Tank-Mix Adjuvants Regulate the Deposition, Absorption, and Permeation Behavior of Pesticide Solutions on Rice Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of EPX and CLR Suspension Concentrates
2.3. Surface Tension Measurements
2.4. Contact Angle Measurements
2.5. Rice Plant Treatment
2.6. Sample Preparation
2.7. Analytical Method
2.8. Statistical Analysis
3. Results
3.1. Surface Tension
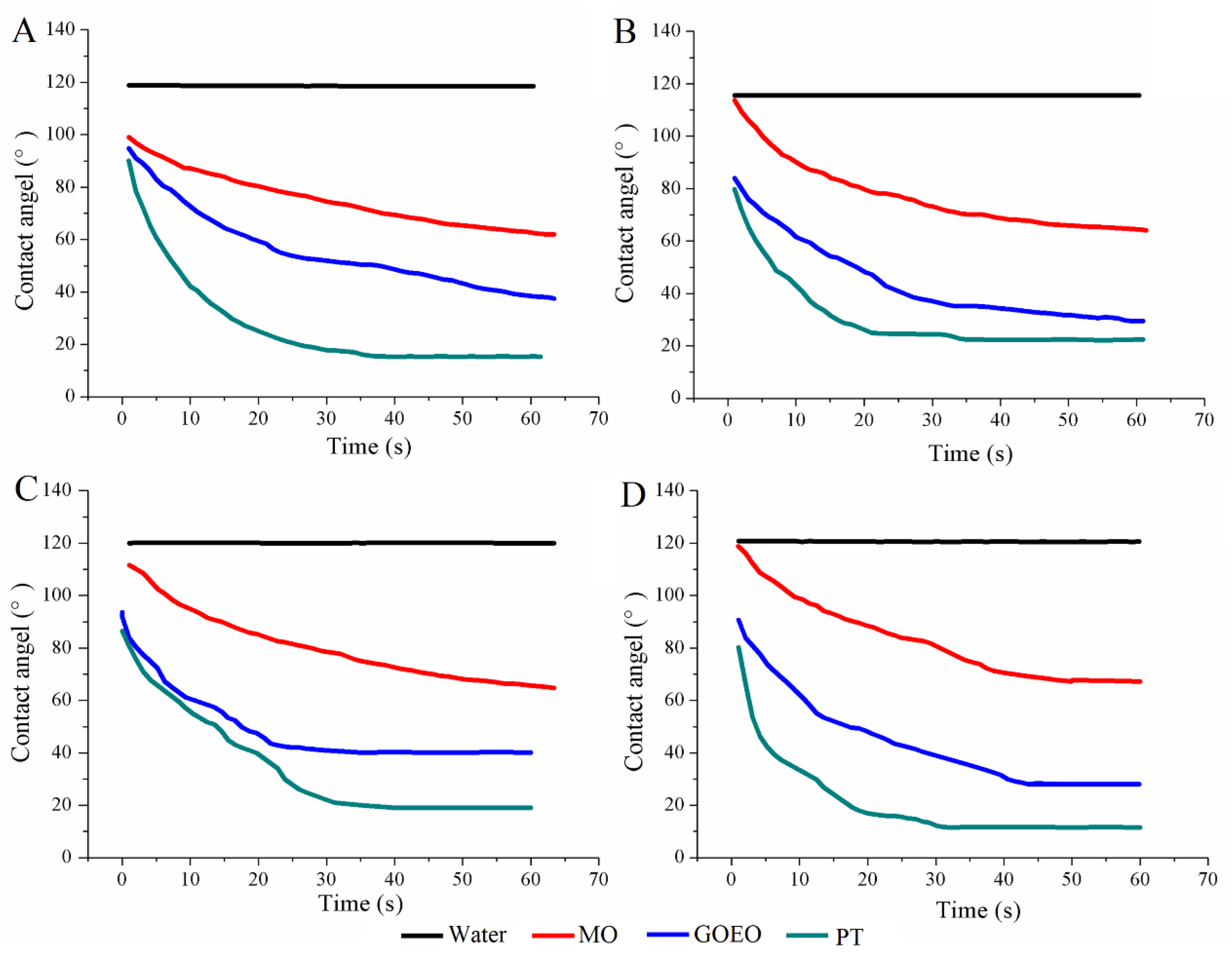
3.2. Contact Angle
3.3. Validation of Analytical Method
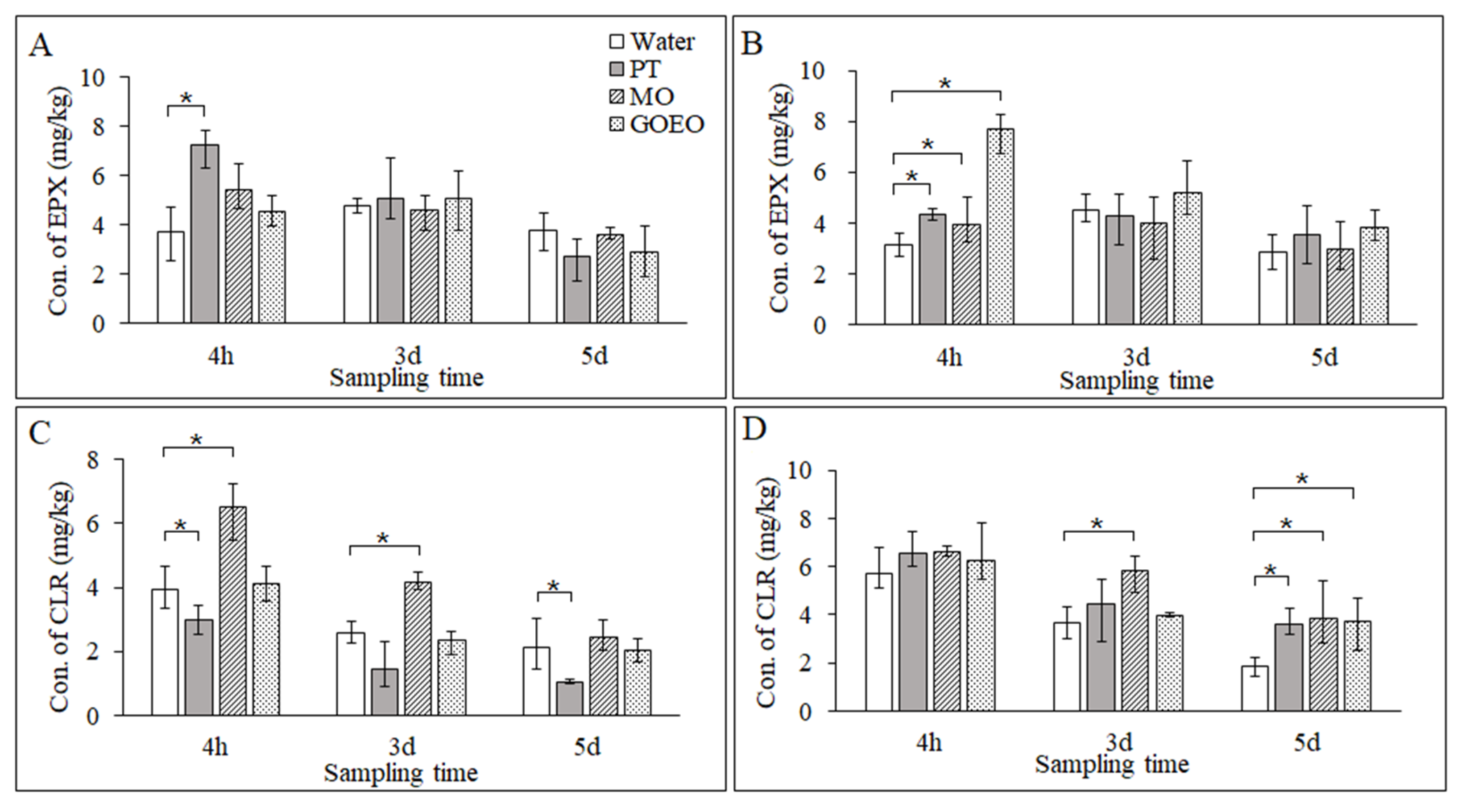
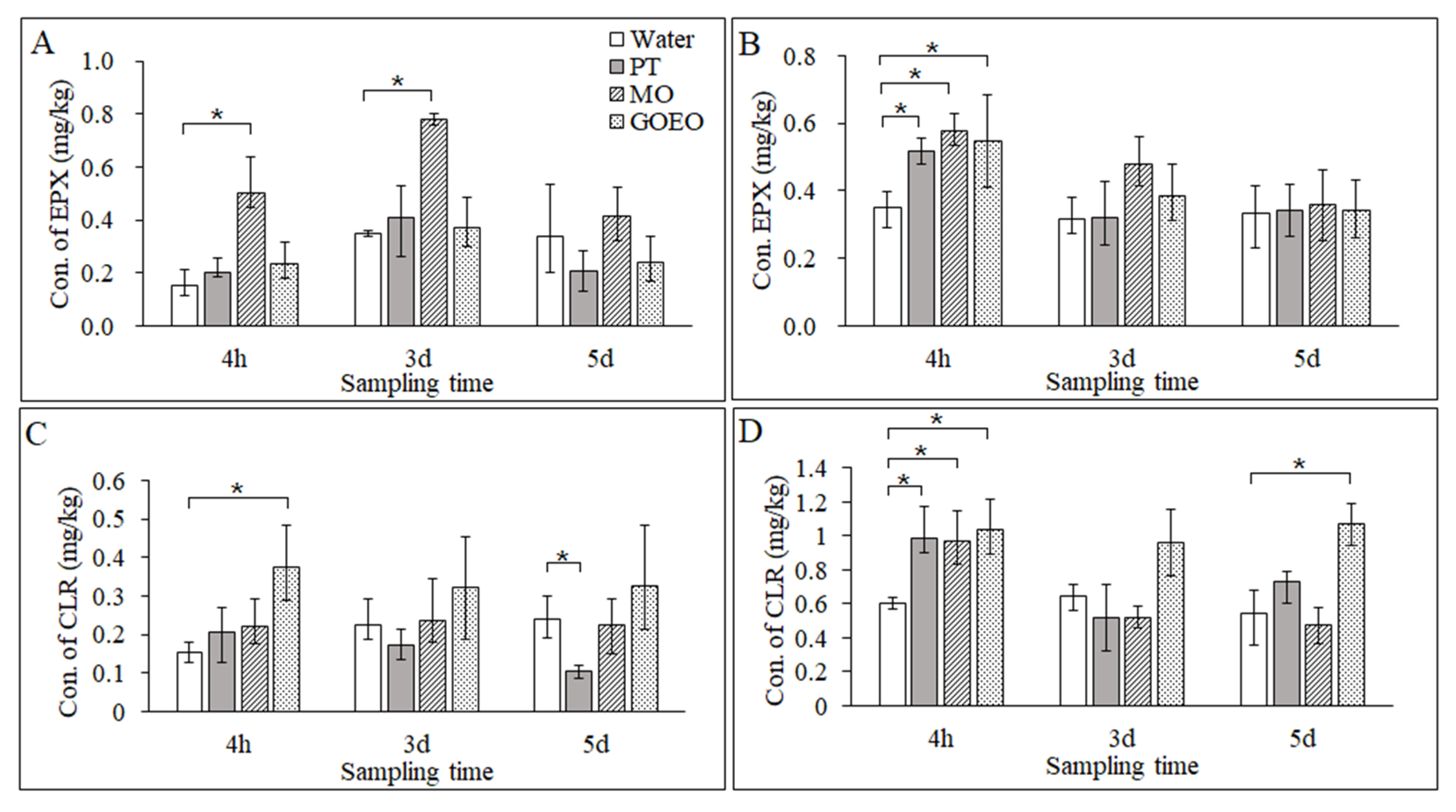
3.4. Deposition of EPX and CLR on the Leaf Surface
3.5. Permeation of EPX and CLR in the Leaves
3.6. Translocation of EPX and CLR to the Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cao, C.; Chen, Z.; Cao, L.; Huang, Q.; Song, B. Efficient pesticide formulation and regulation mechanism for improving the deposition of droplets on the leaves of rice (Oryza sativa L.). Pest Manag. Sci. 2021, 77, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, B.; Zhang, C.; Guo, Y.; Ma, Y.; Li, Z.; Wu, T.; Bao, Z.; Gao, Y.; Du, F. Catechol functionalized hat-shape carriers for prolonging pesticide retention and flush resistance on foliage. Chem. Eng. J. 2021, 420, 127689. [Google Scholar] [CrossRef]
- Holloway, P.J.; Butler Ellis, M.C.; Webb, D.A.; Western, N.M.; Tuck, C.R.; Hayes, A.L.; HMiller, P.C. Effects of some agricultural tank-mix adjuvants on the deposition efficiency of aqueous sprays on foliage. Crop Prot. 2000, 19, 27–37. [Google Scholar] [CrossRef]
- Melo, A.A.; Usano-Alemany, J.; Guedes, J.V.C.; Hunsche, M. Impact of tank-mix adjuvants on deposit formation, cuticular penetration and rain-induced removal of chlorantraniliprole. Crop Prot. 2015, 78, 253–262. [Google Scholar] [CrossRef]
- Hunsche, M.; Alexeenko, A.; Damerow, L.; Noga, G. Rain-induced removal of copper from apple leaves: Influence of rain properties and tank-mix adjuvants on deposit characteristics at the micro scale. Crop Prot. 2011, 30, 495–501. [Google Scholar] [CrossRef]
- Ryckaert, B.; Spanoghe, P.; Heremans, B.; Haesaert, G.; Steurbaut, W. Possibilities to use tank-mix adjuvants for better fungicide spreading on triticale ears. J. Agric. Food Chem. 2008, 56, 8041–8044. [Google Scholar] [CrossRef] [PubMed]
- Aliverdi, A.; Ahmadvand, G. Tank-mix adjuvants to reduce the adverse effect of muddy rain on the activity of paraquat against winter wild oat. Crop Prot. 2020, 28, 105013. [Google Scholar] [CrossRef]
- Yadav, D.B.; Yadav, A.; Punia, S.S.; Chauhand, B.S. Management of herbicide-resistant Phalaris minor in wheat by sequential or tank-mix applications of pre- and post-emergence herbicides in north-western Indo-Gangetic Plains. Crop Prot. 2016, 89, 239–247. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zeng, A.; Song, J.; Xu, T.; Lv, X.; He, X. Effects of adjuvants on spraying characteristics and control efficacy in unmanned aerial application. Agriculture 2022, 12, 138. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, M.; Sun, Z.; Li, L.; Shang, H.; Xi, W.; Li, B.; Li, Y.; Xu, Y.; Wu, X. Using tank-mix adjuvant improves the physicochemical properties and dosage delivery to reduce the use of pesticides in unmanned aerial vehicles for plant protection in wheat. Pest Manag. Sci. 2022, 78, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, L.; Pang, X.; Gao, Y.; Liu, Y.; Zhang, P.; Wei, G.; Mu, W.; Li, B.; Liu, F. Tank-mixing adjuvants enhanced the efficacy of fludioxonil on cucumber anthracnose by ameliorating the penetration ability of active ingredients on target interface. Colloid Surface B 2021, 204, 111804. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Loughin, T.M. Efficacy of Beauveria bassiana for red flour beetle when applied with plant essential oils or in mineral oil and organosilicone carriers. J. Econ. Entomol. 2005, 98, 683–688. [Google Scholar] [CrossRef]
- Xu, J.; Long, X.; Ge, S.; Li, M.; Chen, L.; Hu, D.; Zhang, Y. Deposition amount and dissipation kinetics of difenoconazole and propiconazole applied on banana with two commercial spray adjuvants. RSC Adv. 2019, 9, 19780–19790. [Google Scholar] [CrossRef]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant oil mixtures as a novel botanical pesticide to control gregarious locusts. J. Pest Sci. 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Yuan, W.; Zhao, P.; Chen, H.; Wang, L.; Huang, G.; Cao, L.; Huang, Q. Natural green-peel orange essential oil enhanced the deposition, absorption and permeation of prochloraz in cucumber. RSC Adv. 2019, 9, 20395–20401. [Google Scholar] [CrossRef] [PubMed]
- Hazen, J.L. Adjuvants—terminology, classification, and chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- van Zyl, J.G.; Sieverding, E.G.; Viljoen, D.J.; Fourie, P.H. Evaluation of two organosilicone adjuvants at reduced foliar spray volumes in South African citrus orchards of different canopy densities. Crop Prot. 2014, 64, 198–206. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, Z.; Pang, S.; Yang, T.; Clark, J.M.; He, L. Alteration of the nonsystemic behavior of the pesticide ferbam on tea leaves by engineered gold nanoparticles. Environ. Sci. Technol. 2016, 50, 6216–6223. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Kaczyński, P.; Mojsak, P.; Rusiłowska, J.; Beknazarova, Z.; Ilyasova, G.; Absatarova, D. Systemic and non-systemic pesticides in apples from Kazakhstan and their impact on human health. J. Food Compos. Anal. 2020, 90, 103494. [Google Scholar] [CrossRef]
- Vryzas, Z. The plant as metaorganism and research on next-generation systemic pesticides—Prospects and challenges. Front. Microbiol. 2016, 7, 1968. [Google Scholar] [CrossRef] [PubMed]
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef]
- Hester, S.; Moore, T.; Padgett, W.T.; Murphy, L.; Wood, C.E.; Nesnow, S. The hepatocarcinogenic conazoles: Cyproconazole, epoxiconazole, and propiconazole induce a common set of toxicological and transcriptional responses. Toxicol. Sci. 2012, 127, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Konwick, B.K.; Garrison, A.W.; Avants, J.K.; Fisk, A.T. Bioaccumulation and biotransformation of chiral triazole fungicides in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2006, 80, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Honorato Júnior, J.; Zambolim, L.; Aucique-Pérez, C.E.; Resende, R.S.; Rodrigues, F.A. Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pestic. Biochem. Phys. 2015, 123, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, H.; Fan, S.; Wang, M.; Lu, C.; Yang, Y. Systemicity of chlorantraniliprole in velvetleaf (Abutilon theophrasti). J. AOAC Int. 2013, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef]
- Sijs, R.; Bonn, D. The effect of adjuvants on spray droplet size from hydraulic nozzles. Pest Manag. Sci. 2020, 76, 3487–3494. [Google Scholar] [CrossRef]
- Hilz, E.; Vermeer, A.W. Spray drift review: The extent to which a formulation can contribute to spray drift reduction. Crop Prot. 2013, 44, 75–83. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.; Wang, Z.; Hu, H.; Ma, Y. Surface tension and spreading coefficient of single-and mix-pesticide solutions with aerial spraying organosilicone adjuvant. Int. J. Precis. Agric. Aviat. 2021, 4, 6–13. [Google Scholar] [CrossRef]
- da Silva Santos, R.T.; Vechia, J.F.D.; dos Santos, C.A.M.; Almeida, D.P.; Ferreira, M.D.C. Relationship of contact angle of spray solution on leaf surfaces with weed control. Sci. Rep. 2021, 11, 9886. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Fine, J.D.; Reynolds, R.D.; Frazier, M.T. Toxicological risks of agrochemical spray adjuvants: Organosilicone surfactants may not be safe. Front. Public Health 2016, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Cang, T.; Zhao, X.; Wang, S.; Qi, P.; Wang, X.; Xu, X.; Wang, Q. Positive effects of an oil adjuvant on efficacy, dissipation and safety of pyrimethanil and boscalid on greenhouse strawberry. Ecotoxicol. Environ. Safe 2018, 160, 127–133. [Google Scholar] [CrossRef]
- Forster, W.A.; Kimberley, M.O. The contribution of spray formulation component variables to foliar uptake of agrichemicals. Pest Manag. Sci. 2015, 71, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Lichiheb, N.; Bedos, C.; Personne, E.; Benoit, P.; Bergheaud, V.; Fanucci, O.; Bouhlel, J.; Barriuso, E. Measuring leaf penetration and volatilization of chlorothalonil and epoxiconazole applied on wheat leaves in a laboratory-scale experiment. J. Environ. Qual. 2015, 44, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Sun, Z.; Bird, N.; Gu, Y.; Xu, Y.; Zhang, Z.; Wu, X. Effects of tank-mix adjuvants on physicochemical properties and dosage delivery at low dilution ratios for unmanned aerial vehicle application in paddy fields. Pest Manag. Sci. 2022, 78, 1582–15593. [Google Scholar] [CrossRef]
- Landim, T.N.; da Cunha, J.P.A.R.; Alves, G.S.; Marques, M.G.; Silva, S.M. Interactions between adjuvants and the fungicide azoxystrobin+benzovindiflupyr in hydraulic spraying. Engenharia Agrícola 2019, 39, 600–606. [Google Scholar] [CrossRef]
- Niedobová, J.; Skalský, M.; Ouředníčková, J.; Michalko, R.; Bartošková, A. Synergistic effects of glyphosate formulation herbicide and tank-mixing adjuvants on Pardosa spiders. Environ. Pollut. 2019, 249, 338–344. [Google Scholar] [CrossRef] [PubMed]


| Composition | 125 g/L EPX SC | 200 g/L CLR SC | Function |
|---|---|---|---|
| 95% TC (%) | 13.2 | 21.1 | Technical |
| Agrilan 788 (%) | 3.3 | 4.2 | Dispersing |
| 500 LQ (%) | 0.6 | / | Wetting |
| Sag 1522 (%) | 0.2 | 0.2 | Defoamer |
| Xanthan gum (%) | 4.0 | 2.4 | Thickening |
| Propylene glycol (%) | 5.5 | 5.2 | Antifreeze |
| Deionized water (%) | 73.2 | 66.9 | Solvent |
| Compound | Retention Time (min) | Tube Lens (V) | Quantifying Ions | Qualifying Ions | Collision Energy (V) |
|---|---|---|---|---|---|
| EPX | 1.2 | 93 | 330/121 | 330/101 | 30; 30 |
| CLR | 1.5 | 73 | 484/284 | 484/177 | 5; 5 |
| Spray Adjuvant | EPX | CLR | ||
|---|---|---|---|---|
| 125 mg/L | 187 mg/L | 66.7 mg/L | 100 mg/L | |
| Water | 47.76 (0.15) 1 | 45.82 (0.19) | 45.96 (1.33) | 44.45 (0.70) |
| PT | 21.14 (0.05) | 21.34 (0.13) | 21.36 (0.07) | 21.27 (0.02) |
| MO | 26.71 (0.06) | 26.96 (0.10) | 26.88 (0.09) | 26.66 (0.09) |
| GOEO | 29.45 (0.07) | 29.31 (0.07) | 29.29 (0.08) | 29.30 (0.06) |
| Compound | Sample | Fortified Level (mg/kg) | Average Recovery (%) | RSD (%) | Linear Equation | R2 | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|---|---|
| EPX | Plant | 0.05 | 117.1 | 2.2 | y = 48038820x + 58189 | 0.9995 | 0.33 | 1.03 |
| 0.1 | 99.7 | 5.6 | ||||||
| 0.5 | 96.8 | 3.4 | ||||||
| Root | 0.05 | 101.0 | 7.3 | y = 51725208x + 50006905 | 0.9999 | 0.33 | 1.01 | |
| 0.1 | 91.1 | 4.4 | ||||||
| 0.5 | 91.7 | 5.5 | ||||||
| Water | - | - | - | y = 53076335x − 1125052 | 0.9994 | 0.26 | 0.93 | |
| CLR | Plant | 0.05 | 98.0 | 7.0 | y = 225920x + 791 | 0.9999 | 0.64 | |
| 0.1 | 101.9 | 5.5 | 1.96 | |||||
| 0.5 | 101.1 | 8.2 | ||||||
| Root | 0.05 | 101.4 | 6.0 | y = 467620x + 568 | 0.9999 | 0.45 | 1.41 | |
| 0.1 | 98.2 | 8.6 | ||||||
| 0.5 | 96.4 | 9.0 | ||||||
| Water | - | - | - | y = 512836x + 13591 | 0.9995 | 0.33 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Zheng, L.; Li, Y.; Wang, C.; Cao, L.; Cao, C.; Huang, Q. Tank-Mix Adjuvants Regulate the Deposition, Absorption, and Permeation Behavior of Pesticide Solutions on Rice Plant. Agriculture 2022, 12, 1119. https://doi.org/10.3390/agriculture12081119
Zhao P, Zheng L, Li Y, Wang C, Cao L, Cao C, Huang Q. Tank-Mix Adjuvants Regulate the Deposition, Absorption, and Permeation Behavior of Pesticide Solutions on Rice Plant. Agriculture. 2022; 12(8):1119. https://doi.org/10.3390/agriculture12081119
Chicago/Turabian StyleZhao, Pengyue, Li Zheng, Yuanyuan Li, Chaojie Wang, Lidong Cao, Chong Cao, and Qiliang Huang. 2022. "Tank-Mix Adjuvants Regulate the Deposition, Absorption, and Permeation Behavior of Pesticide Solutions on Rice Plant" Agriculture 12, no. 8: 1119. https://doi.org/10.3390/agriculture12081119






