Changes in the Physiological Adaptation and Regulation Ability in Harmonia axyridis under Chlorpyrifos and Imidacloprid Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Methods
2.2.1. Screening for the Effective concentrations of Imidacloprid and Chlorpyrifos and the Formal Experimental Concentration Design
2.2.2. Phenotypes and Developmental Duration
- (i).
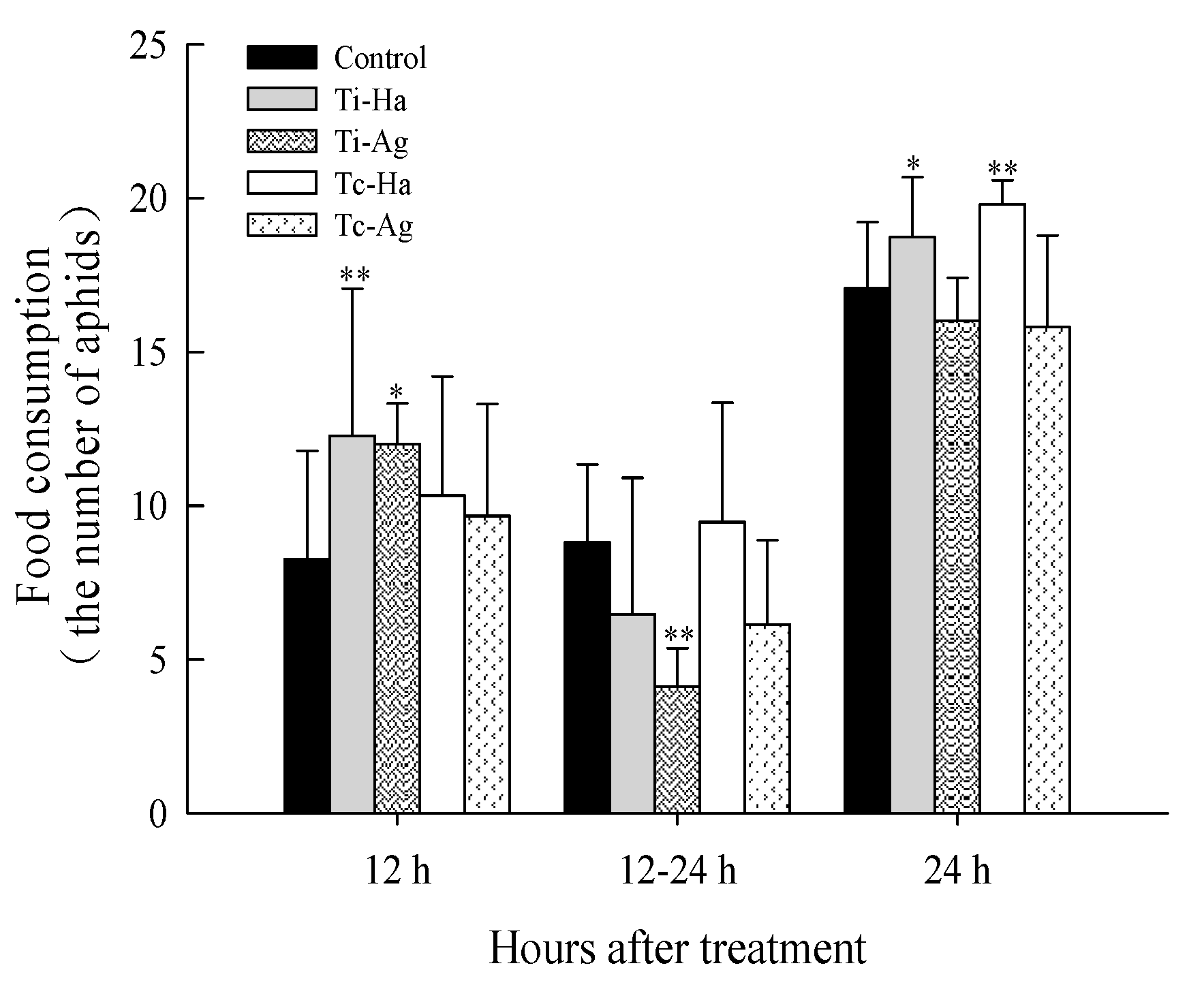
- Food consumption: The 4th instar ladybirds were placed in the tubes and starved for 12 h, then 20 pea aphids were added to feed the ladybirds. The numbers of pea aphids eaten by the ladybirds were recorded at 12 h and 24 h, and three replications per treatment were conducted, with 15 insects per treatment;
- (ii).
- Height and developmental duration: Insects were transferred into weighed Petri dishes and photographs were taken to record the morphological changes among the developmental phases. The time and weight of pupation, the time of emergence, and the weights of adults were also recorded.
- (iii).
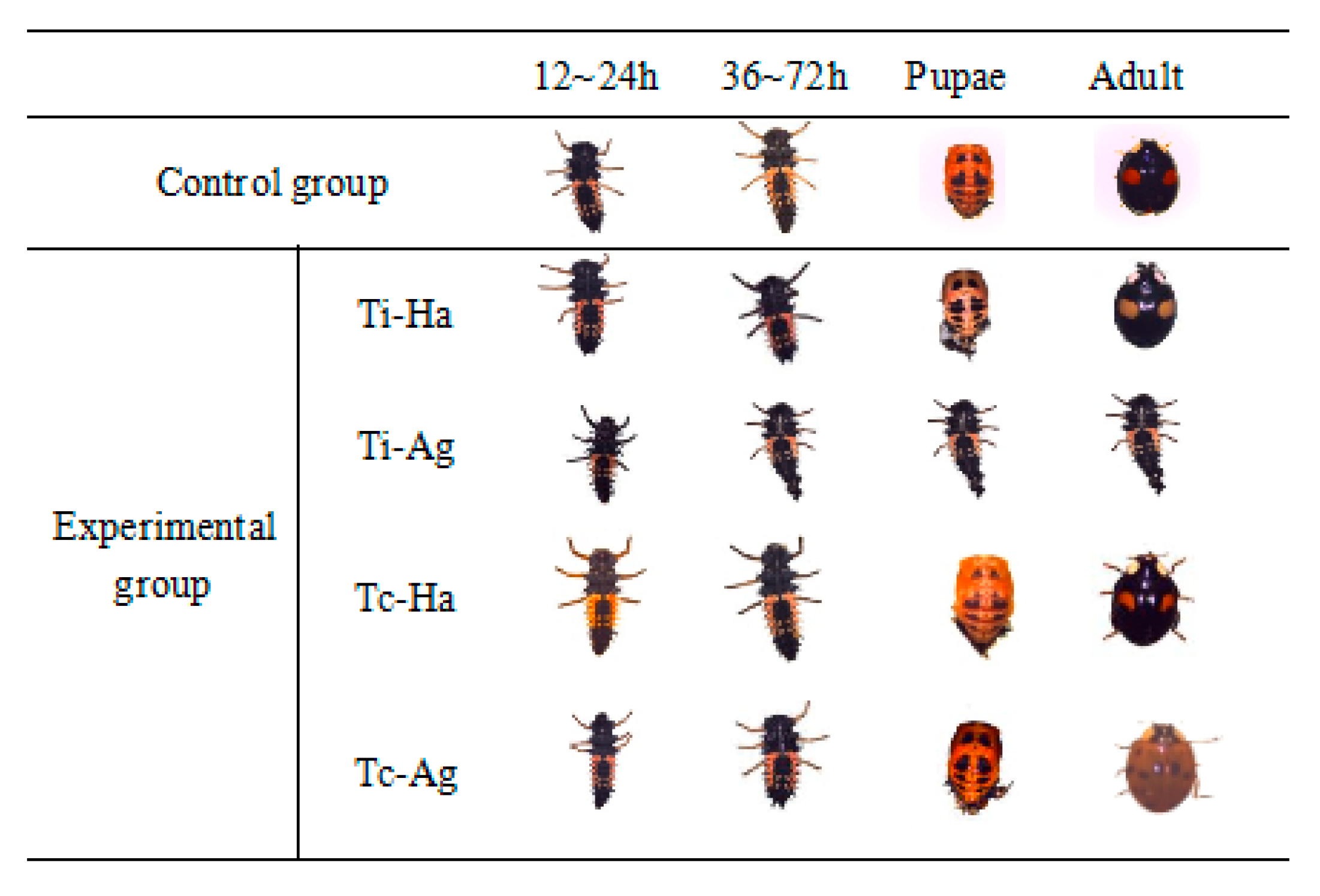
- Phenotypic observation: Imidacloprid and chlorpyrifos treatments were conducted for 12 h, and the developmental phenotypes of experimental insects in different treatment groups were observed and photographed.
2.2.3. Determination of Trehalase Activity and Sugar Content
2.2.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.2.5. Statistical Analyses
3. Results
3.1. Developmental Duration and Phenotypic Changes of Ladybird
3.2. Effect of Food Consumption on Ladybirds
3.3. Single Average Weight and Developmental Duration (from Pupa to Adult) of Ladybirds
3.4. Effect on TRE1 and TRE2 Enzyme Activity
3.5. Changes in Trehalose, Glucose, and Glycogen Contents in Ladybirds
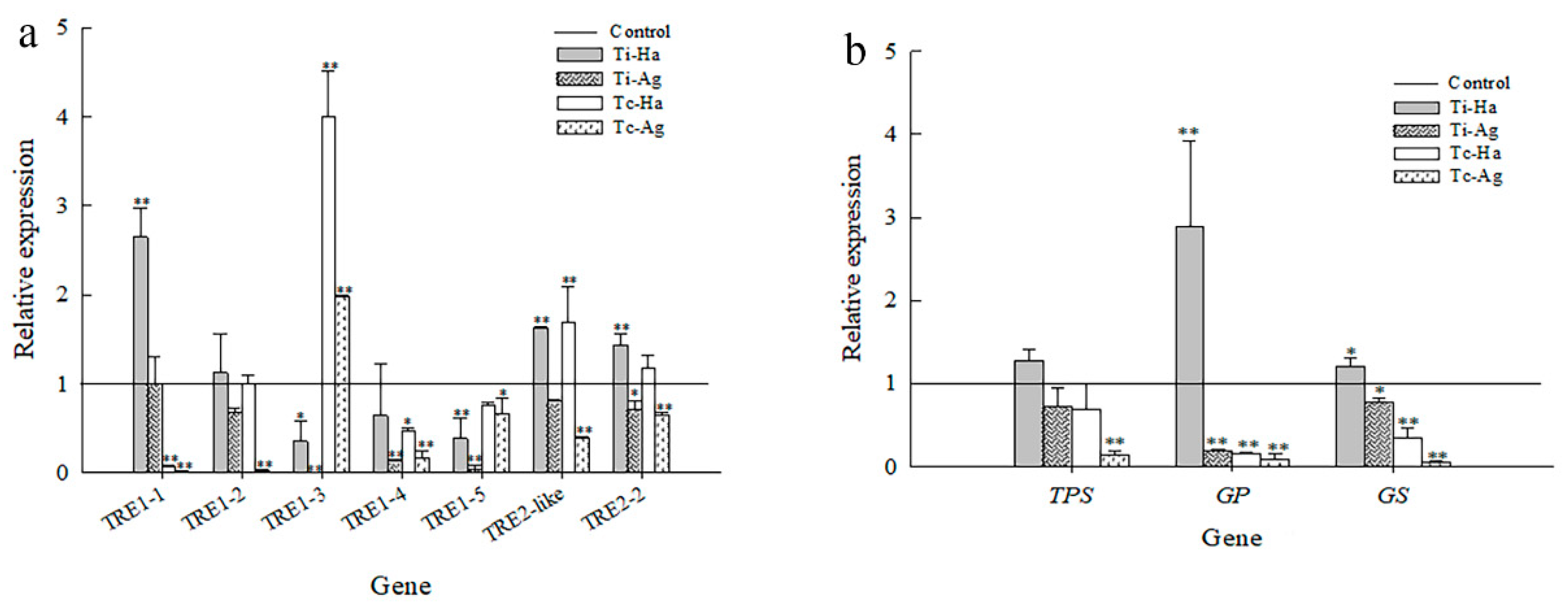
3.6. Relative Expression Levels of Key Genes for Trehalase Metabolism in Ladybirds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di, N.; Zhang, K.; Xu, Q.; Zhang, F.; Harwood, J.D.; Wang, S.; Desneux, N. Predatory Ability of Harmonia axyridis (Coleoptera: Coccinellidae) and Orius sauteri (Hemiptera: Anthocoridae) for Suppression of Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2021, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ren, Y.; Luning, P.A. Factors influencing Chinese farmers’ proper pesticide application in agricultural products—A review. Food Control 2021, 122, 107788. [Google Scholar] [CrossRef]
- Mangan, J.; Mangan, M.S. A comparison of two IPM training strategies in China: The importance of concepts of the rice ecosystem for sustainable insect pest management. Agric. Hum. Values 1998, 15, 209–221. [Google Scholar] [CrossRef]
- Ingegno, B.L.; Tavella, L. Ozone gas treatment against three main pests of stored products by combination of different application parameters. J. Stored Prod. Res. 2022, 95, 101902. [Google Scholar] [CrossRef]
- Vinha, G.L.; Plata-Rueda, A.; Soares, M.A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Deltamethrin-Mediated Effects on Locomotion, Respiration, Feeding, and Histological Changes in the Midgut of Spodoptera frugiperda Caterpillars. Insects 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Guedes, R.N.C. Occurrence and Significance of Insecticide-Induced Hormesis in Insects. In Pesticide Dose: Effects on the Environment and Target and Non-Target Organisms; American Chemical Society: Washington, DC, USA, 2017; Volume 1249, pp. 101–119. [Google Scholar]
- Shi, Y.; Pandit, A.; Nachman, R.J.; Christiaens, O.; Davies, S.A.; Dow, J.A.T.; Smagghe, G. Transcriptome analysis of neuropeptides in the beneficial insect lacewing (Chrysoperla carnea) identifies kinins as a selective pesticide target: A biostable kinin analogue with activity against the peach potato aphid Myzus persicae. J. Pest. Sci. 2022. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Indirect Effect of Pesticides on Insects and Other Arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Diptaningsari, D.; Trisyono, Y.A.; Purwantoro, A.; Wijonarko, A. Inheritance and Realized Heritability of Resistance to Imidacloprid in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae), From Indonesia. J. Econ. Entomol. 2019, 112, 1831–1837. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest. Manag. Sci. 2021, 77, 64–76. [Google Scholar] [CrossRef]
- Begum, A.; Alam, S.N.; Jalal Uddin, M. Management of Pesticides: Purposes, Uses, and Concerns. In Pesticide Residue in Foods: Sources, Management, and Control; Khan, M.S., Rahman, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 53–86. [Google Scholar] [CrossRef]
- Amarasekare, K.G.; Shearer, P.W.; Mills, N.J. Testing the selectivity of pesticide effects on natural enemies in laboratory bioassays. Biol. Control. 2016, 102, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, Z.; Li, Y.; Li, W.; Zhou, Q. Characterization and functional analysis of a carboxylesterase gene associated with chlorpyrifos resistance in Nilaparvata lugens (Stål). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 203, 12–20. [Google Scholar] [CrossRef]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate Trehalose-6-Phosphate Synthase Gene: Genetic Architecture, Biochemistry, Physiological Function, and Potential Applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, X.; Wang, S.-S.; Pan, B.-Y.; Wang, S.-G.; Wang, S.; Tang, B. Evaluation of the Expression and Function of the TRE2-like and TRE2 Genes in Ecdysis of Harmonia axyridis. Front. Physiol. 2019, 10, 1371. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhang, X.-Y.; Mu, X.-R.; Li, X.; Zhou, M.; Song, Y.-H.; Xu, K.-K.; Li, C. Insulin-Like ILP2 Regulates Trehalose Metabolism to Tolerate Hypoxia/Hypercapnia in Tribolium castaneum. Front. Physiol. 2022, 13, 857239. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, Q.; Wang, S.; Li, G.; Wu, Y.; Xu, C.; Tang, B.; Li, C. Regulatory function of the trehalose-6-phosphate synthase gene TPS3 on chitin metabolism in brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2022, 31, 241–250. [Google Scholar] [CrossRef]
- Wang, S.-S.; Li, G.-Y.; Liu, Y.-K.; Luo, Y.-J.; Xu, C.-D.; Li, C.; Tang, B. Regulation of carbohydrate metabolism by trehalose-6-phosphate synthase 3 in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef]
- Wyatt, G. The biochemistry of sugars and polysaccharides in insects. In Advances in Insect Physiology; Elsevier: London, UK, 1967; Volume 4, pp. 287–360. [Google Scholar]
- Tang, B.; Wei, P.; Chen, J.; Wang, S.; Zhang, W. Progress in gene features and functions of insect trehalases. Acta Entomol. Sin. 2012, 55, 1315–1321. [Google Scholar]
- Wegener, G.; Macho, C.; Schlöder, P.; Kamp, G.; Ando, O. Long-term effects of the trehalase inhibitor trehazolin on trehalase activity in locust flight muscle. J. Exp. Biol. 2010, 213, 3852–3857. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.; Shen, Q.; Yang, M.; Xie, G.; Wang, S. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.Q.; Zhao, K.F.; Huang, L.J.; Wu, J.C. The effects of triazophos on the trehalose content, trehalase activity and their gene expression in the brown planthopper Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2011, 100, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pang, R.; Xing, K.; Yuan, L.; Liang, Z.; Zhang, W. Peroxiredoxin alleviates the fitness costs of imidacloprid resistance in an insect pest of rice. PLoS Biol. 2021, 19, e3001190. [Google Scholar] [CrossRef] [PubMed]
- Mokkapati, J.S.; Bednarska, A.J.; Laskowski, R. The development of the solitary bee Osmia bicornis is affected by some insecticide agrochemicals at environmentally relevant concentrations. Sci. Total Environ. 2021, 775, 145588. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.C.; Chang, H.C.; Wu, W.Y.; Chen, Y.W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Xu, Q.; Zou, Q.; Fang, Q.; Wang, S.; Ye, G. Sequencing and characterization of glycogen synthase and glycogen phosphorylase genes from Spodoptera exigua and analysis of their function in starvation and excessive sugar intake. Arch. Insect Biochem. Physiol. 2012, 80, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.N. Trehalose—the insect ‘blood’sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar]
- Tatun, N.; Singtripop, T.; Sakurai, S. Dual control of midgut trehalase activity by 20-hydroxyecdysone and an inhibitory factor in the bamboo borer Omphisa fuscidentalis Hampson. J. Insect Physiol. 2008, 54, 351–357. [Google Scholar] [CrossRef] [PubMed]



| Primer | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| RTHATREH1-1 | CTTCGCCAGTCAAACGTCA | CCGTTTGGGACATTCCAGATA |
| RTHATREH1-2 | TGACAACTTCCAACCTGGTAATG | TTCCTTCGAGACATCTGGCTTA |
| RTHATREH1-3 | ACAGTCCCTCAGAATCTATCGTCA | GGAGCCAAGTCTCAAGCTCATC |
| RTHATREH1-4 | TTACTGCCAGTTTGATGACCATT | CATTTCGCTAATCAGAAGACCCT |
| RTHATREH1-5 | TGATGATGAGGTACGACGAGAA | GTAGCAAGGACCTAACAAACTGC |
| RTHATREH2-like | TTCCAGGTGGGAGATTCAGG | GGGATCAATGTAGGAGGCTGTG |
| RTHATREH2-2 | CAATCAGGGTGCTGTAATGTCG | CGTAGTTGGCTCATTCGTTTCC |
| RTHATPS | GACCCTGACGAAGCCATACC | AAAGTTCCATTACACGCACCA |
| RTHAGP | GCTGAAGCCCTCTACCAACT | CGCCGTACTCGTATCTTATGC |
| RTHAGS | CCCTTAGGATCGGATGTTCTC | CACCAGCCATCTCCCAGTT |
| QHA-arp49- | GCGATCGCTATGGAAAACTC | TACGATTTTGCATCAACAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ni, X.; Liu, M.; Tang, B.; Li, C.; Liu, Y. Changes in the Physiological Adaptation and Regulation Ability in Harmonia axyridis under Chlorpyrifos and Imidacloprid Stress. Agriculture 2022, 12, 1134. https://doi.org/10.3390/agriculture12081134
Li G, Ni X, Liu M, Tang B, Li C, Liu Y. Changes in the Physiological Adaptation and Regulation Ability in Harmonia axyridis under Chlorpyrifos and Imidacloprid Stress. Agriculture. 2022; 12(8):1134. https://doi.org/10.3390/agriculture12081134
Chicago/Turabian StyleLi, Guoyong, Xiaoli Ni, Meikun Liu, Bing Tang, Can Li, and Yangyang Liu. 2022. "Changes in the Physiological Adaptation and Regulation Ability in Harmonia axyridis under Chlorpyrifos and Imidacloprid Stress" Agriculture 12, no. 8: 1134. https://doi.org/10.3390/agriculture12081134





