Rhizobium and Phosphate Solubilizing Bacteria Influence the Soil Nutrient Availability, Growth, Yield, and Quality of Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Initial and Post-Harvest Soil Analysis
2.3. Application of Bio-Fertilizer and Chemical Fertilizer
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Growth Attributes
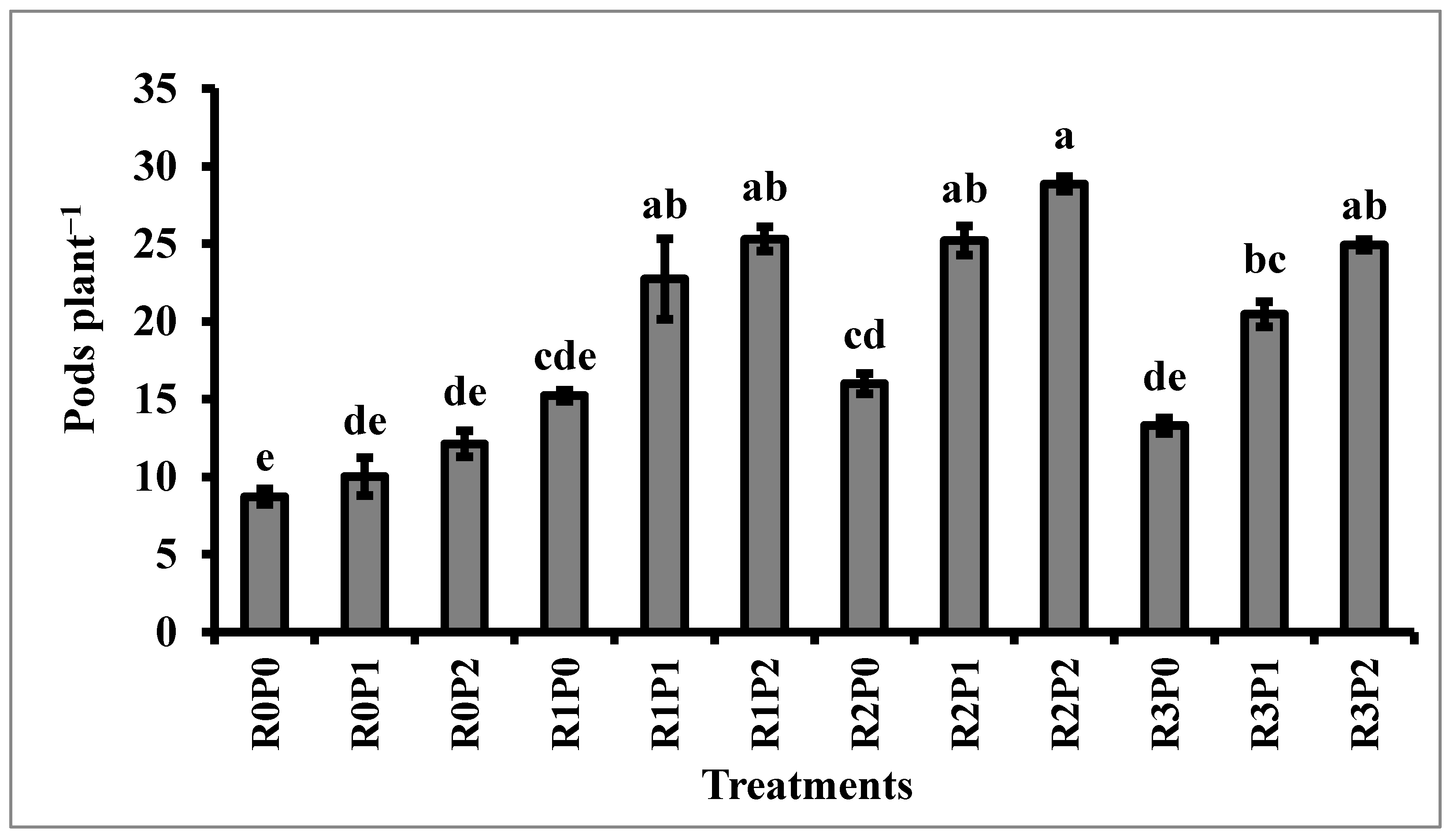
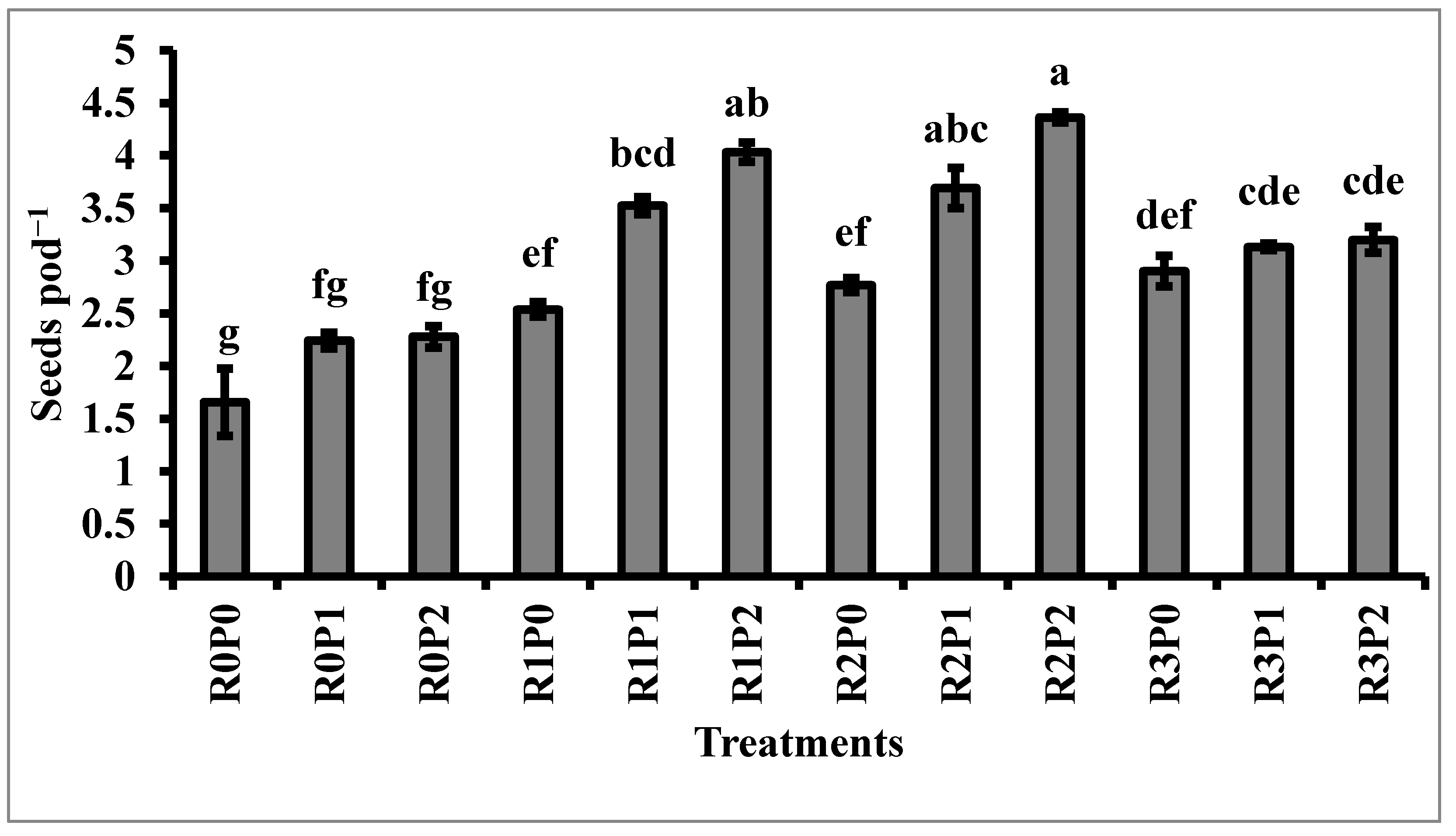
3.2. Yield and Yield Contributing Characters
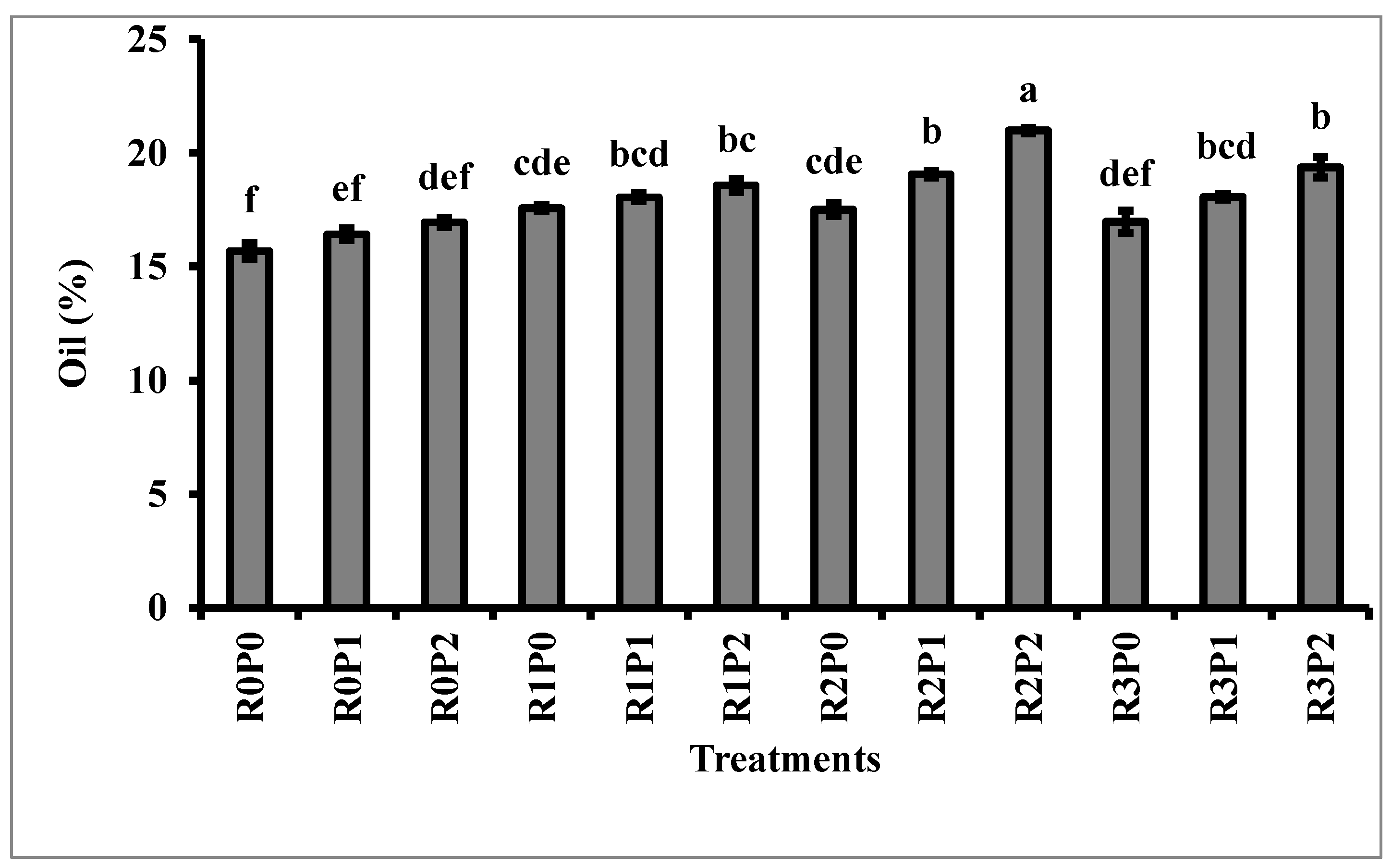
3.3. Protein and Oil Content
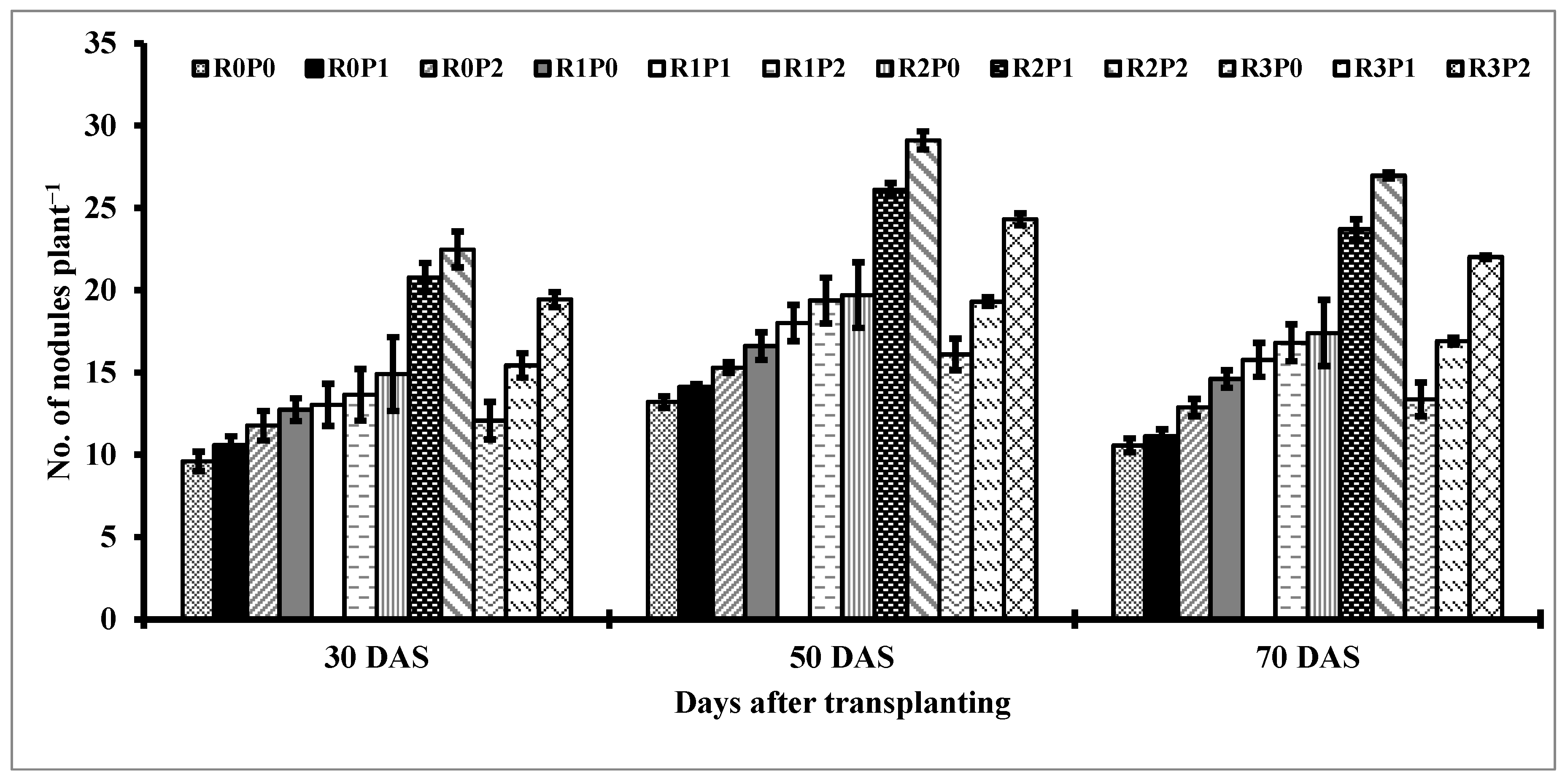
3.4. Nodulation
3.5. Nitrogen and Phosphorus Content in Post-Harvest Soil and Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tairo, E.V.; Ndakidemi, P.A. Bradyrhizobium japonicum Inoculation and Phosphorus Supplementation on Growth and Chlorophyll Accumulation in Soybean (Glycine max L.). Am. J. Plant Sci. 2013, 4, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Nget, R.; Aguilar, E.A.; Cruz, P.C.S.; Reaño, C.E.; Sanchez, P.B.; Reyes, M.R.; Prasad, P.V.V. Responses of Soybean Genotypes to Different Nitrogen and Phosphorus Sources: Impacts on Yield Components, Seed Yield, and Seed Protein. Plants 2022, 11, 298. [Google Scholar] [CrossRef]
- Dabesa, A.; Tana, T. Response of Soybean (Glycine max L. (Merrill)) to Bradyrhizobium Inoculation, Lime, and Phosphorus Applications at Bako, Western Ethiopia. Int. J. Agron. 2021, 2021, 6686957. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.; Salahuddin. Influence of nitrogen and phosphorus on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An eco-friendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cook, J.; Nearing, J.T.; Zhang, J.; Raudonis, R.; Glick, B.R.; Langille, M.G.I.; Cheng, Z. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol. Res. 2021, 245, 126690. [Google Scholar] [CrossRef] [PubMed]
- Murgese, P.; Santamaria, P.; Leoni, B.; Crecchio, C. Ameliorative effects of PGPB on yield, physiological parameters, and nutrient transporter genes expression in Barattiere (Cucumis melo L.). J. Soil Sci. Plant Nutr. 2020, 20, 784–793. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Uni. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Tagore, G.S.; Namdeo, S.L.; Sharma, S.K.; Kumar, N. Effect of Rhizobium and phosphate solubilizing bacterial inoculants on symbiotic traits, nodule leghaemoglobin, and yield of chickpea genotypes. Int. J. Agron. 2013, 2013, 581627. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legume are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kumar, N.; Kumar, S.; Lal, M. Effect of co-inoculation of B. Japonicum, PSB and AM fungi on microbial biomass carbon, nutrient uptake and yield of soybean (Glycine max L. merril). Agriways 2015, 3, 14–18. [Google Scholar]
- Khan, M.S.; Koizumi, N.; Olds, J.L. Biofixation of atmospheric nitrogen in the context of world staple crop production: Policy perspectives. Sci. Total Environ. 2020, 701, 134945. [Google Scholar] [CrossRef] [PubMed]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- Zohaib, A.; Yousaf, S.; Anjum, S.A.; Tabassum, T.; Abbas, T.; Muzaffar, W.; Ikram, W. Growth, allometry and dry matter yield of soybean genotypes in response to seed inoculation with plant growth promoting rhizobacteria. Pak. J. Agric. Res. 2019, 32, 102–109. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Ray, J.D.; Heatherly, L.G.; Fritschi, F.B. Influence of large amounts of nitrogen on non-irrigated and irrigated soybean. Crop Sci. 2006, 46, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Hungria, M.; Mendes, I.C. Nitrogen fixation with soybean: Perfect symbiosis? In Biological Nitrogen Fixation; De Bruijin, F.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 2, pp. 1009–1023. [Google Scholar]
- Hatfield, J.L.; Egli, D.B.; Leggett, J.E.; Peaslee, D.E. Effect of applied nitrogen on the nodulation and early growth of soybean. Agron. J. 1974, 66, 112–114. [Google Scholar] [CrossRef]
- Hardy, R.W.F.; Burns, R.C.; Herbert, R.R.; Jackson, E.K. Biological nitrogen fixation: A key to world protein. Plant Soil 1971, 35, 561–590. [Google Scholar] [CrossRef]
- Aziz, A.; Ahiabor, B.; Opoku, A.; Abaidoo, R. Contributions of rhizobium inoculants and phosphorus fertilizer to biological nitrogen fixation, growth and grain yield of three soybean varieties on a fluvic luvisol. Am. J. Exp. Agric. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Si, Z.; Yang, Q.; Liang, R.; Chen, L.; Chen, D.; Li, Y. Digalactosyldiacylglycerol synthase gene MTDGD1 plays an essential role in nodule development and nitrogen fixation. Mol. Plant Microbe. Interact. 2019, 32, 1196–1209. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of soil phosphorus on legume production. In Legumes for Soil Health and Sustainable Management; Meena, R., Das, A., Yadav, G., Lal, R., Eds.; Springer: Singapore, 2018; pp. 487–510. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, regulation, and agricultural application of plant phosphate transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. Int. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Fatima, T.; Anshu, S.D.; Tripathi, P.; Srivastava, S.; Singh, P.C. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J. King Saud Uni. Sci. 2020, 32, 791–798. [Google Scholar] [CrossRef]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; da Silva, E.F. Plant growth-promoting rhizobacteria-assisted phytoremediation of mine soils. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., de Campos, P.J.F., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 281–295. [Google Scholar] [CrossRef]
- Ahmad, M.; Adil, Z.; Hussain, A.; Mumtaz, M.Z.; Nafees, M.; Ahmad, I.; Jamil, M. Potential of phosphate solubilizing bacillus strains for improving growth and nutrient uptake in mungbean and maize crops. Pak. J. Agric. Sci. 2019, 56, 283–289. [Google Scholar] [CrossRef]
- Gaur, R.; Noam, S.; Johri, B.; Rossi, P.; Aragno, M. Diacetylphloroglucinol-producing pseudomonads do not influence AM fungi in wheat rhizosphere. Curr. Sci. 2004, 88, 453–457. [Google Scholar]
- Alemneh, A.A.; Cawthray, G.R.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Ability to produce indole acetic acid is associated with improved phosphate solubilizing activity of rhizobacteria. Arch. Microbiol. 2021, 203, 3825–3837. [Google Scholar] [CrossRef]
- Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.S.; Mubeen, F.; Aziz, O.; Qian, Z.; Ijaz, R.; Tu, S. Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Commun. Soil Sci. Plant Anal. 2018, 49, 2715–2725. [Google Scholar] [CrossRef]
- Chaudhary, S.R.; Sindhu, S.S. Growth stimulation of clusterbean (Cyamopsis tetragonoloba) by coinoculation with rhizosphere bacteria and Rhizobium. Legume Res. 2016, 39, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Hossain, T. Oilseeds and Products Annual; United States Department of Agriculture: Washington, DC, USA, 2021; pp. 2–7. [Google Scholar]
- Salam, M.A.; Kamruzzaman, M. Comparative and competitive advantage of soybean cultivation in Noakhali and Laxmipur districts of Bangladesh. J. Bangladesh Agril. Univ. 2015, 13, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.S.; Ali, M.M.; Shahrin, S.; Cheesman, S.; Alam, S.N.; Krupnik, T.J. Simple and Effective Management Methods that Can Improve Soybean Production in Bangladesh; CIMMYT: El Batan, Mexico, 2022; pp. 1–19. [Google Scholar]
- FRG. Fertilizer Recommendation Guide; Bangladesh Agricultural Research Council (BARC): Dhaka, Bangladesh, 2018. [Google Scholar]
- Egashira, K.; Hagimine, M.; Moslehuddin, A.Z.M. Fixed ammonium in some Bangladesh soils. Soil Sci. Plant Nutr. 1998, 44, 269–272. [Google Scholar] [CrossRef]
- Mehmood, K.; Baquy, M.A.A.; Xu, R.K. Influence of nitrogen fertilizer forms and crop straw biochars on soil exchange properties and maize growth on an acidic Ultisol. Arch. Agron. Soil Sci. 2018, 64, 834–849. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 29–38. [Google Scholar] [CrossRef]
- Douglas, W.P. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA: Washington, DC, USA, 1954. [Google Scholar]
- Brown, J.G.; Lilleland, O. Rapid determination of potassium and sodium in plant materials and soil extracts by flame photometry. Proc. Amer. Soc. Hort. Sci. 1946, 48, 341–345. [Google Scholar]
- Victor, J.K.; Nearpass, D.C. The Determination of Available Sulfur in Soils. Soil Sci. Soc. Am. J. 1960, 24, 337–340. [Google Scholar] [CrossRef]
- Watson, D.J. Comparative physiological studies on the growth of field crops: I Variation in net assimilation rate and leaf area between species and varieties and between years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Case, V.W. Sampling, Handling, and Analyzing Plant Tissue Samples; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 389–427. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Yao, Y.; You, Q.; Duan, G.; Ren, G.; Chu, S.; Zhao, J.; Li, X.; Zhou, X.; Jiao, Y. Quantitative trait loci analysis of seed oil content and composition of wild and cultivated soybean. BMC Plant Biol. 2020, 20, 51. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Assainar, S.K.; Abbott, L.K.; Mickan, B.S.; Storer, P.J.; Whiteley, A.S.; Siddique, K.H.M.; Solaiman, Z.M. Polymer-coated rock mineral fertilizer has potential to substitute soluble fertilizer for increasing growth, nutrient uptake, and yield of wheat. Biol. Fertil. Soils 2020, 56, 381–394. [Google Scholar] [CrossRef]
- Patra, P.; Pati, B.K.; Ghosh, G.K.; Mura, S.S.; Saha, A. Effect of biofertilizers and sulphur on growth, yield and oil content of hybrid sunflower (Helianthus annuus L.) in a typical lateritic soil. Open Access Sci. Rep. 2013, 2, 603. [Google Scholar] [CrossRef]
- Lamptey, S.; Ahiabor, B.D.K.; Yeboah, S.; Osei, D. Effect of Rhizobium inoculants and reproductive growth stages on shoot biomass and yield f soybean (Glycine max (L.) Merril). J. Agric. Sci. 2014, 6, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Tewari, K.; Ishikawa, S.; Tanaka, K.; Kamiyama, S.; Ono, Y.; Hatano, S.; Ohtake, N.; Sueyoshi, K.; Hasegawa, H.; et al. Role of nitrogen on growth and seed yield of soybean and a new fertilization technique to promote nitrogen fixation and seed yield. In Soybean—The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Mathenge, C.; Thuita, M.; Masso, C.; Gweyi-Onyango, J.; Vanlauwe, B. Variability of soybean response to rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 2019, 194, 104290. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Iqbal, A.; Akhtar, N.; Shakir, M.A.; Khan, A. Coinoculation of phosphate solubilizing bacteria and rhizobia in the presence of L-tryptophan for the promotion of mash bean (Vigna mungo L.). Soil Environ. 2012, 31, 47–54. [Google Scholar]
- Figueiredo, M.V.B.; Martinez, C.R.; Burity, H.A.; Chanway, C.P. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J. Microbiol. Biotechnol. 2008, 24, 1187–1193. [Google Scholar] [CrossRef]
- Yasmeen, S.; Bano, A. Combined effect of phosphate-solubilizing microorganisms, Rhizobium and Enterobacter on root nodulation and physiology of soybean (Glycine max L.). Commun. Soil Sci. Plant Anal. 2014, 45, 2373–2384. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Poll. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sharma, R.; Sindhu, S.; Sehawat, A. Soil fertility improvement by symbiotic rhizobia for sustainable agriculture. In Soil Fertility Management for Sustainable Development; Panpatte, D.G., Jhala, V.K., Eds.; Springer Nature: Singapore; Pte Ltd.: Singapore, 2019; pp. 101–166. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021, 13, 1140. [Google Scholar] [CrossRef]
- Yousefi, A.A.; Khavazi, K.; Moezi, A.A.; Rejali, F.; Nadian, H.A. Phosphate solubilizing bacteria and arbuscular mycorrhizal fungi impacts on inorganic phosphorus fractions and wheat growth. World Appl. Sci. J. 2011, 15, 1310–1318. [Google Scholar]
- Ahmad, E.; Zaidi, A.; Khan, M.S. Response of PSM inoculation to certain legumes and cereal crops. In Phosphate Solubilizing Microorganisms; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Cham, Germany, 2014; pp. 175–205. [Google Scholar] [CrossRef]
- Waghmare, Y.M.; Gokhale, D.N.; Pawar, H.D. Effect of integrated nutrient management on yield, yield attributes and quality of soybean (Glycine max (L.) Merill.). J. Agric. Res. Technol. 2012, 37, 370–372. [Google Scholar]
- Jaga, P.K.; Sharma, S. Effect of bio-fertilizer and fertilizers on productivity of soybean. Ann. Plant Soil Res. 2015, 17, 171–174. [Google Scholar]
- Fatima, Z.; Zia, M.; Chaudhary, F. Interactive effect of Rhizobium strains And P on soybean yield, nitrogen fixation and soil fertility. Pak. J. Bot. 2007, 39, 255–264. [Google Scholar]
- Adesemoye, A.Q.; Torbert, H.A.; Kloepper, J.W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 2008, 54, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Watts, D.B.; Kloepper, J.W.; Torbert, H.A. Effect of microbial based inoculants on nutrient concentrations and early root morphology of corn (Zea mays). J. Plant Nutr. Soil Sci. 2017, 180, 56–70. [Google Scholar] [CrossRef]
- De Sousa, S.M.; de Oliveira, C.A.; Andrade, D.L.; de Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; de Paula, U.G.L.; Gomes, E.A. Tropical Bacillus strains inoculation enhances maize root surface area, dry weight, nutrient uptake and grain yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Filipini, L.D.; Pilatti, F.K.; Meyer, E.; Ventura, B.S.; Lourenzi, C.R.; Lovato, P.E. Application of Azospirillum on seeds and leaves, associated with Rhizobium inoculation, increases growth and yield of common bean. Arch. Microbiol. 2021, 203, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J.B.N. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, S.; Hua, Q.; Qiu, C.; Wu, P.; Liu, X.; Lin, X. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol. 2021, 12, 693535. [Google Scholar] [CrossRef] [PubMed]
- Jain, L.K.; Singh, P.; Singh, P. Growth and nutrient uptake of chickpea (Cicer arietinum L.) as influenced by biofertilizers and phosphorus nutrition. Crop Res. 2003, 25, 410–413. [Google Scholar]
- Sindhu, S.S.; Dadarwal, K.R.; Davis, T.M. Non-nodulating chickpea breeding line for the study of symbiotic nitrogen fixation potential. Indian J. Microbiol. 1992, 32, 175–180. [Google Scholar]
- Tindwa, H.J.; Kachiguma, A.; Mrema, J.P. Incubation of soil with agricultural lime and phosphorus enhances biological nitrogen fixation and yield of soybean (Glycine max (L.) merrill) in an ultisol. J. Cent. Eur. Agric. 2019, 20, 938–952. [Google Scholar] [CrossRef] [Green Version]
- Assainar, S.K.; Abbott, L.K.; Mickan, B.S.; Whiteley, A.S.; Siddique, K.H.M.; Solaiman, Z.M. Response of Wheat to a Multiple Species Microbial Inoculant Compared to Fertilizer Application. Front. Plant Sci. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Míguez-Montero, M.A.; Valentine, A.; Pérez-Fernández, M.A. Regulatory effect of phosphorus and nitrogen on nodulation and plant performance of leguminous shrubs. AoB Plants 2019, 12, plz047. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than Rhizosphere P solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E.; Scherer, H.W. Chapter 16—Nitrogen fixation. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 389–408. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Elkoca, E.; Kantar, F.; Sahin, F. Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J. Plant Nutr. 2008, 31, 157–171. [Google Scholar] [CrossRef]



| Soil Properties | Value |
|---|---|
| pH | 7.2 |
| Soil organic carbon (%) | 0.48 |
| Soil organic matter (%) | 0.96 |
| Nitrogen (%) | 0.057 |
| Available phosphorous (mg kg−1) | 2.83 |
| Available potassium (mg kg−1) | 140 |
| Available sulphur (mg kg−1) | 11 |
| Treatment | Plant Height (cm) | Branch Plant−1 |
|---|---|---|
| Rhizobium japonicum | ||
| R0 | 48.10 c | 4.41 c |
| R1 | 53.70 ab | 6.86 ab |
| R2 | 56.05 a | 8.18 a |
| R3 | 50.20 bc | 6.36 b |
| HSD(0.05) Level of significance | 3.83 ** | 1.48 ** |
| Pseudomonas striata | ||
| P0 | 47.45 c | 4.93 b |
| P1 | 52.17 b | 6.92 a |
| P2 | 56.43 a | 7.50 a |
| HSD(0.05) | 3.00 | 1.16 |
| Level of significance | ** | ** |
| CV (%) | 5.66 | 17.67 |
| Treatment | Leaf Area Index (LAI) | Total Chlorophyll Content (mg g−1 FW) | |
|---|---|---|---|
| 30 DAS | 60 DAS | ||
| R0P0 | 0.37 c | 2.00 d | 4.42 e |
| R0P1 | 0.38 c | 1.99 d | 5.16 de |
| R0P2 | 0.39 bc | 2.17 cd | 5.47 de |
| R1P0 | 0.38 c | 2.34 bc | 5.89 cd |
| R1P1 | 0.42 abc | 2.60 ab | 6.91 bc |
| R1P2 | 0.55 a | 2.69 a | 8.06 ab |
| R2P0 | 0.38 c | 2.23 cd | 5.94 cd |
| R2P1 | 0.52 ab | 2.73 a | 7.59 ab |
| R2P2 | 0.55 a | 2.73 a | 8.39 a |
| R3P0 | 0.40 bc | 2.20 cd | 6.24 cd |
| R3P1 | 0.45 abc | 2.59 ab | 6.83 bc |
| R3P2 | 0.49 abc | 2.60 ab | 7.11 abc |
| HSD(0.05) | 0.14 | 0.31 | 1.32 |
| Level of significance | * | * | * |
| CV (%) | 6.88 | 10.07 | 4.44 |
| Treatment | Pod Length (cm) | 100-Seed Weight (g) |
|---|---|---|
| Rhizobium japonicum | ||
| R0 | 3.66 c | 9.71 c |
| R1 | 4.55 b | 11.13 b |
| R2 | 5.11 a | 11.89 a |
| R3 | 4.36 b | 10.84 b |
| HSD(0.05) | ** | ** |
| Level of significance | 0.28 | 0.58 |
| Pseudomonas striata | ||
| P0 | 4.03 b | 10.07 c |
| P1 | 4.51 a | 11.07 b |
| P2 | 4.72 a | 11.54 a |
| HSD(0.05) | 0.22 | 0.46 |
| Level of significance | ** | ** |
| CV (%) | 4.83 | 4.10 |
| Treatment | Nodule Dry Weight (mg Plant−1) | Nitrogen Percentage of Nodule (60 DAS) | ||
|---|---|---|---|---|
| 30 DAS | 50 DAS | 70 DAS | ||
| Rhizobium japonicum | ||||
| R0 | 29.82 c | 36.05 d | 31.97 d | 3.54 c |
| R1 | 36.01 b | 43.03 c | 39.59 c | 4.46 b |
| R2 | 41.79 a | 66.57 a | 61.48 a | 4.93 a |
| R3 | 37.08 b | 48.56 b | 44.62 b | 4.72 a |
| HSD(0.05) | 2.96 | 3.47 | 3.13 | 0.21 |
| Level of significance | ** | ** | ** | ** |
| Pseudomonas striata | ||||
| P0 | 34.44 b | 46.83 b | 42.93 b | 4.24 b |
| P1 | 36.39 ab | 48.45 ab | 44.28 ab | 4.49 a |
| P2 | 37.69 a | 50.40 a | 46.05 a | 4.51 a |
| HSD(0.05) | 2.32 | 2.72 | 2.45 | 0.16 |
| Level of significance | ** | * | * | ** |
| CV (%) | 6.29 | 5.50 | 5.41 | 3.62 |
| Treatment | Shoot N (%) | Shoot P (%) | Seed P (%) |
|---|---|---|---|
| Rhizobium japonicum | |||
| R0 | 1.48 c | 0.37 | 0.48 c |
| R1 | 2.81 b | 0.39 | 0.60 b |
| R2 | 3.08 a | 0.39 | 0.66 a |
| R3 | 2.93 ab | 0.39 | 0.65 a |
| HSD(0.05) | 0.19 | - | 0.05 |
| Level of significance | ** | NS | ** |
| Pseudomonas striata | |||
| P0 | 2.53 | 0.28 c | 0.38 c |
| P1 | 2.55 | 0.41 b | 0.67 b |
| P2 | 2.65 | 0.47 a | 0.74 a |
| HSD(0.05) | - | 0.03 | 0.04 |
| Level of significance | NS | ** | ** |
| CV(%) | 5.71 | 7.00 | 5.81 |
| Treatment | Soil N (%) | Soil Available Phosphorus (mg kg−1) |
|---|---|---|
| Rhizobium japonicum | ||
| R0 | 0.11 d | 3.81 |
| R1 | 0.16 c | 3.90 |
| R2 | 0.27 a | 4.17 |
| R3 | 0.22 b | 3.88 |
| HSD(0.05) | 0.02 | - |
| Level of significance | ** | NS |
| Pseudomonas striata | ||
| P0 | 0.18 | 2.88 b |
| P1 | 0.19 | 2.99 b |
| P2 | 0.20 | 5.94 a |
| HSD(0.05) | - | 0.31 |
| Level of significance | NS | ** |
| CV (%) | 8.06 | 7.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shome, S.; Barman, A.; Solaiman, Z.M. Rhizobium and Phosphate Solubilizing Bacteria Influence the Soil Nutrient Availability, Growth, Yield, and Quality of Soybean. Agriculture 2022, 12, 1136. https://doi.org/10.3390/agriculture12081136
Shome S, Barman A, Solaiman ZM. Rhizobium and Phosphate Solubilizing Bacteria Influence the Soil Nutrient Availability, Growth, Yield, and Quality of Soybean. Agriculture. 2022; 12(8):1136. https://doi.org/10.3390/agriculture12081136
Chicago/Turabian StyleShome, Swarna, Alak Barman, and Zakaria M. Solaiman. 2022. "Rhizobium and Phosphate Solubilizing Bacteria Influence the Soil Nutrient Availability, Growth, Yield, and Quality of Soybean" Agriculture 12, no. 8: 1136. https://doi.org/10.3390/agriculture12081136






