Different Diets Change Milk Extracellular Vesicle-Protein Profile in Lactating Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Sample Collection
2.2. Isolation of Milk Whey and EVs
2.3. High Abundance Proteins Removal and BCA Assay
2.4. SDS-PAGE
2.5. FASP Digestion and iTRAQ Labeling
2.6. LC-MS/MS Analysis
2.7. Data Processing
2.8. Principal Component Analysis (PCA) and Bioinformatics Analysis
3. Results
3.1. Overview of the Milk Derived EV-Proteins
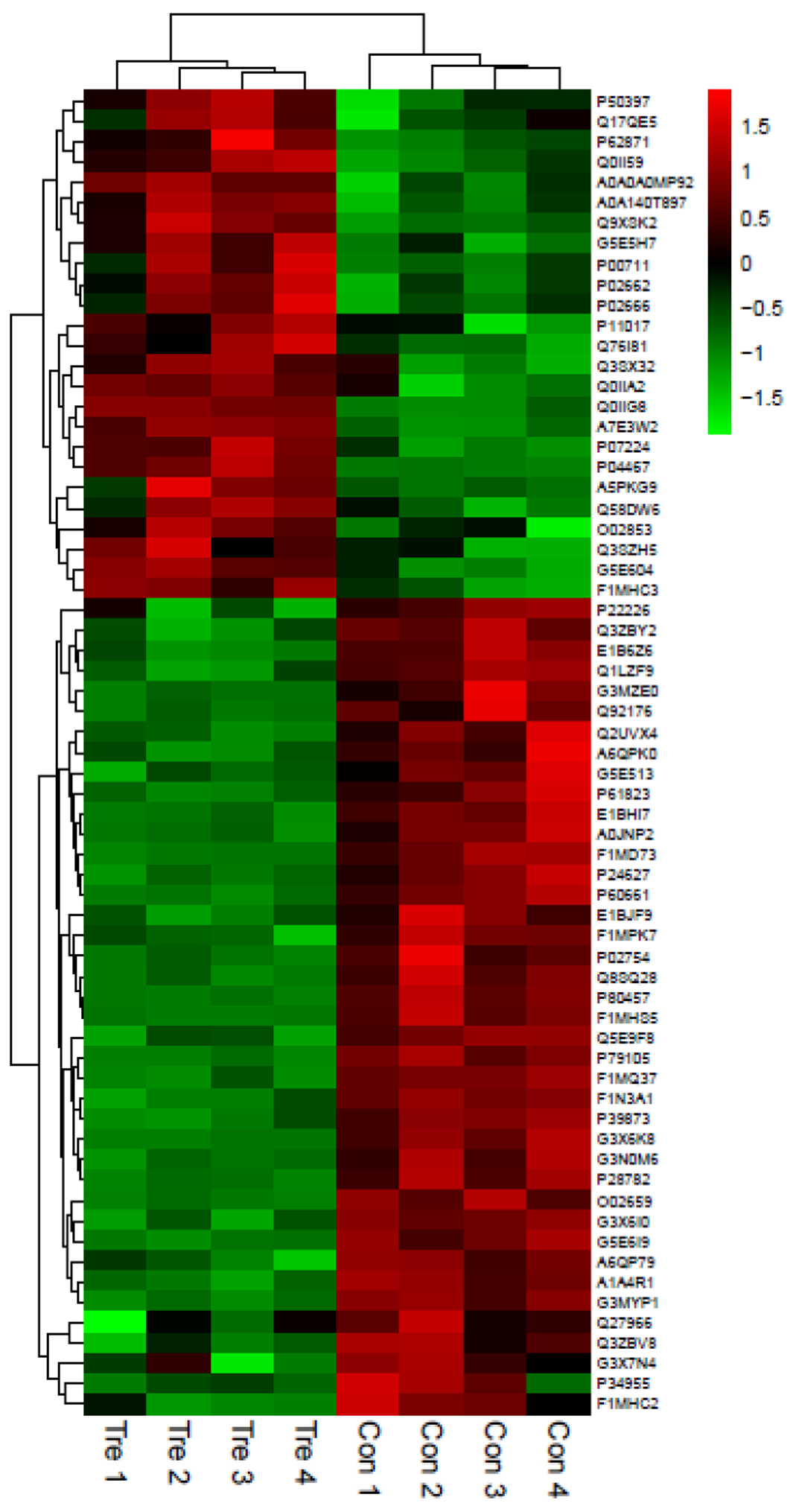
3.2. Statistical Analysis of the Differentially Expressed Proteins
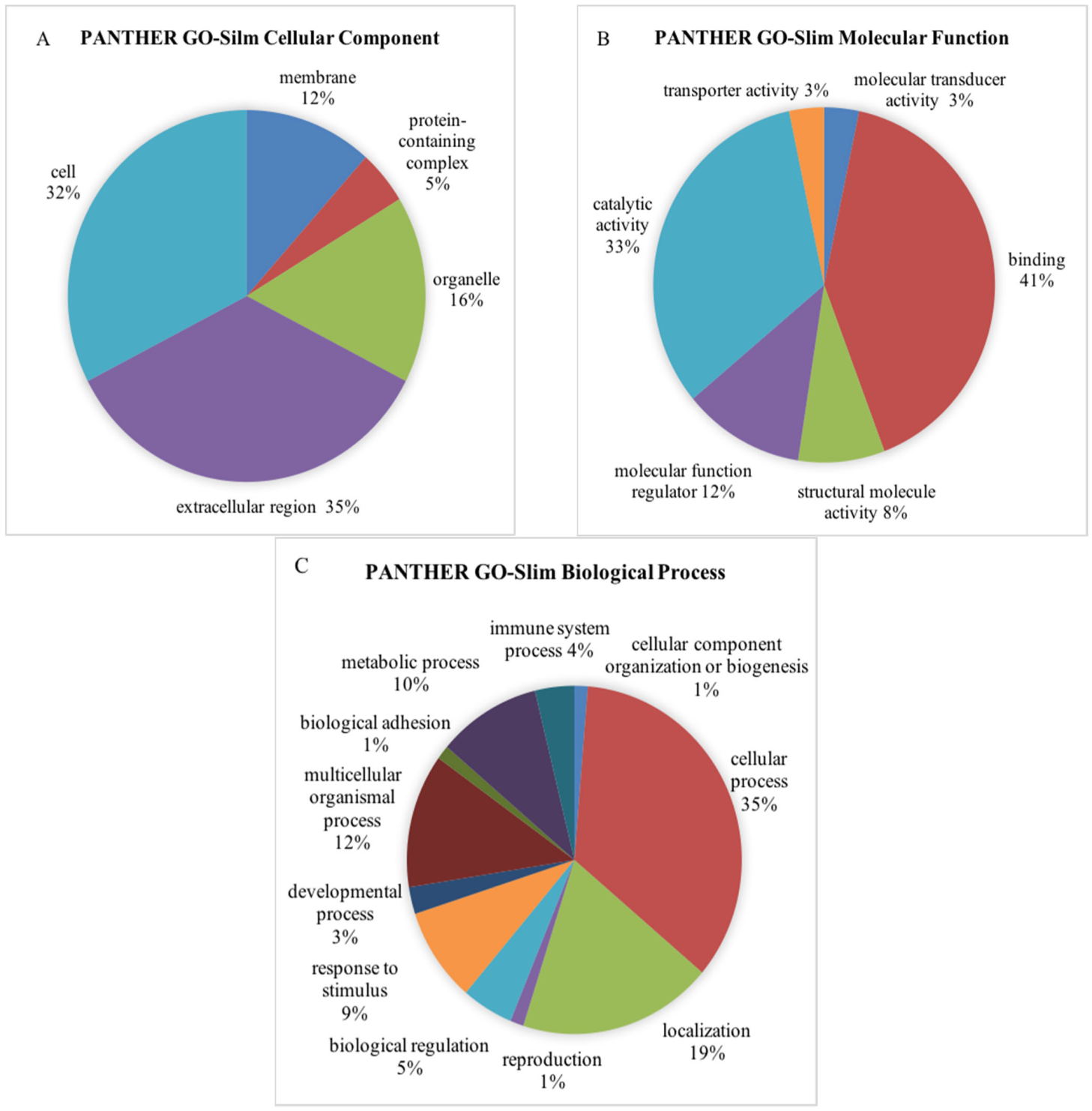
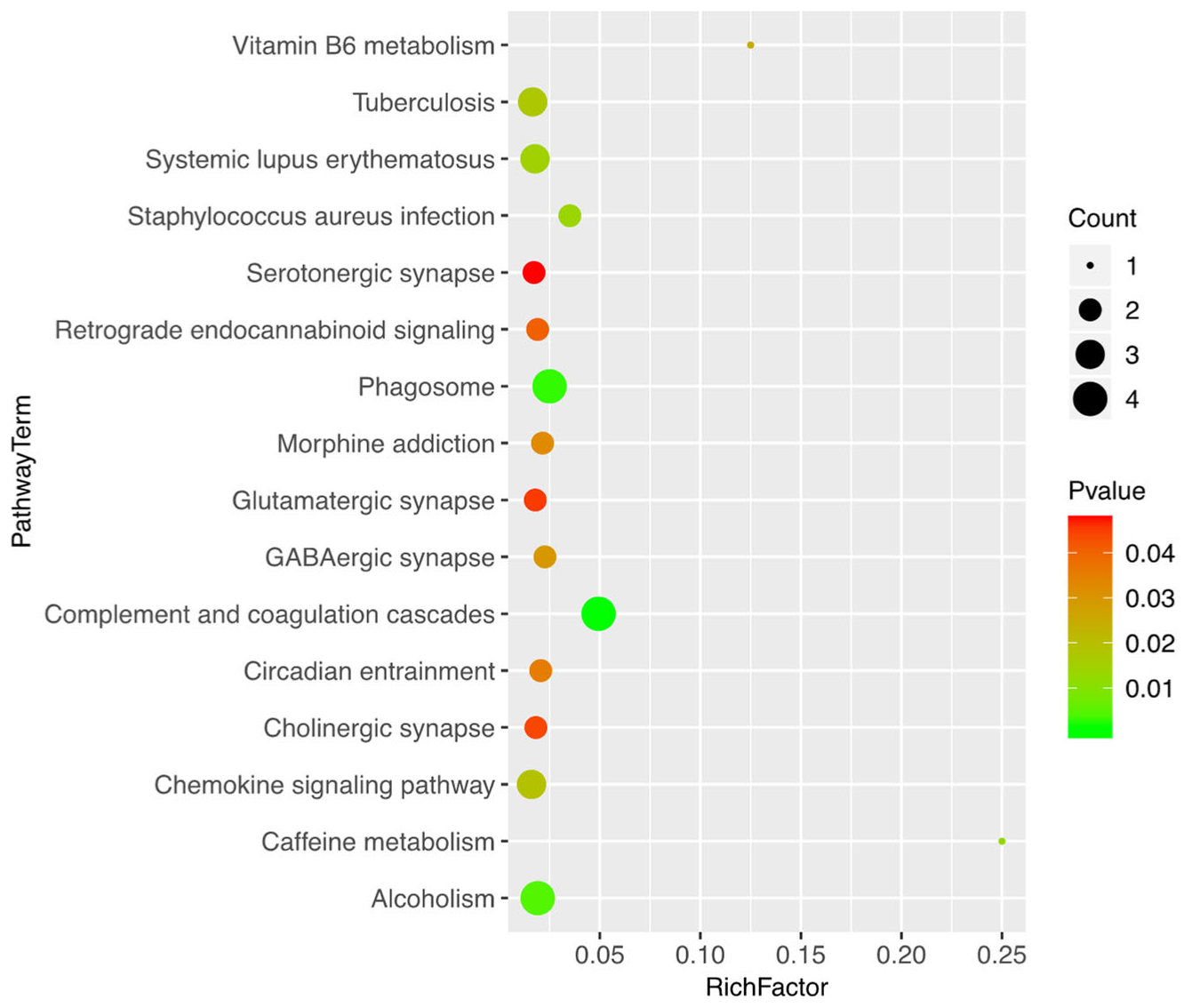
3.3. Bioinformatics Analysis of the Differentially Expressed Proteins
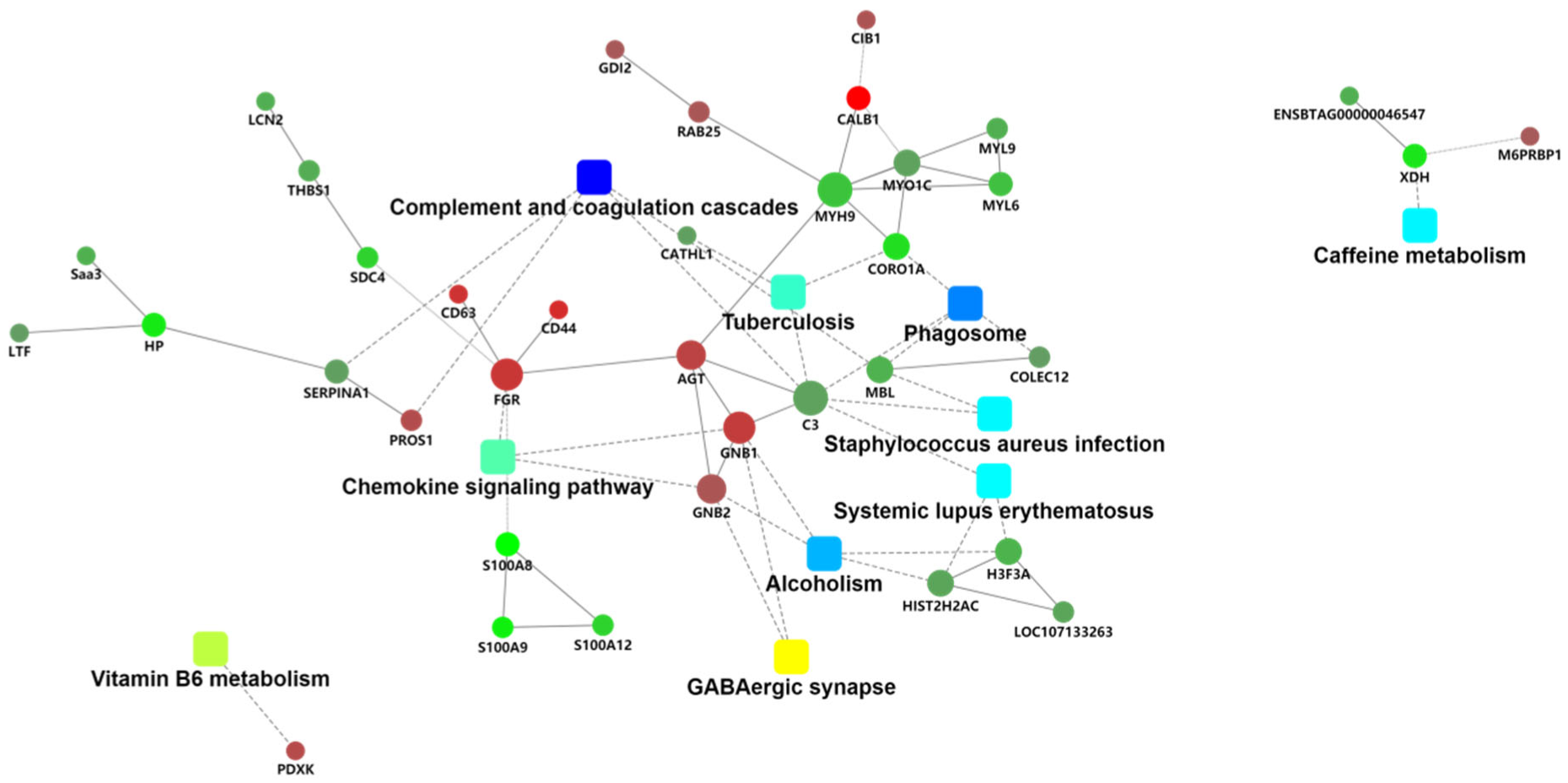
3.4. PPI Analysis of the Differentially Expressed Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NFFS | Non-forage fiber sources |
| EV | Extracellular vesicle |
| ESCRT | Endosomal complex required for transport |
| iTRAQ | Isobaric tags for relative and absolute quantification |
| TMR | Total mixed rations |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| PCA | Principal component analysis |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GO | Gene ontology |
| MBL | Mannose-binding protein C precursor |
| SERPINA1 | Alpha-1-antiproteinase precursor |
| PROS1 | Vitamin K-dependent protein S precursor |
| FGR | Tyrosine-protein kinase |
| MYH9 | myosin-9 |
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Trisha, G. Nature’s first functional food. Science 2014, 345, 747–749. [Google Scholar]
- Korhonen, H.; Marnila, P.; Gill, H.S. Bovine milk antibodies for health. Br. J. Nutr. 2000, 84, 135–146. [Google Scholar] [CrossRef]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Human milk proteins: Key components for the biological activity of human milk. Single Mol. Single Cell Seq. 2004, 554, 11–25. [Google Scholar]
- Goldman, A.S.; Chheda, S.; Garofalo, R.; Schmalstieg, F.C. Cytokines in human milk: Properties and potential effects upon the mammary gland and the neonate. J. Mammary Gland Biol. Neoplasia 1996, 1, 251–258. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Jelen, P.; Jelen, P. Handbook of Milk Composition. Food Sci. Technol. Int. 1995, 6, 1223–1224. [Google Scholar] [CrossRef]
- Van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Hoen, E.N.N.; Garssen, J.; Stahl, B.; Altelaar, A.M.; Redegeld, F.A.; Wauben, M.H. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2017, 39, 501–513. [Google Scholar] [CrossRef]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 2016, 147, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.; Morosi, L.; Chinello, C.; Magni, F.; Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 2011, 11, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.; Mullins, C. Invited review: Strategies for promoting productivity and health of dairy cattle by feeding nonforage fiber sources. J. Dairy Sci. 2012, 95, 4735–4746. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nan, X.; Chu, K.; Tong, J.; Yang, L.; Zheng, S.; Zhao, G.; Jiang, L.; Xiong, B. Shifts of Hydrogen Metabolism from Methanogenesis to Propionate Production in Response to Replacement of Forage Fiber with Non-forage Fiber Sources in Diets in vitro. Front. Microbiol. 2018, 9, 2764. [Google Scholar] [CrossRef]
- Quan, S.-Y.; Nan, X.-M.; Wang, K.; Zhao, Y.-G.; Jiang, L.-S.; Yao, J.-H.; Xiong, B.-H. Replacement of forage fiber with non-forage fiber sources in dairy cow diets changes milk extracellular vesicle-miRNA expression. Food Funct. 2020, 11, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Nan, X.; Wang, K.; Jiang, L.; Yao, J.; Xiong, B. Different Diets Change the Expression of Bovine Serum Extracellular Vesicle-miRNAs. Animals 2019, 9, 1137. [Google Scholar] [CrossRef]
- Committee of Animal Nutrition. Nutrient Requirements of Dairy Cattle; National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2018, 47, D419–D426. [Google Scholar] [CrossRef]
- Sun, N.; Sun, W.; Li, S.; Yang, J.; Yang, L.; Quan, G.; Gao, X.; Wang, Z.; Cheng, X.; Li, Z.; et al. Proteomics Analysis of Cellular Proteins Co-Immunoprecipitated with Nucleoprotein of Influenza A Virus (H7N9). Int. J. Mol. Sci. 2015, 16, 25982–25998. [Google Scholar] [CrossRef]
- Ipharraguerre, I.; Clark, J. Soyhulls as an Alternative Feed for Lactating Dairy Cows: A Review. J. Dairy Sci. 2003, 86, 1052–1073. [Google Scholar] [CrossRef]
- Dann, H.; Carter, M.; Cotanch, K.; Ballard, C.; Takano, T.; Grant, R. Effect of Partial Replacement of Forage Neutral Detergent Fiber with By-Product Neutral Detergent Fiber in Close-Up Diets on Periparturient Performance of Dairy Cows. J. Dairy Sci. 2007, 90, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Zebeli, Q.; Zollitsch, W.; Knaus, W. Feeding of by-products completely replaced cereals and pulses in dairy cows and enhanced edible feed conversion ratio. J. Dairy Sci. 2015, 98, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef]
- Yang, M.; Song, D.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Wu, J.; Yue, X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Res. Int. 2017, 92, 17–25. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef]
- Zhang, L.; Boeren, S.; Hageman, J.A.; Van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Perspective on calf and mammary gland development through changes in the bovine milk proteome over a complete lactation. J. Dairy Sci. 2015, 98, 5362–5373. [Google Scholar] [CrossRef]
- Le, A.; Barton, L.D.; Sanders, J.T.; Zhang, Q. Exploration of Bovine Milk Proteome in Colostral and Mature Whey Using an Ion-Exchange Approach. J. Proteome Res. 2010, 10, 692–704. [Google Scholar] [CrossRef]
- De la Torre Gomez, C.; Goreham, R.V.; Bech-Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xi, Q.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wang, S.-B.; Shu, G.; Wang, L.-N.; Zhu, X.-T.; et al. Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Veter-Res. 2017, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Van Dijk, A.D.J.; Hettinga, K. An interactomics overview of the human and bovine milk proteome over lactation. Proteome Sci. 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Ly, S.; Shan, S.T.; Laugier, J.; Boilard, E.; Gilbert, C.; Provost, P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles 2017, 6, 1401897. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Mol, P.; Kannegundla, U.; Dey, G.; Gopalakrishnan, L.; Dammalli, M.; Kumar, M.; Patil, A.H.; Basavaraju, M.; Rao, A.; Ramesha, K.P.; et al. Bovine Milk Comparative Proteome Analysis from Early, Mid, and Late Lactation in the Cattle Breed, Malnad Gidda (Bos indicus). OMICS 2018, 22, 223–235. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef]
- Kilpatrick, D. Mannan-binding lectin: Clinical significance and applications. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2002, 1572, 401–413. [Google Scholar] [CrossRef]
- De Pascale, G.; Cutuli, S.L.; Pennisi, M.A.; Antonelli, M. The Role of Mannose-Binding Lectin in Severe Sepsis and Septic Shock. Mediat. Inflamm. 2013, 2013, 625803. [Google Scholar] [CrossRef]
- Webb, J.H. Studies on the interaction between vitamin K-dependent protein S and complement regulator C4b-binding protein: Localization of binding sites and identification of a possible function of the complex. Scand. J. Clin. Lab. Investig. 2002, 62, 19–28. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
| Items | Experiment Diet | |
|---|---|---|
| Control Group | Treat Group | |
| Ingredient, % of DM | ||
| Alfalfa hay | 18.67 | 9.70 |
| Corn silage | 27.39 | 24.88 |
| Steam-flaked corn | 23.20 | 24.25 |
| Soybean meal | 8.22 | 8.21 |
| Cottonseed meal | 9.54 | 9.54 |
| Beet pulp | 5.68 | 5.68 |
| DDGS | 3.78 | 3.77 |
| Whole cotton seed | — | 5.72 |
| Soybean hull | — | 4.73 |
| Bergafat T300 | 1.04 | 1.04 |
| Premix | 1.86 | 1.84 |
| Chemical composition, % of DM | ||
| CP | 16.02 | 16.34 |
| EE | 4.20 | 5.18 |
| RDP(%CP) | 58.61 | 56.64 |
| NDF | 31.01 | 31.84 |
| ADF | 21.78 | 22.23 |
| Starch | 25.35 | 25.49 |
| NEL, Mcal/kg | 1.61 | 1.62 |
| Biological Process | Process Hit | Percentage |
|---|---|---|
| Cellular component organization or biogenesis | 5 | 0.84% |
| Cellular process | 207 | 34.62% |
| Localization | 89 | 14.88% |
| Reproduction | 7 | 1.17% |
| Biological regulation | 61 | 10.20% |
| Response to stimulus | 47 | 7.86% |
| Developmental process | 16 | 2.68% |
| Multicellular organismal process | 44 | 7.36% |
| Biological adhesion | 15 | 2.51% |
| Metabolic process | 84 | 14.05% |
| Immune system process | 23 | 3.85% |
| Protein Class | Percentage | Protein ID | Gene Name | Gene Symbol |
|---|---|---|---|---|
| Enzyme modulator | 29.50% | P50397 | Rab GDP dissociation inhibitor beta | GDI2 |
| Q3ZCK2 | RAS-like proto-oncogene A | RALA | ||
| Q3SYU2 | Elongation factor 2 | EEF2 | ||
| P04896 | Guanine nucleotide-binding protein G(s) subunit alpha isoforms short | GNAS | ||
| P11017 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-2 | GNB2 | ||
| P61585 | Transforming protein RhoA | RHOA | ||
| P61223 | Ras-related protein Rap-1b | RAP1B | ||
| P62998 | Ras-related C3 botulinum toxin substrate 1 | RAC1 | ||
| P68103 | Elongation factor 1-alpha 1 | EEF1A1 | ||
| Q7SIH1 | Alpha-2-macroglobulin | A2M | ||
| Q2KJ93 | Cell division control protein 42 homologs | CDC42 | ||
| Q2UVX4 | Complement C3 | C3 | ||
| P62871 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | GNB1 | ||
| Membrane traffic protein | 4.50% | Q3SZA6 | Syndecan binding protein | SDCBP |
| P49951 | Clathrin heavy chain 1 | CLTC | ||
| Chaperone | 6.80% | P63103 | 14-3-3 protein zeta/delta | YWHAZ |
| Q3MHL7 | T-complex protein 1 subunit zeta | CCT6A | ||
| Q76LV2 | Heat shock protein HSP 90-alpha | HSP90AA1 | ||
| Hydrolase | 11.40% | Q3SYU2 | Elongation factor 2 | EEF2 |
| P11017 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-2 | GNB2 | ||
| P68103 | Elongation factor 1-alpha 1 | EEF1A1 | ||
| P62871 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | GNB1 | ||
| F1N647 | Fatty acid synthase | FASN | ||
| Oxidoreductase | 9.10% | Q5E9B1 | L-lactate dehydrogenase B chain | LDHB |
| Q5E947 | Peroxiredoxin-1 | PRDX1 | ||
| Q9BGI3 | Peroxiredoxin-2 | PRDX2 | ||
| F1N647 | Fatty acid synthase | FASN | ||
| Lyase | 2.30% | Q9XSJ4 | Alpha-enolase | ENO1 |
| Transfer/carrier protein | 2.30% | A0A140T897 | Serum albumin | ALB |
| Transferase | 4.50% | P50397 | Rab GDP dissociation inhibitor beta | GDI2 |
| F1N647 | Fatty acid synthase | FASN | ||
| Nucleic acid binding | 6.80% | Q3SYU2 | Elongation factor 2 | EEF2 |
| G5E6I9 | Histone H2B | LOC107133263 | ||
| P68103 | Elongation factor 1-alpha 1 | EEF1A1 | ||
| Ligase | 4.50% | A3KN51 | TSG101 protein | TSG101 |
| F1N647 | Fatty acid synthase | FASN | ||
| Defense/immunity protein | 4.50% | Q7SIH1 | Alpha-2-macroglobulin | A2M |
| Q2UVX4 | Complement C3 | C3 | ||
| Cytoskeletal protein | 9.10% | P31976 | Ezrin | EZR |
| Q2HJ49 | Moesin | MSN | ||
| P60712 | Actin, cytoplasmic 1 | ACTB | ||
| Q3SYV4 | Adenylyl cyclase-associated protein 1 | CAP1 | ||
| Signaling molecule | 4.50% | Q7SIH1 | Alpha-2-macroglobulin | A2M |
| Q2UVX4 | Complement C3 | C3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, S.; Du, C.; Wang, K.; Nan, X.; Xiong, B. Different Diets Change Milk Extracellular Vesicle-Protein Profile in Lactating Cows. Agriculture 2022, 12, 1234. https://doi.org/10.3390/agriculture12081234
Quan S, Du C, Wang K, Nan X, Xiong B. Different Diets Change Milk Extracellular Vesicle-Protein Profile in Lactating Cows. Agriculture. 2022; 12(8):1234. https://doi.org/10.3390/agriculture12081234
Chicago/Turabian StyleQuan, Suyu, Chunmei Du, Kun Wang, Xuemei Nan, and Benhai Xiong. 2022. "Different Diets Change Milk Extracellular Vesicle-Protein Profile in Lactating Cows" Agriculture 12, no. 8: 1234. https://doi.org/10.3390/agriculture12081234





