christensen, M.J.; Xin, X.; Zhang, N. Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland. Agriculture 2022, 12, 961. https://doi.org/10.3390/agriculture12070961
Zhang Y, Nan Z, Christensen MJ, Xin X, Zhang N. Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland. Agriculture. 2022; 12(7):961. https://doi.org/10.3390/agriculture12070961
Chicago/Turabian StyleZhang, Yawen, Zhibiao Nan, Michael John Christensen, Xiaoping Xin, and Nan Zhang. 2022. "Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland" Agriculture 12, no. 7: 961. https://doi.org/10.3390/agriculture12070961
APA StyleZhang, Y., Nan, Z., Christensen, M. J., Xin, X., & Zhang, N. (2022). Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland. Agriculture, 12(7), 961. https://doi.org/10.3390/agriculture12070961







