Nectar Abundance and Nectar Composition in Selected Rubus idaeus L. Varieties
Abstract
:1. Introduction
1.1. Importance of Berry Fruits
1.2. Nectar Secretion and Its Impact on Pollination
1.3. Nectar Chemical Profile and Pollination Syndrome
1.3.1. Qualitative Composition of Nectar Sugars
1.3.2. Nectar Protein
1.3.3. Floral Nectary Amino Acids
2. Materials and Methods
2.1. Plant Material
2.2. Scope of the Study
2.3. Nectar Abundance
2.4. Qualitative and Quantitative Composition of Nectar
2.5. Determination of Total Protein and Amino Acid Content in Nectar
2.6. Statistical Analysis
3. Results
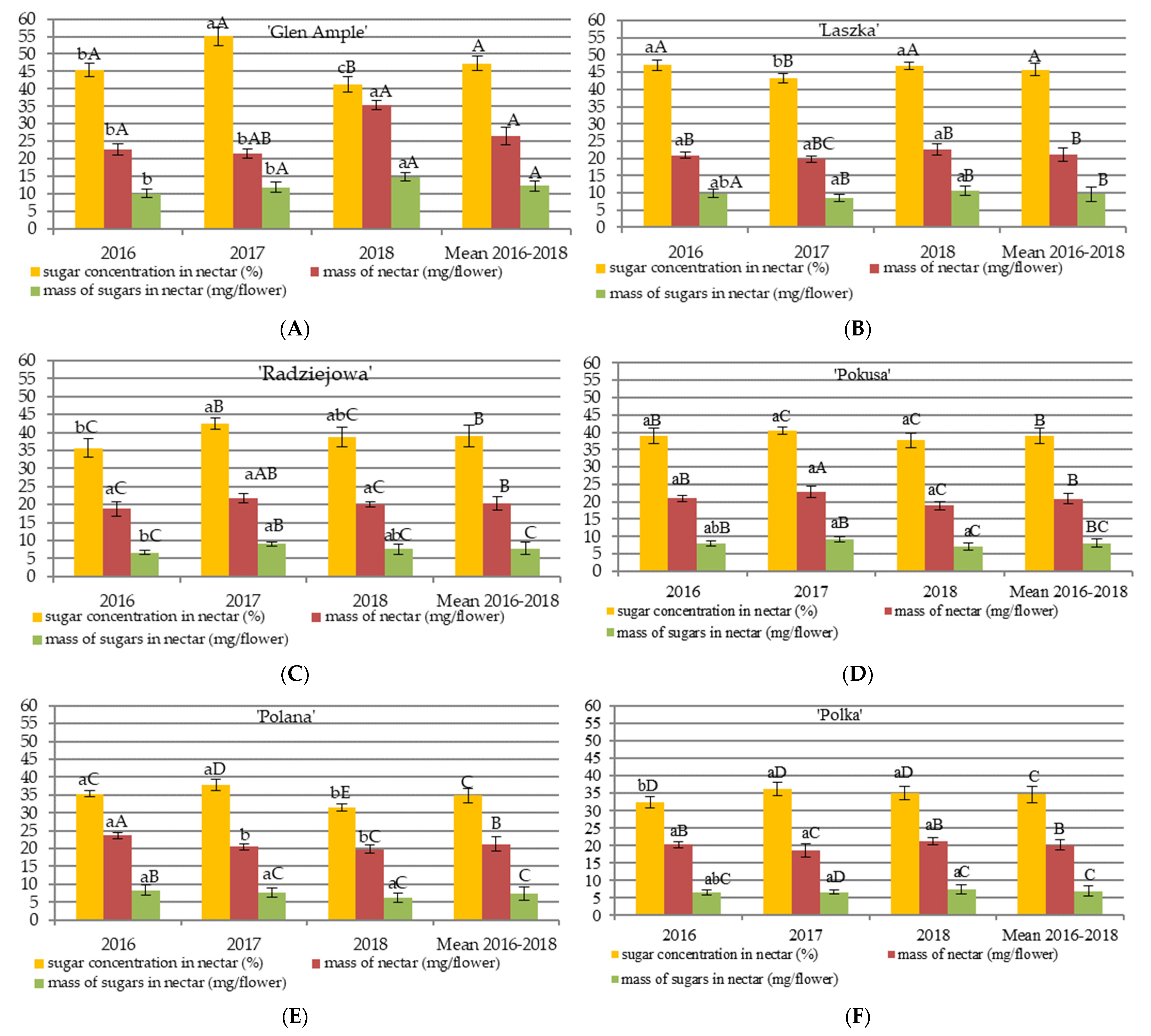
3.1. Nectar Abundance
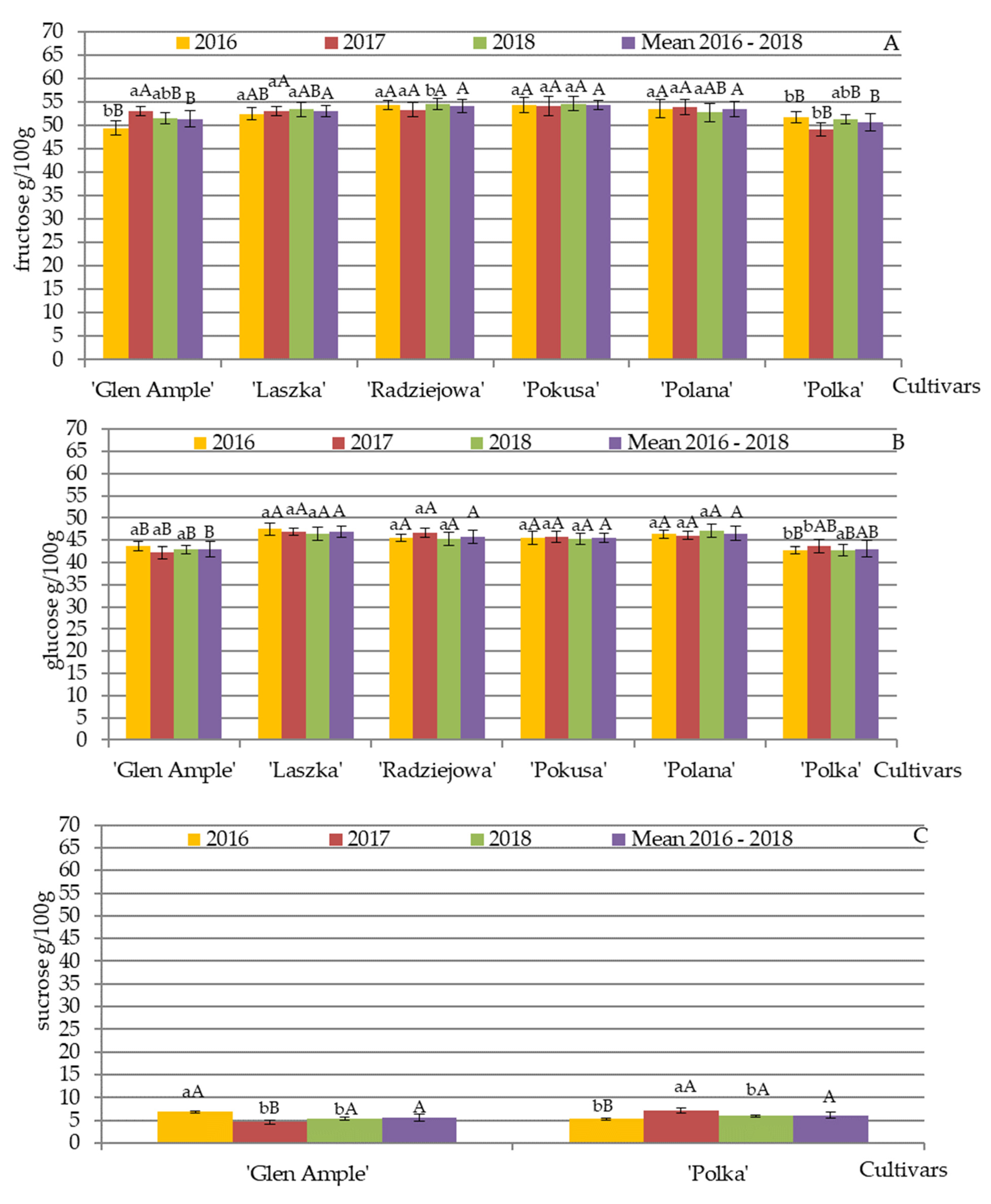
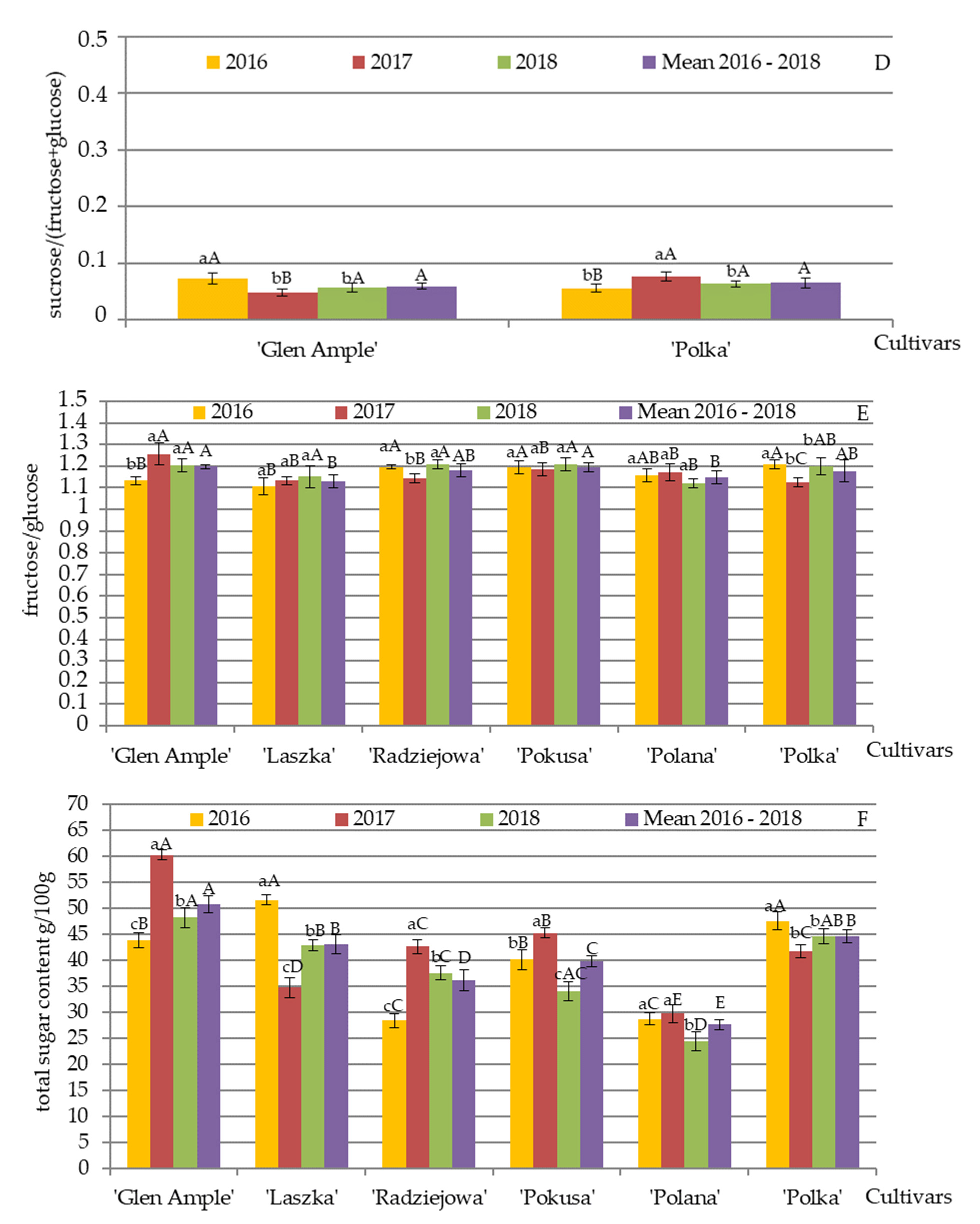
3.2. Qualitative and Quantitative Composition of Nectar Sugars
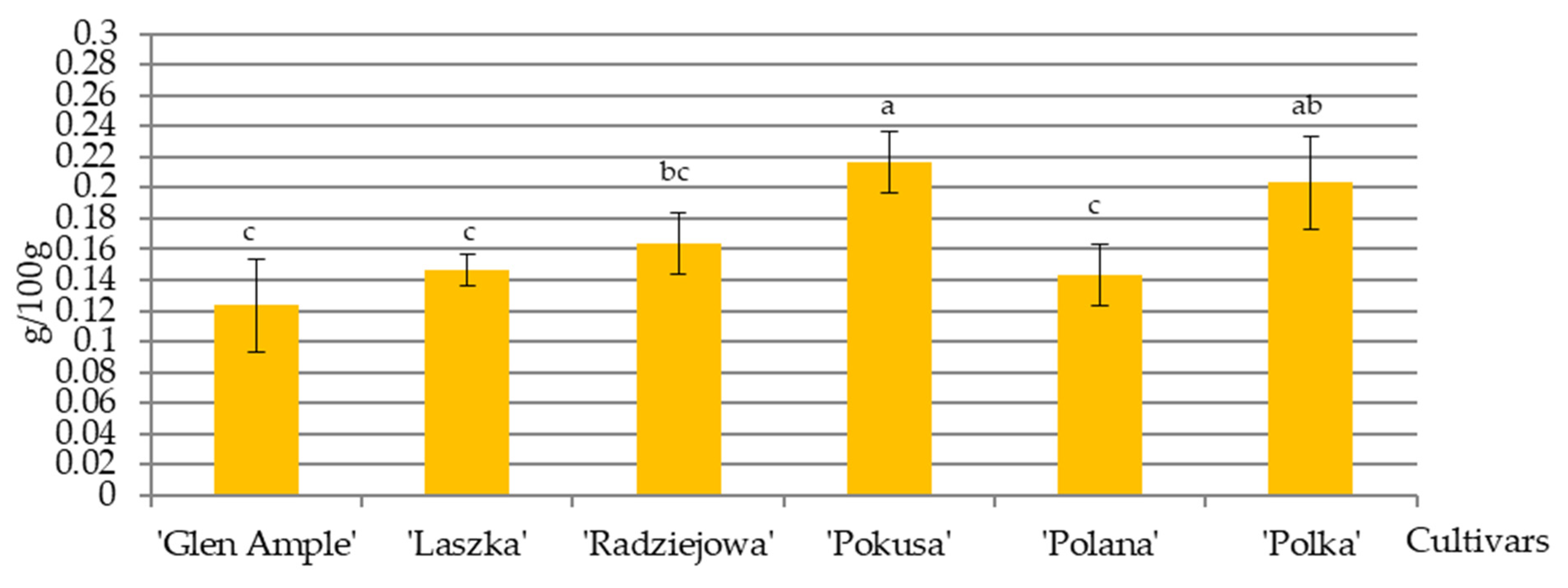
3.3. Nectar Protein Content
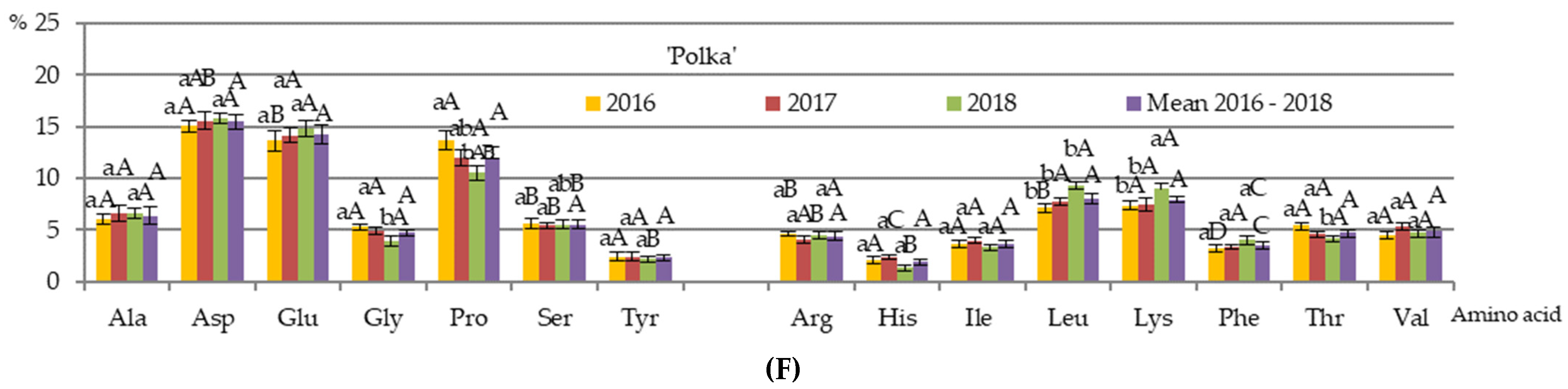
3.4. Qualitative and Quantitative Composition of Nectar Amino Acids
4. Discussion
4.1. Nectar Abundance
4.2. Qualitative and Quantitative Composition of Nectar Sugars
4.3. Nectar Protein Content
4.4. Qualitative and Quantitative Composition of Nectar Amino Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gundesli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. IJAFLS 2019, 3, 350–361. [Google Scholar]
- Hameed, A.; Galli, M.; Adamska-Patruno, E.; Krętowski, A.; Ciborowski, M. Select polyphenol-rich berry consumption to defer or deter diabetes and diabetes-related complications. Nutrients 2020, 12, 2538. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, D.; Gonzalez-Buesa, J.; Oria, R.; Venturini, M.E.; Arias, E. Effect of modified atmosphere packaging (MAP) and UV-C irradiation on postharvest quality of red raspberries. Agriculture 2021, 12, 29. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Myhre, J.B.; Løken, E.B.; Wandel, M.; Andersen, L.F. Meal types as sources for intakes of fruits, vegetables, fish and whole grains among Norwegian adults. Public Health Nutr. 2015, 18, 2011–2021. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 10 July 2022).
- Nilsen, L.; Hopstock, L.A.; Grimsgaard, S.; Carlsen, M.H.; Lundblad, M.W. Intake of vegetables, fruits and berries and compliance to “Five-a-Day” in a general Norwegian population—The tromsø study 2015–2016. Nutrients 2021, 13, 2456. [Google Scholar] [CrossRef]
- Lim, T.K. Rubus idaeus. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 555–569. [Google Scholar] [CrossRef]
- Purgar, D.; Duralija, B.; Voća, S.; Vokurka, A.; Ercisli, S. A comparison of fruit chemical characteristics of two wild grown Rubus species from different locations of Croatia. Molecules 2012, 17, 10390–10398. [Google Scholar] [CrossRef]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from Polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic acid: A review on its natural sources, chemical stability, and therapeutic potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) fruit as a source of bioactive compounds with health-promoting effects-a review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef] [PubMed]
- Mîrza, A. Antioxidant activity of leaf and fruit extracts from Rubus fruticosus, Rubus idaeus and Rubus longobaccus growing in the conditions of the Republic Moldova. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 363–372. [Google Scholar]
- Kewlani, P.; Singh, L.; Belwal, T.; Bhatt, I.D. Optimization of ultrasonic-assisted extraction for bioactive compounds in Rubus ellipticus fruits: An important source for nutraceutical and functional foods. Sustain. Chem. Pharm. 2022, 25, 100603. [Google Scholar] [CrossRef]
- Carew, R.; Kempler, C.; Moore, P.; Walters, T. Developments in raspberry production, cultivar releases, and intellectual property rights: A comparative study of British Columbia and Washington State. Int. J. Fruit Sci. 2009, 9, 54–77. [Google Scholar] [CrossRef]
- Jain, S.M.; Priadyarshan, P.M. Raspberry breeding. In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 233–248. [Google Scholar] [CrossRef]
- FAO. Data Collection, Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 February 2022).
- Kostryco, M.; Chwil, M.; Matraszek-Gawron, R. Comparison of the micromorphology and ultrastructure of pollen grains of selected Rubus idaeus L. cultivars grown in commercial plantation. Plants 2020, 9, 1194. [Google Scholar] [CrossRef]
- Kostryco, M.; Chwil, M. Structure of anther epidermis and endothecium, production of pollen, and content of selected nutrients in pollen grains from six Rubus idaeus L. cultivars. Agronomy 2021, 11, 1723. [Google Scholar] [CrossRef]
- Chagnon, M.; Gingras, J.; De Oliveira, D. Honey bee (Hymenoptera: Apidae) foraging behavior and raspberry pollination. J. Econ. Entomol. 1991, 84, 457–460. [Google Scholar] [CrossRef]
- Cane, J.H. Pollination potential of the bee Osmia aglaia for cultivated red raspberries and blackberries (Rubus: Rosaceae). HortScience 2005, 40, 1705–1708. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Dewenter, I.S.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B: Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Filep, R.; Orosz-Kovács, Z.; Farkas, Á. Patterns of nectar and pollen presentation influence the attractiveness of four raspberry and blackberry cultivars to pollinators. J. Hortic. Sci. 2015, 90, 47–56. [Google Scholar] [CrossRef]
- Żurawicz, E.; Studnicki, M.; Kubik, J.; Pruski, K. A careful choice of compatible pollinizers significantly improves the size of fruits in red raspberry (Rubus idaeus L.). Sci. Hortic. 2018, 235, 253–257. [Google Scholar] [CrossRef]
- Bartual, A.M.; Sutter, L.; Bocci, G.; Moonen, A.C.; Cresswell, J.; Entling, M.; Giffard, M.; Jacot, K.; Jeanneret, P.; Holland, J.; et al. The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 2019, 279, 43–52. [Google Scholar] [CrossRef]
- Markov Ristić, Z.; Popov, S. Raspberry production and economic value off insect pollination of raspberry in Serbia. In Proceedings of the XII International Scientific Agricultural Symposium “Agrosym” 2021 Jahorina, Sarajevo, Bosnia and Herzegovina, 7–10 October 2021; Kovacevic, D., Ed.; Academy of Engineering Sciences of Serbia: Sarajevo, Bosnia and Herzegovina, 2021; pp. 918–924. [Google Scholar]
- Tanda, A.S. Entomophilous crops get better fruit quality and yield: An appraisal. Indian J. Entomol. 2019, 81, 227–234. [Google Scholar] [CrossRef]
- Prodorutti, D.; Frilli, F. Entomophilous pollination of raspberry, red currant and highbush blueberry in a mountain area of Friuli-Venezia-Giulia (North-Eastern Italy). Acta Hortic. 2008, 777, 429–434. [Google Scholar] [CrossRef]
- Lye, G.C.; Jennings, S.N.; Osborne, J.L.; Goulson, D. Impacts of the use of non-native commercial bumble bees for pollinator supplementation in raspberry. J. Econ. Entomol. 2011, 104, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bederska-Łojewska, D.; Pieszka, M.; Marzec, A.; Rudzińska, M.; Grygier, A.; Siger, A.; Cieślik-Boczula, K.; Orczewska-Dudek, S.; Migdał, W. Physicochemical properties, fatty acid composition, volatile compounds of blueberries, cranberries, raspberries, and cuckooflower seeds obtained using sonication method. Molecules 2021, 26, 7446. [Google Scholar] [CrossRef] [PubMed]
- Zambon, V.; Agostini, K.; Nepi, M.; Rossi, M.L.; Martinelli, A.P.; Sazima, M. The role of nectar traits and nectary morphoanatomy in the plant-pollinator interaction between Billbergia distachia (Bromeliaceae) and the hermit Phaethornis eurynome (Trochilidae). Bot. J. Linn. 2020, 192, 816–827. [Google Scholar] [CrossRef]
- Pozo, M.I.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Peck, R.L., Ed.; Nova Science Publishers Inc: Hauppauge, NY, USA, 2015; pp. 1–45. [Google Scholar]
- Nepi, M.; Calabrese, D.; Guarnieri, M.; Giordano, E. Evolutionary and ecological considerations on nectar-mediated tripartite interactions in angiosperms and their relevance in the mediterranean basin. Plants 2021, 10, 507. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006, 97, 413–421. [Google Scholar] [CrossRef]
- Wolff, D. Nectar sugar composition and volumes of 47 species of Gentianales from a southern Ecuadorian montane forest. Ann. Bot. 2006, 97, 767–777. [Google Scholar] [CrossRef]
- Bertazzini, M.; Forlani, G. Intraspecific variability of floral nectar volume and composition in rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Petanidou, T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005, 31, 1065–1088. [Google Scholar] [CrossRef]
- Nepi, M. Nectary structure and ultrastructure. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 129–166. [Google Scholar]
- Ågren, J.; Elmqvist, T.; Tunlid, A. Pollination by deceit, floral sex ratios and seed set in dioecious Rubus chamaemorus L. Oecologia 1986, 70, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Gyan, K.Y.; Woodell, S.R.J. Nectar production, sugar content, amino acids and potassium in Prunus spinosa L., Crataegus monogyna Jacq. and Rubus fruticosus L. at Wytham, Oxfordshire. Funct. Ecol. 1987, 1, 251–259. [Google Scholar] [CrossRef]
- Schmidt, K.; Orosz-Kovács, Z.S.; Farkas, Á. The effect of blossom structure and nectar composition of some raspberry and blackberry cultivars on the behaviour of pollinators. J. Plant Reprod. Biol. 2008, 1, 1–6. [Google Scholar]
- Nagy Tóth, E.N.; Szabó, L.G.; Botz, L.; Orosz-Kovács, Z. Effect of rootstocks on floral nectar composition in apple cultivars. Plant. Syst. Evol. 2003, 23, 43–55. [Google Scholar] [CrossRef]
- Afik, O.; Dag, A.; Kerem, Z.; Shafi, S. Analyses of avocado (Persea americana) nectar properties and their perception by honey bees (Apis mellifera). J. Chem. Ecol. 2006, 32, 1949–1963. [Google Scholar] [CrossRef]
- Cakmak, I.; Song, D.S.; Mixson, T.A.; Serrano, E.; Clement, M.L.; Savitski, A.; Johnson, G.; Giray, T.; Abramson, C.I.; Barthell, J.F.; et al. Foraging response of Turkish honey bee subspecies to flower color choices and reward consistency. J. Insect Behav. 2010, 23, 100–116. [Google Scholar] [CrossRef]
- Twidle, A.M.; Mas, F.; Harper, A.R.; Horner, R.M.; Welsh, T.J.; Suckling, D.M. Kiwifruit flower odor perception and recognition by honey bees, Apis mellifera. J. Agric. Food Chem. 2015, 63, 5597–5602. [Google Scholar] [CrossRef]
- Gould, J.L. Specializations in honey bee learning. In Neuroethological Studies of Cognitive and Perceptual Processes; Moss, C.F., Shettleworth, S.J., Eds.; Routledge: New York, NY, USA, 2018; pp. 11–30. [Google Scholar] [CrossRef]
- Howard, S.R.; Garcia, J.E.; Dyer, A.G. Comparative psychophysics of colour preferences in two species of non-eusocial Australian native halictid bees. J. Comp. Physiol. A 2021, 207, 657–666. [Google Scholar] [CrossRef]
- Kevan, P.; Giurfa, M.; Chittka, L. Why are there so many and so few white flowers? Trends Plant Sci. 1996, 1, 252. [Google Scholar] [CrossRef]
- Hill, P.S.; Wells, P.H.; Wells, H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997, 54, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Karahan, A.; Cakmak, I.; Hranitz, J.M.; Karaca, I.; Wells, H. Sublethal imidacloprid effects on honey bee flower choices when foraging. Ecotoxicology 2015, 24, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.L. Pattern learning by honey bees. Anim. Behav. 1986, 34, 990–997. [Google Scholar] [CrossRef]
- Wykes, G.R. The preferences of honey bees for solutions of various sugars which occur in nectar. J. Exp. Biol. 1952, 29, 511–518. [Google Scholar] [CrossRef]
- Maurizio, A.; Grafl, I. Das Trachtpflanzenbuch: Nektar und Pollen—Die Wichtigsten Nahrungsquellen der Honigbiene; Ehrenwirth Verlag: München, Germany, 1969; 228p. [Google Scholar]
- Farkas, A.; Orosz-Kovács, Z.S. Primary and secondary attractants of flowers in pear Pyrus betulifolia. Acta Hortic. 2004, 636, 317–324. [Google Scholar] [CrossRef]
- Chwil, M.; Kostryco, M.; Matraszek-Gawron, R. Comparative studies on structure of the floral nectaries and the abundance of nectar production of Prunus laurocerasus L. Protoplasma 2019, 256, 1705–1726. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- Park, S.; Thornburg, R.W. Biochemistry of nectar proteins. J. Plant Biol. 2009, 52, 27–34. [Google Scholar] [CrossRef]
- Song, Y.Q.; Milne, R.I.; Zhou, H.X.; Ma, X.L.; Fang, J.Y.; Zha, H.G. Floral nectar chitinase is a potential marker for monofloral honey botanical origin authentication: A case study from loquat (Eriobotrya japonica Lindl.). Food Chem. 2019, 282, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Smeets, K.; Van Nerum, K.; Van Leuven, F.; Van Damme, E.J. Lectin and alliinase are the predominant proteins in nectar from leek (Allium porrum L.) flowers. Planta 1997, 201, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, R.W.; Carter, C.; Powell, A.; Mittler, R.; Rizhsky, L.; Horner, H.T. A major function of the tobacco floral nectary is defense against microbial attack. Plant Syst. Evol. 2003, 238, 211–218. [Google Scholar] [CrossRef]
- Poulis, B.A.D.; O’Leary, S.J.B.; Haddow, J.D.; von Aderkas, P. Identification of proteins present in the Douglas fir ovular secretion: An insight into conifer pollen selection and development. Int. J. Plant Sci. 2005, 166, 733–739. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar] [CrossRef]
- Harper, A.D.; Stalnaker, S.H.; Wells, L.; Darvill, A.; Thornburg, R.; York, W.S. Interaction of Nectarin 4 with a fungal protein triggers a microbial surveillance and defense mechanism in nectar. Phytochemistry 2010, 71, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Soligo, C.; Nocentini, D.; Abate, M.; Guarnieri, M.; Cai, G.; Pacini, E. Amino acids and protein profile in floral nectar: Much more than a simple reward. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 475–481. [Google Scholar] [CrossRef]
- Heil, M.; Rattke, J.; Boland, W. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 2005, 308, 560–563. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Zhao, F.; Zha, H.; Yang, L.; Lu, Y.; Wang, G.; Shi, J.; Chen, J. Floral nectary morphology and proteomic analysis of nectar of Liriodendron tulipifera Linn. Front. Plant Sci. 2016, 7, 826. [Google Scholar] [CrossRef]
- Schmitt, A.J.; Sathoff, A.E.; Holl, C.; Bauer, B.; Samac, D.A.; Carter, C.J. The major nectar protein of Brassica rapa is a non-specific lipid transfer protein, BrLTP2. 1, with strong antifungal activity. J. Exp. Bot. 2018, 69, 5587–5597. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 2004, 9, 320–324. [Google Scholar] [CrossRef]
- Carter, C.; Shafir, S.; Yehonatan, L.; Palmer, R.G.; Thornburg, R. A novel role for proline in plant floral nectars. Naturwissenschaften 2006, 93, 72–79. [Google Scholar] [CrossRef]
- Carter, C.; Healy, R.; Nicole, M.; Naqvi, S.S.; Ren, G.; Park, S.; Beattie, G.A.; Horner, H.T.; Thornburg, W.T. Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiol. 2007, 143, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kram, B.W.; Bainbridge, E.A.; Perera, M.A.D.; Carter, C. Identification, cloning and characterization of a GDSL lipase secreted into the nectar of Jacaranda mimosifolia. Plant Mol. Biol. 2008, 68, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Wielsch, N.; Kessler, D.; Svatos, A.; Park, C.M.; Baldwin, I.T.; Kim, S.G. Natural variation in floral nectar proteins of two Nicotiana attenuata accessions. BMC Plant Biol. 2013, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Thornburg, R.W. The nectary-specific pattern of expression of the tobacco Nectarin I promoter is regulated by multiple promoter elements. Plant Mol. Biol. 2003, 51, 451–457. [Google Scholar] [CrossRef]
- Carter, C.J.; Thornburg, R.W. Tobacco Nectarin III is a bifunctional enzyme with monodehydroascorbate reductase and carbonic anhydrase activities. Plant Mol. Biol. 2004, 54, 415–425. [Google Scholar] [CrossRef]
- Zha, H.G.; Liu, T.; Zhou, J.J.; Sun, H. MS-desi, a desiccation-related protein in the floral nectar of the evergreen velvet bean (Mucuna sempervirens Hemsl): Molecular identification and characterization. Planta 2013, 238, 77–89. [Google Scholar] [CrossRef]
- Bordin, D.M.; Latgé, S.G.; Pyke, G.; Kalman, J.; Doble, P.; Genta, F.A.; Blanes, L. A simple approach to analyze sugar nectar composition in flowers using capillary electrophoresis and enzymatic assays. J. Braz. Chem. Soc. 2020, 31, 2129–2134. [Google Scholar] [CrossRef]
- Ruhlmann, J.M.; Kram, B.W.; Carter, C.J. CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis. J. Exp. Bot. 2010, 61, 395–404. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The effects of soil fertilizer on amino acids in the floral nectar of corncockle, Agrostemma githago (Caryophyllaceae). Oikos 2001, 92, 101–106. [Google Scholar] [CrossRef]
- Roguz, K.; Bajguz, A.; Chmur, M.; Gołębiewska, A.; Roguz, A.; Zych, M. Diversity of nectar amino acids in the Fritillaria (Liliaceae) genus: Ecological and evolutionary implications. Sci. Rep. 2019, 9, 15209. [Google Scholar] [CrossRef]
- Gottsberger, G.; Schrauwen, J.; Linskens, F. Amino acids and sugars in nectar, and their putative. Plant Syst. Evol. 1984, 145, 55–77. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. Analyzing variability in nectar amino acids: Composition is less variable than concentration. J. Chem. Ecol. 2001, 27, 2545–2558. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.; Ellis, W.N.; Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 2006, 115, 155–169. [Google Scholar] [CrossRef]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The taste of nectar—A neglected area of pollination ecology. Oikos 2002, 98, 552–557. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. The occurrence and significance of amino acids in floral nectar. Plant Syst. Evol. 1986, 151, 175–186. [Google Scholar] [CrossRef]
- Mevi-Schutz, J.; Erhardt, A. Amino acids in nectar enhance butterfly fecundity: A long-awaited link. Am. Nat. 2005, 165, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Opler, P.A.; Krizek, G.O. Butterflies East of the Great Plains; John Hopkins University Press: Baltimore, MD, USA, 1984. [Google Scholar]
- Boggs, C.L. Dynamics of reproductive allocation from juvenile and adult feeding: Radiotracer studies. Ecology 1997, 78, 192–202. [Google Scholar] [CrossRef]
- Cahenzli, F.; Erhardt, A. Nectar amino acids enhance reproduction in male butterflies. Oecologia 2013, 171, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.W. Pollination in Impatiens capensis and Impatiens pallida (Balsaminacceae). Bull. Torrey Bot. Club 1977, 104, 361–367. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Shiraishi, A.; Kuwabra, M. The effects of amino acids on the labellar hair chemosensorycells of the fly. J. Gen. Physiol. 1970, 56, 768–782. [Google Scholar] [CrossRef]
- Dadd, R.H. Insect nutrition: Current developments and metabolic implications. Annu. Rev. Entomol. 1973, 18, 381–420. [Google Scholar] [CrossRef]
- Brue, R.N. Nutrition. In Avian Medicine: Principles and Application; Ritchie, B., Harrison, G., Harrison, L., Eds.; Wingers Publishing: Lake Worth, FL, USA, 1994; pp. 63–95. [Google Scholar]
- Tamm, S.; Gass, C.L. Energy intake rates and nectar concentration preferences by hummingbirds. Oecologia 1986, 70, 20–23. [Google Scholar] [CrossRef]
- Blüthgen, N.; Gottsberger, G.; Fiedler, K. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecol. 2004, 29, 418–429. [Google Scholar] [CrossRef]
- Hainsworth, F.R.; Wolf, L.L. Nectar characteristics and food selection by hummingbirds. Oecologia 1976, 25, 101–113. [Google Scholar] [CrossRef]
- Inouye, D.W.; Waller, G.D. Responses of honeybees (Apis mellifera) to amino acid solutions mimicking floral nectars. Ecology 1984, 65, 618–625. [Google Scholar] [CrossRef]
- Rusterholz, H.P.; Erhardt, A. Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia 1998, 113, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, B. Notes of the method to investigate nectar secretion rates in flowers. J. Apic. Sci. 2002, 46, 117–125. [Google Scholar]
- Hossain, M.S.; Yeasmin, F.; Rahman, M.M.; Akhtar, S.; Hasnat, M.A. Role of insect visits on cucumber (Cucumis sativus L.) yield. J. Biodivers. Conserv. Bioresour. Manag. 2018, 4, 81–88. [Google Scholar] [CrossRef]
- Percival, M. Floral Biology; Elsevier Science: London, UK, 2013; 260p. [Google Scholar]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonised methods of the European honey commission. Apidologie 1997, 1–59, (extra issue). [Google Scholar]
- Rybak-Chmielewska, H.; Szczęsna, T. Determination of saccharides in multifloral honey by means of HPLC. J. Apic. Sci. 2003, 47, 93–101. [Google Scholar]
- Rybak-Chmielewska, H. High performance liquid chromatography (HPLC) study of sugar composition in some kinds of natural honey and winter stores processed by bees from starch syrup. J. Apic. Sci. 2007, 51, 39–48. [Google Scholar]
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The nitrogen content of pollen protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Davies, M.G.; Thomas, A.J. An investigation of hydrolytic techniques for the amino acid analysis of foodstuffs. J. Sci. Food Agric. 1973, 24, 1525–1540. [Google Scholar] [CrossRef]
- Szklanowska, K.; Wieniarska, J. Beekeeping value and fruit crop of ten raspberry cultivars (Rubus idaeus L.). Pszczeln. Zesz. Nauk. 1985, 29, 231–251. (In Polish) [Google Scholar]
- Szklanowska, K. Nectar secretion and honey yields of raspberry (Rubus idaeus L.) and blackberries (Rubus fruticosus L.) in the forest environment (in Polish). Pszczeln. Zesz. Nauk. 1972, 16, 133–145. [Google Scholar]
- Willmer, P.G.; Bataw, A.A.M.; Hughes, J.P. The superiority of bumblebees to honeybees as pollinators: Insect visits to raspberry flowers. Ecol. Entomol. 1994, 19, 271–284. [Google Scholar] [CrossRef]
- Schmidt, K.; Orosz-Kovács, Z.; Farkas, Á. Nectar secretion dynamics in some raspberry and blackberry cultivars. Int. J. Plant Biol. 2012, 4, 147–150. [Google Scholar] [CrossRef]
- Bataw, A.A. Pollination Ecology of Cultivated and Wild Raspberry (Rubus idaeus) and the Behaviour of Visiting Insects. Ph.D. Thesis, University of St. Andrews, St. Andrews, UK, 1996; p. 24. [Google Scholar]
- Simidchiev, T. Studies of nectar and honey production in raspberry (Rubus idaeus L.) and blackberry (Rubus fruticosus L.). Gradinar. Lozar. Nauk. 1976, 13, 42–49. [Google Scholar]
- Pamminger, T.; Becker, R.; Himmelreich, S.; Schneider, C.W.; Bergtold, M. The nectar report: Quantitative review of nectar sugar concentrations offered by bee visited flowers in agricultural and non-agricultural landscapes. PeerJ 2019, 7, e6329. [Google Scholar] [CrossRef] [PubMed]
- Szklanowska, K. Nectar secretion and honey yields of some trees and shrubs in Polish conditions. Pszczeln. Zesz. Nauk. 1978, 22, 17–128. (In Polish) [Google Scholar]
- Whitney, G.G. The reproductive biology of raspberries and plant-pollinator community structure. Am. J. Bot. 1984, 71, 887–894. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops, 2nd. ed.; Academic Press: New York, NY, USA, 1993; pp. 263–270. [Google Scholar]
- McGregor, S.E. Insect Pollination of Cultivated Crop Plants; Agricultural Research Service; U.S. Government Publishing Office: Washington, DC, USA, 1976; 411p. [Google Scholar]
- Shanks, C.H.J. Pollination of raspberries by honeybees. J. Apic. Res. 1969, 8, 19–21. [Google Scholar] [CrossRef]
- Hanley, M.E.; Awbi, A.J.; Franco, M. Going native? Flower use by bumblebees in English urban gardens. Ann. Bot. 2014, 113, 799–806. [Google Scholar] [CrossRef]
- Crailsheim, K. The flow of jelly within a honeybee colony. J. Comp. Physiol. B. 1992, 162, 681–689. [Google Scholar] [CrossRef]
- Solomon, R.J.; Santhi, V.S.; Jayaraj, V. Prevalence of antibiotics in nectar and honey in South Tamilnadu, India. Integr. Biosci. 2006, 10, 163–167. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological stoichiometry of the honeybee: Pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E. Nectar production for the Hungarian honey industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Bukovics, P.; Orosz-Kovács, Z.; Szabó, L.G.; Farkas, Á.; Bubán, T. Composition of floral nectar and its seasonal variability in sour cherry cultivars. Acta Bot. Hung. 2003, 45, 259–271. [Google Scholar] [CrossRef]
- Percival, M.S. Types of nectar in angiosperms. New Phvtol. 1961, 60, 235–281. [Google Scholar] [CrossRef]
- Battaglini, M.; Battaglini, N. Characteristics of the sugar fraction of the nectar from five tree species. Ann. Tocalta 1974, 29, 441–455. [Google Scholar]
- Meheriuk, M.; Lane, W.D.; Hall, J.W. Influence of cultivar on nectar sugar content in several species of tree fruits. HortScience 1987, 22, 448–450. [Google Scholar]
- Chwil, M. Structure of Flower Nectaries and Beekeeping Value of Plant Factors from the Subfamily Prunoideae (Rosaceae). Habilitation Thesis, Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie, Lublin, Poland, 2013; 108p. (In Polish). [Google Scholar]
- Bordács, M.M.; Botz, L.; Orosz-Kovács, Z.; Kerek, M.M. The composition of nectar in apricot cultivars. Acta Hortic. 1995, 384, 367–371. [Google Scholar] [CrossRef]
- Nicolson, S.W. Pollination by passerine birds: Why are the nectars so dilute? Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2002, 131, 645–652. [Google Scholar] [CrossRef]
- Lichtenberg-Kraag, B. Evidence for correlation between invertase activity and sucrose content during the ripening process of honey. J. Apic. Res. 2014, 53, 364–373. [Google Scholar] [CrossRef]
- Braun, P.G.; Hildebrand, P.D.; Jamieson, A.R. Resistance of raspberry cultivars to fire blight. HortScience 2004, 39, 1189–1192. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Chemical constituents of nectar in relations to pollination mechanism and phylogeny. In Biochemical Aspects of Evolutionary Biology; Nitecki, M.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1982; pp. 131–171. [Google Scholar]
- Wykes, G.R. The sugar content of nectars. Biochem. J. 1953, 53, 294–296. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, B. Effect of an amino acid on feeding preferences and learning behavior in the honey bee, Apis mellifera. J. Insect Physiol. 2000, 46, 793–801. [Google Scholar] [CrossRef]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Heinrich, B. Energetics of pollination. Annu. Rev. Ecol. Evol. Syst. 1975, 6, 139–170. [Google Scholar] [CrossRef]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; HarperCollins Publishers: London, UK, 1996; 479p. [Google Scholar]
- Roces, F.; Blatt, J. Haemolymph sugars and the control of the proventriculus in the honey bee Apis mellifera. J. Insect Physiol. 1999, 45, 221–229. [Google Scholar] [CrossRef]
- White, J.S. Sucrose, HFCS, and fructose: History, manufacture, composition, applications, and production. In Fructose, High Fructose Corn Syrup, Sucrose and Health; Rippe, J., Ed.; Humana Press: New York, NY, USA, 2014; pp. 13–33. [Google Scholar] [CrossRef]
- Petanidou, T.; Goethals, V.; Smets, E. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community does flowering time matter? Plant Syst. Evol. 2000, 225, 103–118. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Nepi, M.; Pacini, E. Nectar consumers. In Nectaries and Nectar; Nicolson, S.W., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 312–322. [Google Scholar]
- McWhorter, T.J.; Powers, D.R.; Martínez del Rio, C. Are hummingbirds facultatively ammonotelic? Nitrogen excretion and requirements as a function of body size. Physiol. Biochem. Zool. 2003, 76, 731–743. [Google Scholar] [CrossRef]
- Bender, R.L.; Fekete, M.L.; Klinkenberg, P.M.; Hampton, M.; Bauer, B.; Malecha, M.; Lindgren, K.; Maki, A.J.; Perera, M.; Nikolau, B.J.; et al. PIN6 is required for nectary auxin response and short stamen development. Plant J. 2013, 74, 893–904. [Google Scholar] [CrossRef]
- Klinkenberg, P. A Sucrose Transporter and Proper Hormone Response are Essential for Nectary Function in the Brassicaceae. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2013. Available online: https://hdl.handle.net/11299/177035 (accessed on 3 March 2022).
- Seo, H.J.; Song, J.; Yoon, H.J.; Lee, K.Y. Effects of nectar contents on the foraging activity of honeybee (Apis mellifera) on Asian pear (Pyrus pyrifolia Nakai). Sci. Hortic. 2019, 245, 185–192. [Google Scholar] [CrossRef]
- Venjakob, C.; Ruedenauer, F.A.; Klein, A.M.; Leonhardt, S.D. Variation in nectar quality across 34 grassland plant species. Plant Biol. 2022, 24, 134–144. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, A.; Kang, D.; Kwon, H.Y.; Park, Y.; Kim, M.S. Analysis of floral nectar characteristics of Korean and Chinese hawthorns (Crataegus pinnatifida Bunge). J. Apic. Res. 2018, 57, 119–128. [Google Scholar] [CrossRef]
- Caldwell, D.L.; Gerhardt, K.O. Chemical analysis of peach extrafloral nectary exudate. Phytochemistry 1986, 25, 411–413. [Google Scholar] [CrossRef]
- Somme, L.; Vanderplanck, M.; Michez, D.; Lombaerde, I.; Moerman, R.; Wathelet, B.; Wattiez, B.; Lognay, G.; Jacquemart, A.L. Pollen and nectar quality drive the major and minor floral choices of bumble bees. Apidologie 2015, 46, 92–106. [Google Scholar] [CrossRef]
- Sharifani, M.; Jackson, J.F. Nectar analysis, pollen production and anthocyanin measurements, revealed distinct variations in pears. Acta Hort. 2004, 636, 415–422. [Google Scholar] [CrossRef]
- Hrassnigg, N.; Leonhard, B.; Crailsheim, K. Free amino acids in the haemolymph of honey bee queens (Apis mellifera L.). Amino Acids 2003, 24, 205–212. [Google Scholar] [CrossRef]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling role of glutamate in plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef]
- Anraku, M.; Shintomo, R.; Taguchi, K.; Kragh-Hansen, U.; Kai, T.; Maruyama, T.; Otagiri, M. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015, 134, 36–41. [Google Scholar] [CrossRef]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X.; et al. Dietary supplementation with L-glutamate and L-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef]
- Lanza, J.; Smith, G.C.; Sack, S.; Cash, A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 1995, 102, 113–119. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hua, X.J.; May, M.; Van Montagu, M. Environmental and developmental signals modulate proline homeostasis: Evidence for a negative transcriptional regulator. Proc. Natl. Acad. Sci. USA 1996, 93, 8787–8791. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Chaudhary, E.; Tiwari, P.; Uniyal, P.L. Morphology and pollen chemistry of several bee forage taxa of family Rosaceae from Garhwal Himalaya, Uttarakhand. India. J. Apic. Res. 2018, 62, 167–177. [Google Scholar] [CrossRef]
- Kaczorowski, R.L.; Gardener, M.C.; Holtsford, T.P. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. Am. J. Bot. 2005, 92, 1270–1283. [Google Scholar] [CrossRef]
- Linskens, H.F.; Schrauwen, J. The release of free amino acids from germinating pollen. Acta Bot. Neerl. 1969, 18, 605–614. [Google Scholar] [CrossRef]
- Gottsberger, G.; Arnold, T.; Linskens, H.F. Variation in floral nectar amino acids with aging of flowers, pollen contamination, and flower damage. Israel J. Bot. 1990, 39, 167–176. [Google Scholar]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Alm, J.; Thomas, E.; Lanza, J.; Vriesenga, L. Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 1990, 84, 53–57. [Google Scholar] [CrossRef]
- Hansen, K.; Wacht, S.; Seebauer, H.; Schnuch, M. New aspects of chemoreception in flies. Ann. N. Y. Acad. Sci. 1998, 855, 143–147. [Google Scholar] [CrossRef]
- Nepi, M.; von Aderkas, P.; Wagner, R.; Mugnaini, S.; Coulter, A.; Pacini, E. Nectar and pollination drops: How different are they? Ann. Bot. 2009, 104, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef]
- Otvos, L.; Otvos, I.; Rogers, M.E.; Consolvo, P.J.; Condie, B.A.; Lovas, S.; Bulet, P.; Blaszczyk-Thurin, M. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry 2000, 39, 14150–14159. [Google Scholar] [CrossRef]
- Rolland, J.L.; Abdelouahab, M.; Dupont, J.; Lefevre, F.; Bachere, E.; Romestand, B. Stylicins, a new family of antimicrobial peptides from the Pacific blue shrimp Litopenaeus stylirostris. Mol. Immunol. 2010, 47, 1269–1277. [Google Scholar] [CrossRef]
- Avitabile, C.; D’Andrea, L.D.; Romanelli, A. Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci. Rep. 2014, 4, 4293. [Google Scholar] [CrossRef]
- De Souza Cândido, E.; Cardoso, M.H.S.; Sousa, D.A.; Viana, J.C.; de Oliveira-Júnior, N.G.; Miranda, V.; Franco, O.L. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 2014, 55, 65–78. [Google Scholar] [CrossRef]
- Baker, H.G.; Opler, P.A.; Baker, I. A comparison of the amino acid complements of floral and extrafloral nectars. Bot. Gaz. 1978, 139, 322–332. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Oxman, K.L.; Shafir, S. Amino acid and carbohydrate tradeoffs by honey bee nectar foragers and their implications for plant–pollinator interactions. J. Insect Physiol. 2014, 69, 56–64. [Google Scholar] [CrossRef]
- Ligoxygakis, P.; Pelte, N.; Hoffmann, J.A.; Reichhart, J.M. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 2002, 297, 114–116. [Google Scholar] [CrossRef]
- Vannette, R.L.; Mohamed, A.; Johnson, B.R. Forager bees (Apis mellifera) highly express immune and detoxification genes in tissues associated with nectar processing. Sci. Rep. 2015, 5, 16224. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef]
- McCaughey, W.F.; Gilliam, M.; Standifer, L.N. Amino acids and protein adequacy for honey bees of pollens from desert plants and other floral sources. Apidologie 1980, 11, 75–86. [Google Scholar] [CrossRef]
- Bıliková, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Šimúth, J. Apisimin, a new serine–valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef]
- Groot, A.P.D. Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Thawley, A.R. The components of honey and their effects on its properties: A review. Bee World 1969, 50, 51–60. [Google Scholar] [CrossRef]
- Bose, G.; Battaglini, M. Gas chromatographic analysis of free and protein amino acids in some unifloral honeys. J. Apic. Res. 1978, 17, 152–166. [Google Scholar] [CrossRef]
- Chatt, E.C.; Von Aderkas, P.; Carter, C.J.; Smith, D.; Elliott, M.; Nikolau, B.J. Sex-dependent variation of pumpkin (Cucurbita maxima cv. Big Max) nectar and nectaries as determined by proteomics and metabolomics. Front. Plant Sci. 2018, 9, 860. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Leonhardt, S.D.; Lunau, K.; Spaethe, J. Bumblebees are able to perceive amino acids via chemotactile antennal stimulation. J. Comp. Physiol. 2019, 205, 321–331. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostryco, M.; Chwil, M. Nectar Abundance and Nectar Composition in Selected Rubus idaeus L. Varieties. Agriculture 2022, 12, 1132. https://doi.org/10.3390/agriculture12081132
Kostryco M, Chwil M. Nectar Abundance and Nectar Composition in Selected Rubus idaeus L. Varieties. Agriculture. 2022; 12(8):1132. https://doi.org/10.3390/agriculture12081132
Chicago/Turabian StyleKostryco, Mikołaj, and Mirosława Chwil. 2022. "Nectar Abundance and Nectar Composition in Selected Rubus idaeus L. Varieties" Agriculture 12, no. 8: 1132. https://doi.org/10.3390/agriculture12081132
APA StyleKostryco, M., & Chwil, M. (2022). Nectar Abundance and Nectar Composition in Selected Rubus idaeus L. Varieties. Agriculture, 12(8), 1132. https://doi.org/10.3390/agriculture12081132




