Honey Bee Foraging Decisions Influenced by Pear Volatiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pear Orchard and Honey Bees
2.2. Behavioral Observation Experiment
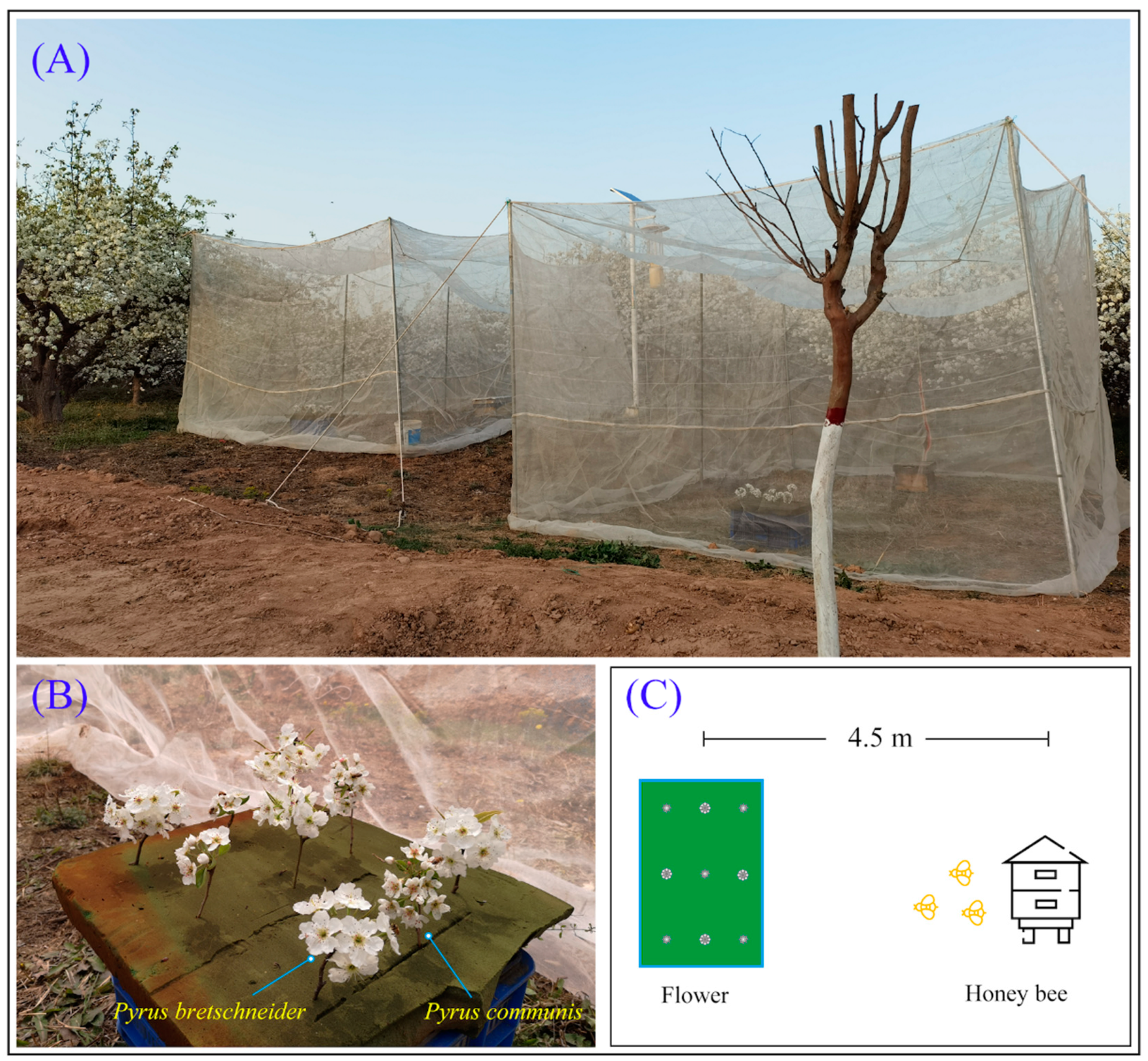
2.2.1. The Flight Cage Arrangement
2.2.2. Behavioral Observation
2.3. Flower Volatile Collection
2.4. Y-Tube Olfactometer Bioassays
2.5. GC–MS and GC–EAD
2.6. Data Statistics
3. Results
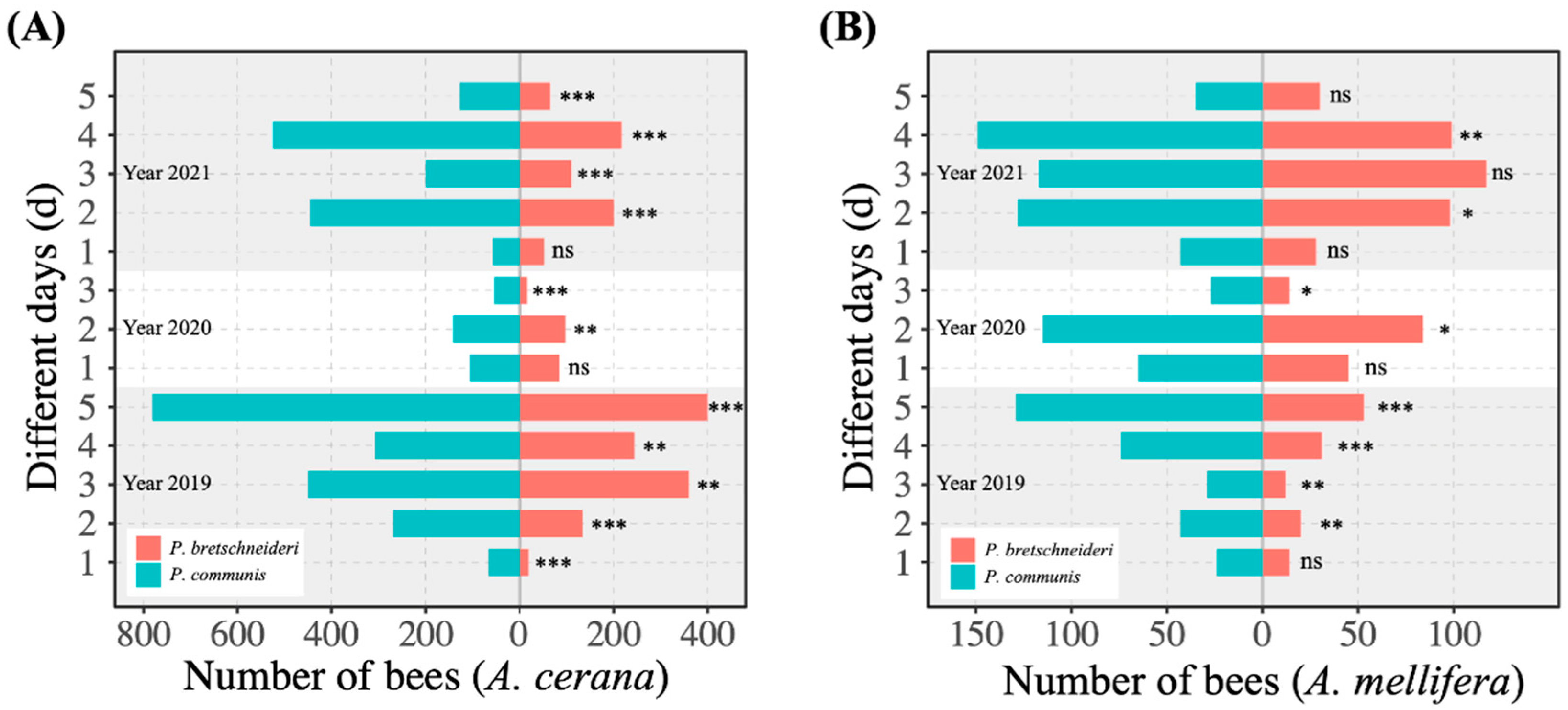
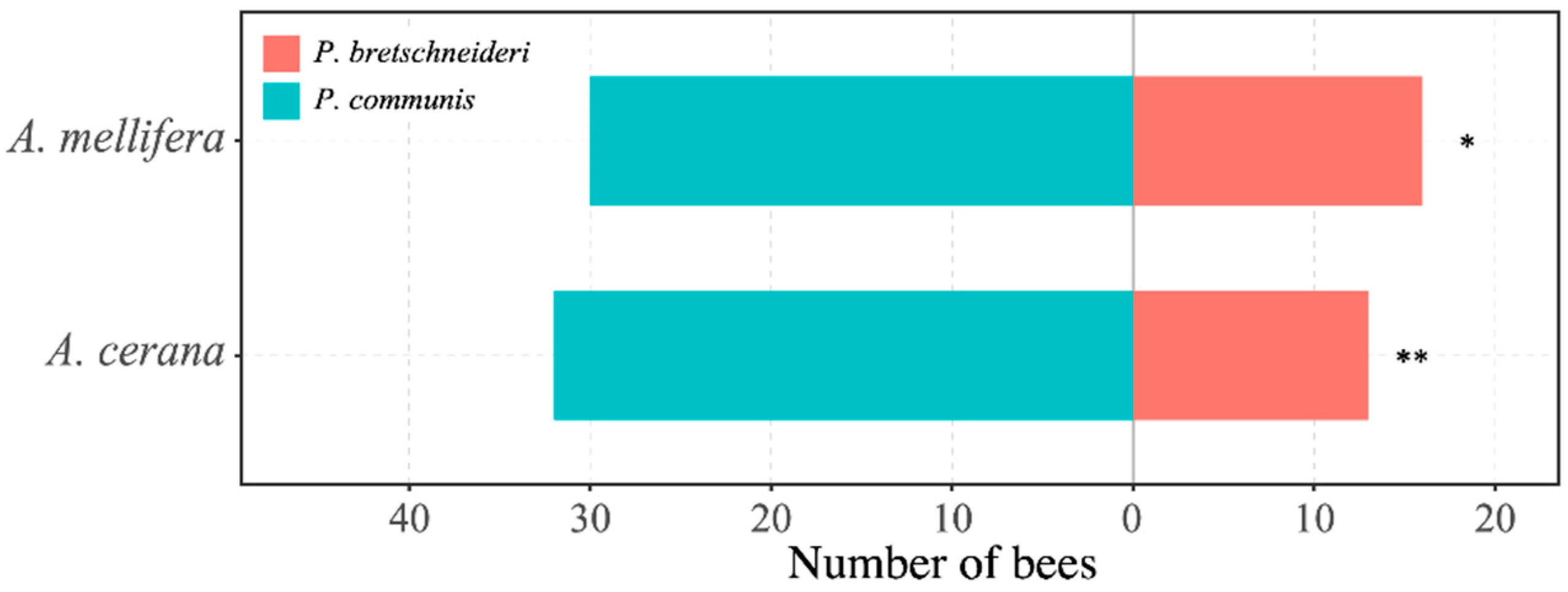
3.1. Foraging Choices of Honey Bees on Pears
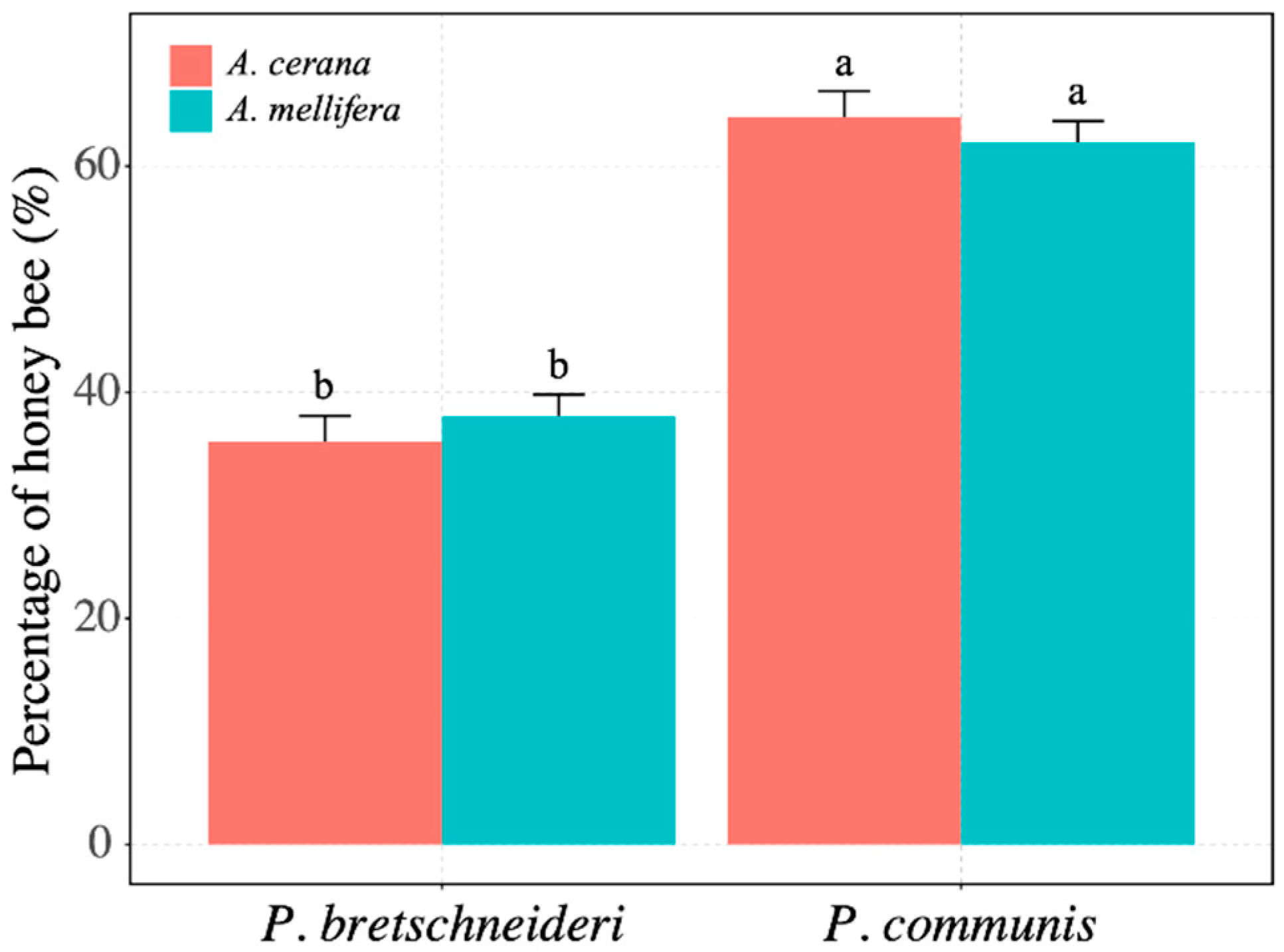
3.2. Comparison of the Proportion of Honey Bees Foraging
3.3. Y-Tube Olfactometer Experiment
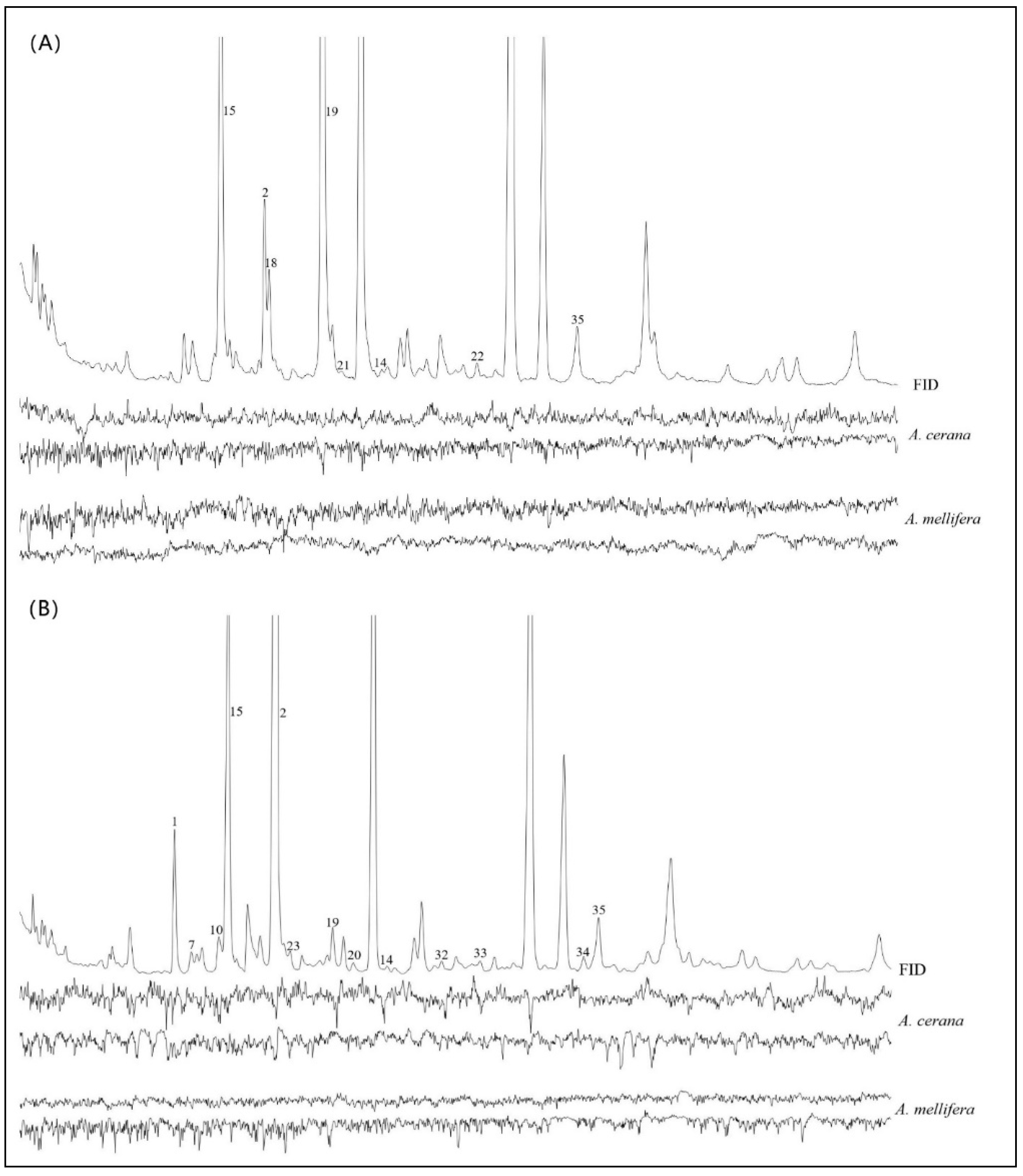
3.4. GC–MS and GC–EAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, L.A.; Aizen, M.A.; Cunningham, S.; Klein, A.M. Pollinator shortage and global crop yield: Looking at the whole spectrum of pollinator dependency. Commun. Integr. Biol. 2009, 2, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Wilbert, S.M.; Bradshaw, H.D.; Otto, K.G.; Schemske, D.W. Genetic mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus). Nature 1995, 376, 762–765. [Google Scholar]
- Kaiser, L.; De Jong, R. Multi-odour memory influenced by learning order. Behav. Process. 1993, 30, 175–183. [Google Scholar] [CrossRef]
- Hill, P.S.M.; Wells, P.H.; Wells, H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997, 54, 615–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beekman, M. How long will honey bees (Apis mellifera L.) be stimulated by scent to revisit past-profitable forage sites? J. Comp. Physiol. A 2005, 191, 1115–1120. [Google Scholar] [CrossRef]
- Zhang, S.W.; Schwarz, S.; Pahl, M.; Zhu, H.; Tautz, J. Honeybee memory: A honeybee knows what to do and when. J. Exp. Biol. 2006, 209, 4420–4428. [Google Scholar] [CrossRef] [Green Version]
- Quinet, M.; Jacquemart, A. Cultivar placement affects pollination efficiency and fruit production in European pear (Pyrus communis) orchards. Eur. J. Agron. 2017, 91, 84–92. [Google Scholar] [CrossRef]
- Grass, I.; Meyer, S.; Taylor, P.J.; Foord, S.H.; Hajek, P.; Tscharntke, T. Pollination limitation despite managed honeybees in South African macadamia orchards. Agric. Ecosyst. Environ. 2018, 260, 11–18. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Parra, L.; Quiroz, A.; Isaacs, R. Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: Implications for flower visitation by bees. Ann. Bot. 2011, 107, 1377–1390. [Google Scholar] [CrossRef]
- Adler, L.S.; Irwin, R.E. What you smell is more important than what you see? Natural selection on floral scent. New Phytol. 2012, 195, 510–511. [Google Scholar] [CrossRef]
- Maccagnani, B.; Ladurner, E.; Santi, F.; Burgio, G. Osmia cornuta (Hymenoptera, Megachilidae) as a pollinator of pear (Pyrus communis): Fruit- and seed-set. Apidologie 2003, 34, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Quinet, M.; Warzée, M.; Vanderplanck, M.; Michez, D.; Lognay, G.; Jacquemart, A. Do floral resources influence pollination rates and subsequent fruit set in pear (Pyrus communis L.) and apple (Malus x domestica Borkh) cultivars? Eur. J. Agron. 2016, 77, 59–69. [Google Scholar] [CrossRef]
- Monzón, V.; Bosch, J.; Retana, J. Foraging behavior and pollinating effectiveness of Osmia cornuta (Hymenoptera: Megachilidae) and Apis mellifera (Hymenoptera: Apidae) on “Comice” pear. Apidologie 2004, 35, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Zisovich, A.H.; Goldway, M.; Schneider, D.; Steinberg, S.; Stern, E.; Stern, R.A. Adding bumblebees (Bombus terrestris L., Hymenoptera: Apidae) to pear orchards increases seed number per fruit, fruit set, fruit size and yield. J. Hortic. Sci. Biotech. 2012, 87, 353–359. [Google Scholar] [CrossRef]
- Gemeda, T.K.; Shao, Y.Q.; Wu, W.Q.; Yang, H.P.; Huang, J.X.; Wu, J. Native honey bees outperform adventive honey bees in increasing Pyrus bretschneideri (Rosales: Rosaceae) pollination. J. Econ. Entomol. 2017, 110, 2290–2294. [Google Scholar] [CrossRef]
- Faoro, I.D.; Orth, A.I. Flower visiting insects during the bloom period of Japanese pear orchards in Brazil. Acta Hortic. 2015, 1094, 275–279. [Google Scholar] [CrossRef]
- Ma, W.H.; Shao, Y.Q.; Zhao, H.T.; Tian, S.H.; Meng, J.; Yang, S.S.; Du, Y.L.; Jiang, Y.S. Using bee attractants to improve honeybee foraging on Dangshan Pear (Pyrus communis L.). J. Agric. Sci. Technol. 2015, 17, 1551–1558. [Google Scholar]
- Gemeda, T.K.; Li, J.L.; Luo, S.D.; Yang, H.P.; Jin, T.T.; Huang, J.X.; Wu, J. Pollen trapping and sugar syrup feeding of honey bee (Hymenoptera: Apidae) enhance pollen collection of less preferred flowers. PLoS ONE 2018, 13, e203648. [Google Scholar] [CrossRef] [Green Version]
- Colda, A.; Bossaert, S.; Verreth, C.; Vanhoutte, B.; Honnay, O.; Keulemans, W.; Lievens, B. Inoculation of pear flowers with Metschnikowia reukaufii and Acinetobacter nectaris enhances attraction of honeybees and hoverflies, but does not increase fruit and seed set. PLoS ONE 2021, 16, e250203. [Google Scholar]
- Ma, W.H.; Long, D.L.; Wang, Y.; Li, X.Y.; Huang, J.X.; Shen, J.S.; Su, W.T.; Jiang, Y.S.; Li, J. Electrophysiological and behavioral responses of Asian and European honeybees to pear flower volatiles. J. Asia-Pac. Entomol. 2021, 24, 221–228. [Google Scholar] [CrossRef]
- Lukas, K.; Harig, T.; Schulz, S.; Hadersdorfer, J.; Dötterl, S. Flowers of European pear release common and uncommon volatiles that can be detected by honey bee pollinators. Chemoecology 2019, 29, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y. The pear industry and research in China. Acta Hortic. 2011, 909, 161–170. [Google Scholar] [CrossRef]
- Qin, G.H.; Tao, S.T.; Zhang, H.P.; Huang, W.J.; Wu, J.Y.; Xu, Y.L.; Zhang, S.L. Evolution of the aroma volatiles of pear fruits supplemented with fatty acid metabolic precursors. Molecules 2014, 19, 20183–20196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.J. Rosaceae. In Flora of China; Editorial Committee of Flora of China, Chinese Academy of Sciences, Ed.; Science Press: Beijing, China, 1974; Volume 36, p. 361. [Google Scholar]
- Koetz, A. Ecology, behaviour and control of Apis cerana with a focus on relevance to the Australian incursion. Insects 2013, 4, 558–592. [Google Scholar] [CrossRef] [Green Version]
- Díaz, P.C.; Arenas, A.; Fernández, V.M.; Susic Martin, C.; Basilio, A.M.; Farina, W.M. Honeybee cognitive ecology in a fluctuating agricultural setting of apple and pear trees. Behav. Ecol. 2013, 24, 1058–1067. [Google Scholar] [CrossRef] [Green Version]
- Smessaert, J.; Honnay, O.; Keulemans, W. Monitoring pollinator activity in an apple and pear orchard, linked with the analysis of the nectar composition. Acta Hortic. 2019, 1231, 59–66. [Google Scholar] [CrossRef]
- Wenqing, W.; Yuan, G.; Jinshan, S.; Weihua, M.; Guibo, G.; Youquan, S. Present situation investigation of bee pollination for pear. Apic. China 2011, 62, 40–44. [Google Scholar]
- Krishna, S.; Keasar, T. Morphological complexity as a floral signal: From perception by insect pollinators to co-evolutionary implications. Int. J. Mol. Sci. 2018, 19, 1681. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.C.; Kuang, H.; Wang, S.S.; Wang, J.; Liu, W.; Wu, Z.H.; Tian, Y.Y.; Huang, Z.Y.; Miao, X.Q. Comparative sucrose responsiveness in Apis mellifera and A. cerana foragers. PLoS ONE 2013, 8, e79026. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Ding, G.L.; Ma, W.H.; Jiang, Y.S.; Huang, J.X. Pollen source affects development and behavioral preferences in honey bees. Insects 2021, 12, 130. [Google Scholar] [CrossRef]
- Pang, C.; Dong, K.; Guo, Y.; Ding, G.; Lu, Y.; Guo, Z.; Wu, J.; Huang, J. Effects of three types of pollen on the growth and development of honey bee Larvae (Hymenoptera, Apidae). Front. Ecol. Environ. 2022, 10, 870081. [Google Scholar] [CrossRef]
- Vaziritabar, S.; Oshidari, S.; Esmaeilzade, S.M. Comparative foraging behavior of eastern honeybee, (Apis cerana F.) and western honeybee, (Apis mellifera carnica) in pollinating pear and apricot flowers in Taleghan, Iran. J. Biol. Envion. Sci. 2015, 7, 136–149. [Google Scholar]
- Seo, H.; Song, J.; Yoon, H.J.; Lee, K.Y. Effects of nectar contents on the foraging activity of honeybee (Apis mellifera) on Asian pear (Pyrus pyrifolia Nakai). Sci. Hortic. 2019, 245, 185–192. [Google Scholar] [CrossRef]
- Canto, A.; Herrera, C.M.; Medrano, M.; Perez, R.; Garcia, I.M. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae): An experimental test. Am. J. Bot. 2008, 95, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Arenas, A.; Kohlmaier, M.G. Nectar source profitability influences individual foraging preferences for pollen and pollen-foraging activity of honeybee colonies. Behav. Ecol. Sociobiol. 2019, 73, 34. [Google Scholar] [CrossRef]
- Jacquemart, A.L.; Michotte-Van Der Aa, A.; Raspé, O. Compatibility and pollinator efficiency tests on Pyrus communis L. cv. ‘Conference’. J. Hortic. Sci. Biotech. 2006, 81, 827–830. [Google Scholar] [CrossRef]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in plant-insect mutualistic relationships: From food reward to partner manipulation. Front. Plant Sci. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Cook, S.M.; Awmack, C.S.; Murray, D.A.; Williams, I.H. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 2003, 28, 622–627. [Google Scholar] [CrossRef]
- Kunert, K.; Crailsheim, K. Seasonal changes in carbohydrate, lipid and protein content in emerging worker honeybees and their mortality. J. Apicult. Res. 1988, 27, 13–21. [Google Scholar] [CrossRef]
- Zaytoon, A.A.T.U.; Matsuka, M.; Sasaki, M. Feeding efficiency of pollen substitutes in a honeybee colony: Effect of feeding site on royal jelly and queen production. Appl. Entomol. Zool. 1988, 23, 481–487. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Schmidt, J.O.; Rao, H.; Wang, W.Y.; Xu, L.G. Feeding preference and survival of young worker honey bees (Hymenoptera: Apidae) fed rape, sesame, and sunflower pollen. J. Econ. Entomol. 1995, 88, 1591–1595. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Rachersberger, M.; Cordeiro, G.D.; Schäffler, I.; Dötterl, S. Honeybee pollinators use visual and floral scent cues to find apple (Malus domestica) flowers. J. Agric. Food Chem. 2019, 67, 13221–13227. [Google Scholar] [CrossRef] [PubMed]
- Milet-Pinheiro, P.; Ayasse, M.; Schlindwein, C.; Dobson, H.E.M.; Dötterl, S. Host location by visual and olfactory floral cues in an oligolectic bee: Innate and learned behavior. Behav. Ecol. 2012, 23, 531–538. [Google Scholar] [CrossRef]
- Giurfa, M.; Núñez, J.; Backhaus, W. Odour and colour information in the foraging choice behaviour of the honeybee. J. Comp. Physiol. A 1994, 175, 773–779. [Google Scholar] [CrossRef]
- Twidle, A.M.; Mas, F.; Harper, A.R.; Horner, R.M.; Welsh, T.J.; Suckling, D.M. Kiwifruit flower odor perception and recognition by honey bees, Apis mellifera. J. Agric. Food Chem. 2015, 63, 5597–5602. [Google Scholar] [CrossRef]
- Mas, F.; Horner, R.M.; Brierley, S.; Butler, R.C.; Suckling, D.M. Selection of key floral scent compounds from fruit and vegetable crops by honey bees depends on sensory capacity and experience. J. Insect Physiol. 2020, 121, 104002. [Google Scholar] [CrossRef]
- Henning, J.A.; Peng, Y.; Montague, M.A.; Teuber, L.R. Honey bee (Hymenoptera: Apidae) behavioral response to primary alfalfa (Rosales: Fabaceae) floral volatiles. J. Econ. Entomol. 1992, 85, 233–239. [Google Scholar] [CrossRef]
- Blight, M.M.; Le Métayer, M.; Delègue, M.P.; Pickett, J.A.; Marion-Poll, F.; Wadhams, L.J. Identification of floral volatiles involved in recognition of oilseed rape flowers, Brassica napus by honeybees, Apis mellifera. J. Chem. Ecol. 1997, 23, 1715–1727. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Jürgens, A.; Glück, U.; Aas, G.; Dötterl, S. Diel fragrance pattern correlates with olfactory preferences of diurnal and nocturnal flower visitors in Salix caprea (Salicaceae). Bot. J. Linn. Soc. 2014, 175, 624–640. [Google Scholar] [CrossRef] [Green Version]
- Schiestl, F.P. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef] [PubMed]
| Bee Species | Factors | Foraging Proportion | |
|---|---|---|---|
| Wald χ2 | p-Value | ||
| Apis cerana and Apis mellifera | Bee species | 3.2411 × 10−29 | 0.260 |
| Pear species | 175.67 | <0.001 | |
| Year | 2.287 × 10−28 | 1.000 | |
| No. | Volatile Compounds | KRI | Relative Peak Area (%) | Electrophysiological Activity | ||
|---|---|---|---|---|---|---|
| P. communis | P. bretschneideri | A. cerana | A. mellifera | |||
| Nitrogen-containing compounds | ||||||
| 1 | L-valine, methyl ester | 900 | 7.7 ± 2.575 | √ | √ | |
| 2 | Methyl L-isoleucinate | 999 | 17.949 ± 3.584 | 52.574 ± 2.073 | √ | √ |
| 3 | Methyl nicotinate | 1054 | 1.965 ± 0.762 | |||
| 4 | Benzothiazole | 1208 | 0.549 ± 0.045 | |||
| 5 | 1-butanamine, N-(2-pyridinylmethylene)- | 1401 | 0.959 ± 0.557 | |||
| 6 | Benzoic acid, 4-ethoxy-, ethyl ester | 1448 | 0.509 ± 0.037 | |||
| Aldehyde compounds | ||||||
| 7 | Benzaldehyde | 982 | 0.678 ± 0.283 | √ | √ | |
| 8 | Decanal | 1204 | 0.145 ± 0.103 | 0.422 ± 0.188 | ||
| 9 | Dodecanal | 1402 | 0.266 ± 0.032 | |||
| Ketone compounds | ||||||
| 10 | 6-methyl-5-hepten-2-one | 938 | 1.033 ± 0.316 | √ | √ | |
| 11 | 5,9-undecadien-2-one, 6,10-dimethyl- | 1420 | 0.769 ± 0.192 | |||
| 12 | 2,6-di-tert-butyl-p-benzoquinone | 1633 | 0.291 ± 0.03 | |||
| 13 | 2,5-cyclohexadiene-1,4-dione, 2,5-diphenyl- | 2353 | 0.163 ± 0.129 | 0.589 ± 0.018 | ||
| Alcohol compounds | ||||||
| 14 | 1-nonanol | 1104 | 0.665 ± 0.47 | 0.165 ± 0.059 | √ | |
| Ester compounds | ||||||
| 15 | Methyl 2-hydroxy-3-methylpentanoate | 983 | 30.675 ± 3.582 | 15.254 ± 3.358 | √ | |
| 16 | Tris(1-chloro-2-propyl) phosphate | – | 0.272 ± 0.582 | |||
| Phenol compounds | ||||||
| 17 | Phenol | 901 | 5.188 ± 2.018 | 4.078 ± 3.545 | ||
| Terpenoid compounds | ||||||
| 18 | β-ocimene | 976 | 2.005 ± 0.499 | √ | √ | |
| 19 | Linalool | 1082 | 22.917 ± 9.032 | 0.969 ± 0.786 | √ | √ |
| 20 | Isophorone | 1097 | 0.414 ± 0.063 | √ | ||
| 21 | 4-oxoisophorone | 1268 | 3.502 ± 0.449 | √ | ||
| 22 | Lilac alcohol D | 1251 | 0.224 ± 0.074 | √ | ||
| Alkane compounds | ||||||
| 23 | Octane, 2-methyl | 1156 | 0.261 ± 0.02 | √ | ||
| 24 | Dodecane | 1214 | 0.705 ± 0.504 | 1.957 ± 0.434 | ||
| 25 | Dodecane, 2,6,11-trimethyl- | 1320 | 0.248 ± 0.024 | 0.662 ± 0.163 | ||
| 26 | Tridecane | 1313 | 0.361 ± 0.029 | |||
| 27 | Tetradecane | 1413 | 1.504 ± 0.209 | 3.233 ± 0.009 | ||
| 28 | Hexadecane | 1612 | 1.594 ± 0.344 | 0.426 ± 0.109 | ||
| 29 | Heptadecane | 1711 | 3.309 ± 0.639 | |||
| 30 | Nonadecane | 1653 | 0.488 ± 0.076 | 1.15 ± 0.022 | ||
| 31 | Heneicosane | 2109 | 0.892 ± 0.161 | 0.949 ± 0.127 | ||
| Alkene compounds | ||||||
| 32 | Longicyclene | 1184 | 0.31 ± 0.079 | √ | ||
| 33 | Longifolene | 1398 | 0.057 ± 0.019 | √ | ||
| 34 | Caryophyllene | 1494 | 0.054 ± 0.007 | √ | ||
| 35 | α-farnesene | 1458 | 0.245 ± 0.121 | 0.498 ± 0.303 | √ | |
| 36 | 7-hexadecene, (Z)- | 1620 | 0.138 ± 0.008 | 0.149 ± 0.033 | ||
| 37 | (E,E,E)-3,7,11,15-tetramethylhexadeca-1,3,6,10,14-pentaene | 1940 | 1.244 ± 0.399 | |||
| 38 | 9-eicosene, (E)- | 2017 | 1.390 ± 0.286 | 1.556 ± 0.943 | ||
| 39 | (E)-4,8-dimethylnona-1,3,7-triene | – | 1.093 ± 0.767 | |||
| Unknowns | ||||||
| 40 | Unknown1 | 0.375 ± 0.08 | ||||
| 41 | Unknown2 | 0.149 ± 0.024 | 2.122 ± 0.378 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Ma, W.; Zhang, Q.; Hu, X.; Ding, G.; Jiang, Y.; Huang, J. Honey Bee Foraging Decisions Influenced by Pear Volatiles. Agriculture 2022, 12, 1074. https://doi.org/10.3390/agriculture12081074
Su W, Ma W, Zhang Q, Hu X, Ding G, Jiang Y, Huang J. Honey Bee Foraging Decisions Influenced by Pear Volatiles. Agriculture. 2022; 12(8):1074. https://doi.org/10.3390/agriculture12081074
Chicago/Turabian StyleSu, Wenting, Weihua Ma, Qi Zhang, Xiao Hu, Guiling Ding, Yusuo Jiang, and Jiaxing Huang. 2022. "Honey Bee Foraging Decisions Influenced by Pear Volatiles" Agriculture 12, no. 8: 1074. https://doi.org/10.3390/agriculture12081074






