Modification of Cuticular Wax Composition and Biosynthesis by Epichloë gansuensis in Achnatherum inebrians at Different Growing Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Leaf Cuticular Wax Extraction
2.3. Chemical Analysis
2.4. Transcriptome Analysis
2.5. Statistical Analysis
3. Results
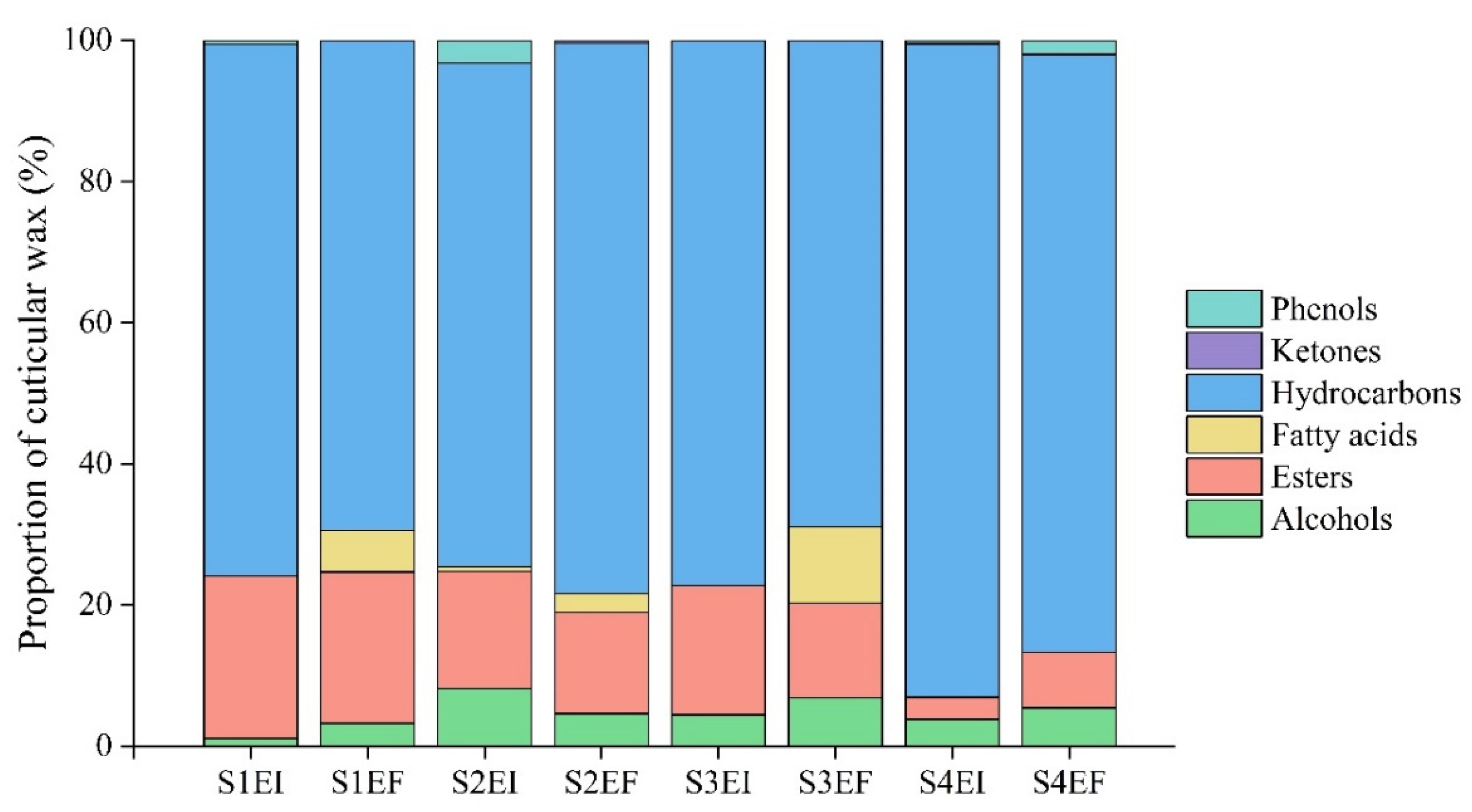
3.1. Composition of Leaf Cuticular Wax
3.2. Carbon Chain Length of Leaf Cuticular Wax
3.3. Differentially Expressed Gene (DEG) Analysis
3.4. KEGG Pathway Enrichment Analysis of the DEGs
3.5. GO Functional-Enrichment Analysis of the DEGs
3.6. Biosynthesis Pathway of Cuticular Wax
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kosma, D.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [Green Version]
- Krauss, P.; Markstadter, C.; Riederer, M. Attenuation of UV radiation by plant cuticles from woody species. Plant Cell Environ. 1997, 20, 1079–1085. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Agati, G.; Cerovic, Z.G. Optical properties of plant surfaces. In Annual Plant Reviews Volume 23: Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell: Oxford, UK, 2006; pp. 216–249. [Google Scholar]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The formation and functions of a fundamental plant tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Lemieux, B. Molecular genetics of epicuticular wax biosynthesis. Trends Plant Sci. 1996, 1, 312–318. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism; The American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 11. [Google Scholar]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Post-Beittenmiller, D. Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 405–430. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.; Barthlott, W.; Koch, S.; Hommes, A.; Wandelt, K.; Mamdouh, W.; De-Feyter, S.; Broekmann, P. Structural analysis of wheat wax (Triticum aestivum, c.v. ‘Naturastar’ L.): From the molecular level to three dimensional crystals. Planta 2006, 223, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Javelle, M.; Vernoud, V.; Depège-Fargeix, N.; Arnould, C.; Oursel, D.; Domergue, F.; Sarda, X.; Rogowsky, P.M. Overexpression of the epidermis-specific homeodomain-leucine zipper IV transcription factor OUTER CELL LAYER1 in maize identifies target genes involved in lipid metabolism and cuticle biosynthesis. Plant Physiol. 2010, 154, 273–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avato, P.; Mikkelsen, J.D.; Wettstein-knowles, P.V. Synthesis of epicuticular primary alcohols and intracellular fatty acids by tissue slices from cer-j59 barley leaves. Carlsberg Res. Commun. 1982, 47, 377–390. [Google Scholar] [CrossRef]
- Yao, L.H.; Ni, Y.; Guo, N.; He, Y.J.; Gao, J.H.; Guo, Y.J. Leaf cuticular waxes in Poa pratensis and their responses to altitudes. Acta Prataculturae Sin. 2018, 27, 97–105. [Google Scholar]
- Tulloch, A.P. Composition of leaf surface waxes of Triticum species: Variation with age and tissue. Phytochemistry 1973, 12, 2225–2232. [Google Scholar] [CrossRef]
- Avato, P.; Bianchi, G.; Pogna, N. Chemosystematics of surface lipids from maize and some related species. Phytochemistry 1990, 29, 1571–1576. [Google Scholar] [CrossRef]
- Gülz, P.-G.; Prasad, R.B.N.; Müller, E. Surface structure and chemical composition of epicuticular waxes during leaf development of Tilia Tomentosa moench. Z. Nat. C 1991, 46, 743–749. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.H.; Chai, G.Q.; Li, C.L.; Hu, Y.G.; Chen, X.H.; Wang, Z.H. Developmental changes in composition and morphology of cuticular waxes on leaves and spikes of glossy and glaucous wheat (Triticum aestivum L.). PLoS ONE 2015, 10, e0141239. [Google Scholar] [CrossRef]
- Busta, L.; Hegebarth, D.; Kroc, E.; Jetter, R. Changes in cuticular wax coverage and composition on developing Arabidopsis leaves are influenced by wax biosynthesis gene expression levels and trichome density. Planta 2017, 245, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Wang, Y.; Wu, H.Q.; Xu, J.; Li, T.T.; Hegebarth, D.; Jetter, R.; Chen, L.T.; Wang, Z.H. Three TaFAR genes function in the biosynthesis of primary alcohols and the response to abiotic stresses in Triticum aestivum. Sci. Rep. 2016, 6, 25008. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, M.L.; Sun, Y.L.; Hegebarth, D.; Li, T.T.; Jetter, R.; Wang, Z.H. Molecular characterization of TaFAR1 involved in primary alcohol biosynthesis of cuticular wax in hexaploid wheat. Plant Cell Physiol. 2015, 56, 1944–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, M.L.; Sun, Y.L.; Wang, Y.T.; Li, T.T.; Chai, G.Q.; Jiang, W.H.; Shan, L.W.; Li, C.L.; Xiao, E.S.; et al. FAR5, a fatty acyl-coenzyme A reductase, is involved in primary alcohol biosynthesis of the leaf blade cuticular wax in wheat (Triticum aestivum L.). J. Exp. Bot. 2015, 66, 1165–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuffer, M.G.; Coe, E.H.; Wessler, S.R. Mutants of maize. Q. Rev. Biol. 1998, 73, 207. [Google Scholar]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.; Xiong, L. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef]
- Qin, B.-X.; Tang, D.; Huang, J.; Li, M.; Wu, X.-R.; Lu, L.-L.; Wang, K.-J.; Yu, H.-X.; Chen, J.-M.; Gu, M.-H.; et al. Rice OsGL1-1 Is Involved in Leaf Cuticular Wax and Cuticle Membrane. Mol. Plant 2011, 4, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.Y.; Li, L.Z.; Xiang, J.H.; Gao, G.F.; Xu, F.X.; Liu, A.L.; Zhang, X.W.; Peng, Y.; Chen, X.B.; Wan, X.Y. OsGL1-3 is involved in cuticular wax biosynthesis and tolerance to water deficit in rice. PLoS ONE 2015, 10, e116676. [Google Scholar] [CrossRef]
- Siegel, M.R.; Latch, G.C.M.; Johnson, M.C. Fungal endophytes of grasses. Annu. Rev. Phytopathol. 1987, 25, 293–315. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Christensen, M.; Voisey, C. The biology of the endophyte/grass partnership. NZGA Res. Pract. Ser. 2007, 13, 123–133. [Google Scholar] [CrossRef]
- Bacon, C.W.; Porter, J.K.; Robbins, J.D.; Luttrell, E.S. Epichloë typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 1977, 34, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochem. 2007, 68, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seed borne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.B.; Li, C.J. Neotyphodium in Native Grasses in China and Observations on Endophyte/Host Interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000; pp. 41–50. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Young, C.A.; Sugawara, K.; Leuchtmann, A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Li, C.J.; Nan, Z.B.; Paul, V.H.; Dapprich, P.D.; Liu, Y. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 2004, 90, 141–147. [Google Scholar]
- Kou, M.Z.; Bastías, D.; Christensen, M.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, P.; Christensen, M.J.; Zhang, X.; Li, C.; Nan, Z. Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of Achnatherum inebrians under various NaCl concentrations. Plant Soil 2018, 435, 57–68. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, X.X.; Feng, Y.; Christensen, M.J.; Nan, Z.B. An Epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecol. 2016, 22, 26–34. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Nan, Z. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.; Nan, Z.B.; Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- Chen, N.; He, R.L.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Liu, Y.; Paul, V.H.; Peter, D. Methodology of Endophyte Detection of Drunken Horse Grass (Achnatherum Inebrians). In Proceedings of the Annual Meeting of Chinese Society for Plant Pathology, Guangzhou, China, 21–27 July 2008; pp. 129–132. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2001. Available online: https://webbook.nist.gov/chemistry/ (accessed on 24 May 2022).
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, J.H.; Wang, Y.; Wang, Z.h.; Quan, L. Analysis of cuticular wax components and crystal structures of barley. J. Triticeae Crops 2018, 38, 693–700. [Google Scholar]
- Zhang, Y.Y.; Li, T.T.; Sun, Y.L.; Wang, Y.T.; Wang, M.L.; Wang, Y.; Shi, X.; Quan, L.; Li, C.L.; Wang, Z.H. Analysis on composition and content of leaf cuticular waxes of wheat (Triticum Aestivum) detected by GC-MS. J. Triticeae Crops 2014, 34, 963–968. [Google Scholar]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef]
- Inuma, T.; Khodaparast, S.A.; Takamatsu, S. Multilocus phylogenetic analyses within Blumeria graminis, a powdery mildew fungus of cereals. Mol. Phylogenetics Evol. 2007, 44, 741–751. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef]
- Edwards, P.; Nelsen, S.J.; Metz, J.G.; Dehesh, K. Cloning of the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli. FEBS Lett. 1997, 402, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Magnuson, K.; Carey, M.R.; Cronan, J.E. The putative fabJ gene of Escherichia coli fatty acid synthesis is the fabF gene. J. Bacteriol. 1995, 177, 3593–3595. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.Q.; Rowland, O.; Kunst, L. Disruptions of the Arabidopsis Enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 2005, 17, 1467–1481. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.-D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, D.; Beisson, F.; Brigham, A.; Shin, J.; Greer, S.; Jetter, R.; Kunst, L.; Wu, X.M.; Yephremov, A.; Samuels, L. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J. 2007, 52, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Pighin, J.A.; Zheng, H.Q.; Balakshin, L.J.; Goodman, I.P.; Western, T.L.; Jetter, R.; Kunst, L.; Samuels, A.L. Plant cuticular lipid export requires an ABC transporter. Science 2004, 306, 702–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoport, T.A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 2007, 450, 663–669. [Google Scholar] [CrossRef] [PubMed]





| Alcohols | Esters | Fatty Acids | Hydrocarbons | Ketones | Phenols | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | df | F | P | F | P | F | P | F | P | F | P | F | P |
| E | 1 | 0.12 | 0.734 | 0.137 | 0.716 | 15.812 | 0.001 | 2.158 | 0.161 | 1 | 0.332 | 1.172 | 0.295 |
| S | 3 | 0.902 | 0.462 | 6.317 | 0.005 | 3.741 | 0.033 | 8.552 | 0.001 | 1 | 0.418 | 2.007 | 0.154 |
| E × S | 3 | 0.54 | 0.662 | 0.564 | 0.647 | 4.073 | 0.025 | 1.813 | 0.185 | 1 | 0.418 | 3.372 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Tian, M.; Zeng, P.; Christensen, M.J.; Kou, M.; Nan, Z.; Zhang, X. Modification of Cuticular Wax Composition and Biosynthesis by Epichloë gansuensis in Achnatherum inebrians at Different Growing Periods. Agriculture 2022, 12, 1154. https://doi.org/10.3390/agriculture12081154
Zhao Z, Tian M, Zeng P, Christensen MJ, Kou M, Nan Z, Zhang X. Modification of Cuticular Wax Composition and Biosynthesis by Epichloë gansuensis in Achnatherum inebrians at Different Growing Periods. Agriculture. 2022; 12(8):1154. https://doi.org/10.3390/agriculture12081154
Chicago/Turabian StyleZhao, Zhenrui, Mei Tian, Peng Zeng, Michael J. Christensen, Mingzhu Kou, Zhibiao Nan, and Xingxu Zhang. 2022. "Modification of Cuticular Wax Composition and Biosynthesis by Epichloë gansuensis in Achnatherum inebrians at Different Growing Periods" Agriculture 12, no. 8: 1154. https://doi.org/10.3390/agriculture12081154
APA StyleZhao, Z., Tian, M., Zeng, P., Christensen, M. J., Kou, M., Nan, Z., & Zhang, X. (2022). Modification of Cuticular Wax Composition and Biosynthesis by Epichloë gansuensis in Achnatherum inebrians at Different Growing Periods. Agriculture, 12(8), 1154. https://doi.org/10.3390/agriculture12081154









