Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material and Experimental Design
2.3. Plant Measurements
2.4. Determination of Hay, Grain, and Straw Yield
2.5. Grain Quality Analyses
2.6. Statistical Analysis
3. Results
3.1. Plant Density
3.2. Plant Height
3.3. Stem Diameter
3.4. Hay Yield
3.5. Grain Yield
3.6. Straw Yield
3.7. Grain PC
3.8. TGW
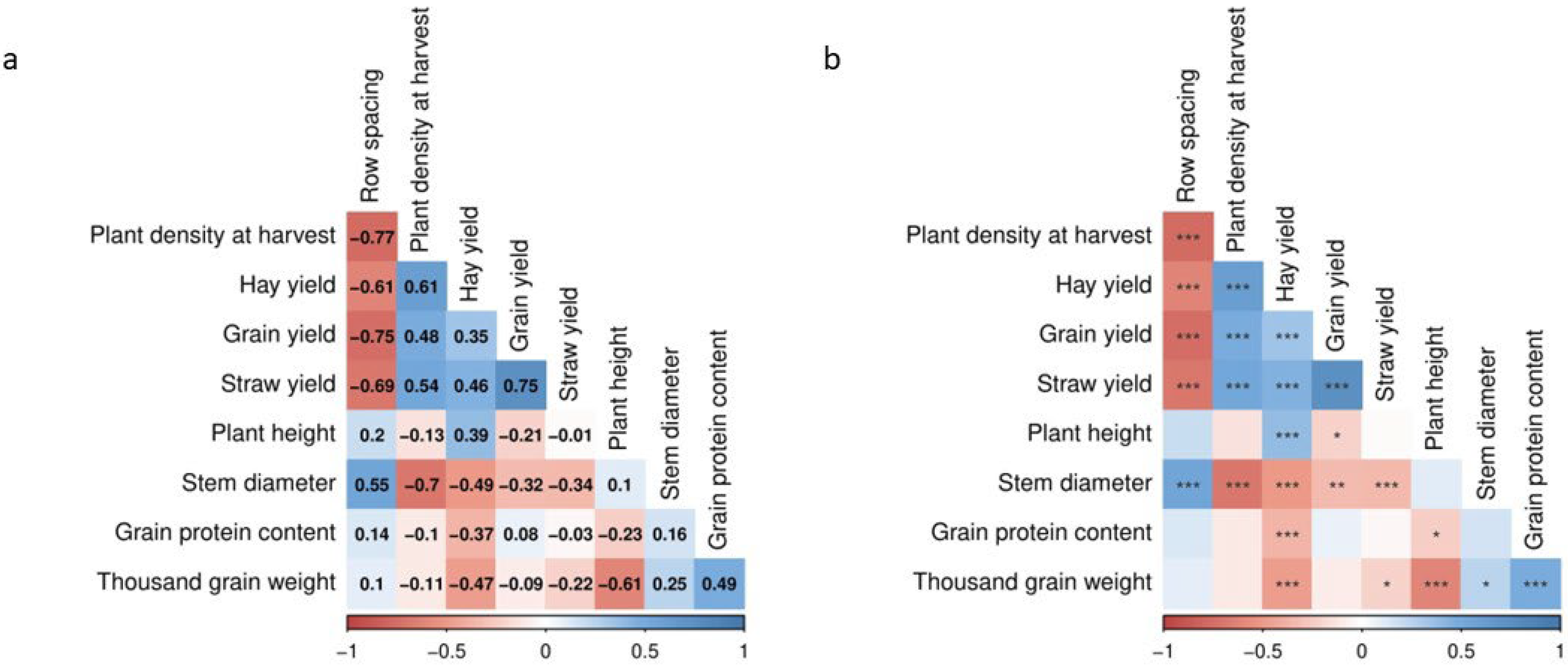
3.9. Correlations between Traits
4. Discussion
4.1. Effect of Row Spacing on Yield Parameters
4.2. Effect of Row Spacing on Grain-Quality Parameters
4.3. Effect of Row Spacing on Plant Growth Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World—International Year of Quinoa 2013; FAO & CIRAD: Rome, Italy, 2015. [Google Scholar]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global Expansion of Quinoa and Challenges for the Andean Region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Jaikishun, S.; Li, W.; Yang, Z.; Song, S. Quinoa: In Perspective of Global Challenges. Agronomy 2019, 9, 176. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and Health Benefits of Quinoa (Chenopodium Quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Ceyhun Sezgin, A.; Sanlier, N. A New Generation Plant for the Conventional Cuisine: Quinoa (Chenopodium Quinoa Willd.). Trends Food Sci. Technol. 2019, 86, 51–58. [Google Scholar] [CrossRef]
- Yeşil, S.; Levent, H. The Influence of Fermented Buckwheat, Quinoa and Amaranth Flour on Gluten-Free Bread Quality. LWT 2022, 160, 113301. [Google Scholar] [CrossRef]
- Aloisi, I.; Parrotta, L.; Ruiz, K.B.; Landi, C.; Bini, L.; Cai, G.; Biondi, S.; del Duca, S. New Insight into Quinoa Seed Quality under Salinity: Changes in Proteomic and Amino Acid Profiles, Phenolic Content, and Antioxidant Activity of Protein Extracts. Front. Plant Sci. 2016, 7, 656. [Google Scholar] [CrossRef]
- Brend, Y.; Galili, L.; Badani, H.; Hovav, R.; Galili, S. Total Phenolic Content and Antioxidant Activity of Red and Yellow Quinoa (Chenopodium Quinoa Willd.) Seeds as Affected by Baking and Cooking Conditions. Food Nutr. Sci. 2012, 3, 1150–1155. [Google Scholar] [CrossRef]
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Editorial: Protein Crops: Food and Feed for the Future. Front. Plant Sci. 2017, 8, 6–9. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Alon, E.; Shapira, O.; Azoulay-Shemer, T.; Rubinovich, L. Shading Nets Reduce Canopy Temperature and Improve Photosynthetic Performance in ‘Pinkerton’ Avocado Trees during Extreme Heat Events. Agronomy 2022, 12, 1360. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F.; Tassone, S. Fatty Acid Profile and Nutritive Value of Quinoa (Chenopodium Quinoa Willd.) Seeds and Plants at Different Growth Stages. Anim. Feed Sci. Technol. 2013, 183, 56–61. [Google Scholar] [CrossRef]
- Ramos, N.; Cruz, A.M. Evaluation of Seven Seasonal Crops for Forage Production during the Dry Season in Cuba. Cuba. J. Agric. Sci. 2002, 36, 271–276. [Google Scholar]
- Ebeid, H.M.; Kholif, A.E.; El-Bordeny, N.; Chrenkova, M.; Mlynekova, Z.; Hansen, H.H. Nutritive Value of Quinoa (Chenopodium Quinoa) as a Feed for Ruminants: In Sacco Degradability and in Vitro Gas Production. Environ. Sci. Pollut. Res. 2022, 29, 35241–35252. [Google Scholar] [CrossRef]
- Darwinkel, A. Understanding the Quinoa Crop: Guidelines for Growing in Temperate Regions of N.W. Europe; EC: Brussels, Belgium, 1997. [Google Scholar]
- Van Schooten, H.; van Schooten, H.; Pinxterhuis, I. Quinoa as an Alternative Forage Crop in Organic Dairy Farming. Available online: https://www.wur.nl/nl/landingspagina-redacteuren/nl/onderzoek-resultaten/onderzoeksinstituten/livestock-research/show-wlr/handboek-kwantitatieve-informatie-veehouderij-kwin.htm (accessed on 28 June 2022).
- Tamminga, S.; Bannink, A.; Dijkstra, J.; Zom, R. Feeding Strategies to Reduce Methane Loss in Cattle; Animal Sciences Group: Wageningen, The Netherlands, 2007. [Google Scholar]
- Patra, A.K.; Saxena, J. The Effect and Mode of Action of Saponins on the Microbial Populations and Fermentation in the Rumen and Ruminant Production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef]
- Bodas, R.; López, S.; Fernández, M.; García-González, R.; Rodríguez, A.B.; Wallace, R.J.; González, J.S. In Vitro Screening of the Potential of Numerous Plant Species as Antimethanogenic Feed Additives for Ruminants. Anim. Feed Sci. Technol. 2008, 145, 245–258. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa (Chenopodium Quinoa) Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Reguera, M. Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions. Plants 2021, 10, 955. [Google Scholar] [CrossRef]
- Filik, G. Biodegradability of Quinoa Stalks: The Potential of Quinoa Stalks as a Forage Source or as Biomass for Energy Production. Fuel 2020, 266, 117064. [Google Scholar] [CrossRef]
- Eisa, S.S.; El-Samad, E.H.A.; Hussin, S.A.; Ali, E.A.; Ebrahim, M.; González, J.A.; Ordano, M.; Erazzú, L.E.; El-Bordeny, N.E.; Abdel-Ati, A.A. Quinoa in Egypt—Plant Density Effects on Seed Yield and Nutritional Quality in Marginal Regions. Middle East J. Appl. Sci. 2018, 8, 515–522. [Google Scholar]
- Bertero, H.D.; de La Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and Genotype-by-Environment Interaction Effects for Grain Yield and Grain Size of Quinoa (Chenopodium Quinoa Willd.) as Revealed by Pattern Analysis of International Multi-Environment Trials. Field Crops Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Agronomic Practices and Performances of Quinoa under Field Conditions: A Systematic Review. Plants 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Spehar, C.R.; da Rocha, J.E.S. Effect of Sowing Density on Plant Growth and Development of Quinoa, Genotype 4.5, in the Brazilian Savannah Highlands. Biosci. J. 2009, 25, 53–58. [Google Scholar]
- Risi, J.; Galwey, N.W. Effects of Sowing Date and Sowing Rate on Plant Development and Grain Yield of Quinoa (Chenopodium Quinoa) in a Temperate Environment. J. Agric. Sci. 1991, 117, 325–332. [Google Scholar] [CrossRef]
- Sief, A.S.; El-Deepah, H.R.A.; Kamel, A.S.M.; Ibrahim, J.F. Effect of Various Inter and Intra Spaces on the Yield and Quality of Quinoa (Chenopodium Quinoa Willd.). J. Plant Prod. Mansoura Univ. 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Van Minh, N.; Hoang, D.T.; van Loc, N.; Long, N.V. Effects of Plant Density on Growth, Yield and Seed Quality of Quinoa Genotypes under Rain-Fed Conditions on Red Basalt Soil Regions. Aust. J. Crop Sci. 2020, 14, 1977–1982. [Google Scholar] [CrossRef]
- Baird, R.; Eaton, A.; Rice, E. (Eds.) 4500-Norg NITROGEN (ORGANIC). In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; p. 498. [Google Scholar]
- Volenec, J.J.; Cherney, J.H.; Johnson, K.D. Yield Components, Plant Morphology, and Forage Quality of Alfalfa as Influenced by Plant Population. Crop Sci. 1987, 47907, 321–326. [Google Scholar] [CrossRef]
- Prommarak, S. Response of Quinoa to Emergence Test and Row Spacing in Chiang Mai-Lumphun Valley Lowland Area. Khon Kaen Agric. J. 2014, 42, 8–14. [Google Scholar]
- Jacobsen, S.E.; Stølen, O.; Jørgensen, I. Cultivation of Quinoa (Chenopodium Quinoa) under Temperate Climatic Conditions in Denmark. J. Agric. Sci. 1994, 122, 47–52. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Shock, C.C.; Meng, C.; Qiao, L. Effects of Management Practices on Quinoa Growth, Seed Yield, and Quality. Agronomy 2020, 10, 445. [Google Scholar] [CrossRef]
- Hunter, M.C.; Sheaffer, C.C.; Culman, S.W.; Lazarus, W.F.; Jungers, J.M. Effects of Defoliation and Row Spacing on Intermediate Wheatgrass II: Forage Yield and Economics. Agron. J. 2020, 112, 1862–1880. [Google Scholar] [CrossRef]
- Albayrak, S.; Türk, M.; Yüksel, O. Effect of Row Spacing and Seeding Rate on Hungarian Vetch Yield and Quality. Turk. J. Field Crops 2011, 16, 54–58. [Google Scholar]
- Zulkadir, G.; Çiftçi, S.; Selenay Gökçe, M.; Karaburu, E.; Bozdağ, E.; İdikut, L. The Effect of Row Distances on Quinoa Yield and Yield Components in The Effect of Row Distances on Quinoa Yield and Yield Components in the Late Planting Period. Int. J. Res. Publ. Rev. 2020, 1, 37–42. [Google Scholar]
- Bellalou, A.; Daklo-Keren, M.; Abu Aklin, W.; Sokolskaya, R.; Rubinovich, L.; Asher, A.; Galili, S. Germination of Chenopodium Quinoa Cv. ‘Mint Vanilla’ Seeds under Different Abiotic Stress Conditions. Seed Sci. Technol. 2022, 50, 41–45. [Google Scholar] [CrossRef]
- Bellalou, A.; Daklo-Keren, M.; Abu-Aklin, W.; Sadan, G.; Sokolskia, R.; Rubinovich, L.; Asher, A.; Londner, A.; Amir-Segev, O.; Farber, A.; et al. Influence of Sowing Date of Quinoa Mother Plants on Seed Germination (in Hebrew). Nir Vatelem 2020, 1–7. [Google Scholar]
- Zhang, T.; Wang, X.; Han, J.; Wang, Y.; Mao, P.; Majerus, M. Effects of Between-Row and within-Row Spacing on Alfalfa Seed Yields. Crop Sci. 2008, 48, 794–803. [Google Scholar] [CrossRef]
- Turgut, I.; Duman, A.; Bilgili, U.; Acikgoz Authorsõ, E. Alternate Row Spacing and Plant Density Effects on Forage and Dry Matter Yield of Corn Hybrids (Zea Mays L.). J. Agron. Crop Sci. 2005, 191, 146–151. [Google Scholar] [CrossRef]
- Sher, A.; Khan, A.; Ashraf, U.; Liu, H.H.; Li, J.C. Characterization of the Effect of Increased Plant Density on Canopy Morphology and Stalk Lodging Risk. Front. Plant Sci. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant Adaptation to Dynamically Changing Environment: The Shade Avoidance Response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Roig-Villanova, I.; Paulišić, S.; Martinez-Garcia, J.F. Shade Avoidance and Neighbor Detection. Methods Mol. Biol. 2019, 2026, 157–168. [Google Scholar] [CrossRef]
- Schwalbert, R.; Amado, T.J.C.; Horbe, T.A.N.; Stefanello, L.O.; Assefa, Y.; Prasad, P.V.V.; Rice, C.W.; Ciampitti, I.A. Corn Yield Response to Plant Density and Nitrogen: Spatial Models and Yield Distribution. Agron. J. 2018, 110, 970–982. [Google Scholar] [CrossRef]
| 2018 to 2019 | 2019 to 2020 | |||||
|---|---|---|---|---|---|---|
| Month | Rainfall (mm) | Min. Temp (°C) | Max. Temp (°C) | Rainfall (mm) | Min. Temp (°C) | Max. Temp (°C) |
| October | 11.1 | 7.8 | 34.1 | 7.4 | 13.3 | 37.3 |
| November | 30.0 | 8.4 | 29.5 | 14.2 | 3.3 | 30.6 |
| December | 140.3 | 4.9 | 20.5 | 207.4 | 3.9 | 22.6 |
| January | 182.0 | 2.3 | 20.0 | 220.7 | 1.0 | 17.1 |
| February | 156.1 | 3.1 | 22.4 | 93.0 | 0.4 | 20.1 |
| March | 106.0 | 3.5 | 23.6 | 80.9 | 4.5 | 26.2 |
| April | 40.1 | 5.8 | 30.7 | 44.5 | 6.0 | 30.1 |
| May | 0.4 | 7.3 | 41.0 | 29.8 | 8.2 | 40.9 |
| June | 0.1 | 13.4 | 37.1 | 0.0 | 11.7 | 34.0 |
| Total | 665.7 | 697.9 | ||||
| Row Spacing (cm) | Accession | Plant Density at Harvest (Plant m−2) | Plant Height (cm) | Stem Diameter (mm) | Hay Yield (kg DM ha−1) | Grain Yield (kg ha−1) | Straw Yield (kg DM ha−1) | Grain Protein Content (%) | Thousand Grain Weight (mg) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | 3 | 255 ± 20 a | 78 ± 2 | 6.4 ± 0.1 c | 9037 ± 946 a | 3191 ± 239 ab | 3070 ± 254 a | 13.3 ± 0.3 | 3099 ± 68 |
| 4E | 198 ± 12 b | 77 ± 3 | 7.0 ± 0.3 c | 7035 ± 634 ab | 4266 ± 470 a | 3214 ± 348 a | 14.2 ± 0.5 | 3111 ± 86 | |
| 26 | 3 | 131 ± 8 c | 81 ± 4 | 8.8 ± 0.4 b | 6914 ± 669 ab | 3656 ± 280 a | 2756 ± 207 ab | 12.6 ± 0.5 | 3350 ± 83 |
| 4E | 61 ± 2 d | 77 ± 4 | 9.1 ± 0.5 ab | 5458 ± 377 b | 3270 ± 312 ab | 1831 ± 205 bc | 12.8 ± 0.7 | 3328 ± 101 | |
| 80 | 3 | 42 ± 3 d | 81 ± 3 | 9.4 ± 0.4 ab | 2259 ± 75 c | 1980 ± 106 b | 1287 ± 109 c | 11.7 ± 0.9 | 3294 ± 76 |
| 4E | 22 ± 3 d | 87 ± 2 | 10.5 ± 0.5 a | 2306 ± 239 c | 1958 ± 259 b | 1275 ± 194 c | 12.1 ± 0.5 | 3446 ± 204 | |
| F-test | Accession | *** | ns | ns | * | ns | ns | ns | ns |
| Row spacing | *** | ns | *** | *** | *** | *** | * | ns |
| Row Spacing (cm) | Accession | Plant Density at Harvest (Plant m−2) | Plant Height (cm) | Stem Diameter (mm) | Hay Yield (kg DM ha−1) | Grain Yield (kg ha−1) | Straw Yield (kg DM ha−1) | Grain Protein Content (%) | Thousand Grain Weight (mg) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | 3 | 260 ± 24 a | 77 ± 4 | 6.8 ± 0.2 c | 11,100 ± 1212 a | 2973 ± 425 ab | 2640 ± 425 ab | 10.3 ± 1.1 | 3103 ± 86 ab |
| 4E | 190 ± 21 b | 81 ± 2 | 7.4 ± 0.2 bc | 10,361 ± 1090 abc | 3501 ± 250 a | 3205 ± 110 a | 11.9 ± 0.8 | 2775 ± 46 b | |
| 26 | 3 | 165 ± 10 bc | 86 ± 4 | 8.2 ± 0.4 bc | 10,710 ± 1820 ab | 2431 ± 442 ab | 2634 ± 239 ab | 9.9 ± 0.3 | 3064 ± 82 ab |
| 4E | 116 ± 4 cd | 86 ± 4 | 8.2 ± 0.5 bc | 9233 ± 1390 abc | 3249 ± 90 a | 3020 ± 92 a | 14.1 ± 1.7 | 2839 ± 46 b | |
| 80 | 3 | 57 ± 4 de | 86 ± 3 | 8.5 ± 0.4 b | 5149 ± 256 c | 1827 ± 128 b | 1398 ± 129 c | 11.9 ± 1.1 | 3312 ± 137 a |
| 4E | 40 ± 4 e | 91 ± 1 | 10.3 ± 0.4 a | 5670 ± 524 bc | 2383 ± 111 ab | 1979 ± 99 bc | 14.1 ± 1.5 | 2768 ± 79 b | |
| F-test | Accession | ** | ns | ** | ns | * | * | ** | *** |
| Row spacing | *** | * | *** | *** | ** | *** | ns | ns |
| Row Spacing (cm) | Accession | Plant Density at Harvest (Plant m−2) | Plant Height (cm) | Stem Diameter (mm) | Hay Yield (kg DM ha−1) | Grain Yield (kg ha−1) | Straw Yield (kg DM ha−1) | Grain Protein Content (%) | Thousand Grain Weight (mg) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | 3 | 259 ± 14 a | 96 ± 7 | 7.8 ± 0.3 | 13,586 ± 1208 a | 3635 ± 142 a | 3597 ± 645 a | 5.3 ± 0.1 ab | 2418 ± 26 abc |
| 4E | 103 ± 27 b | 97 ± 3 | 8.7 ± 0.1 | 11,804 ± 1071 ab | 3575 ± 135 a | 3197 ± 387 ab | 5.4 ± 0.1 ab | 2324 ± 30 c | |
| 26 | 3 | 185 ± 29 a | 98 ± 10 | 8.1 ± 0.6 | 12,007 ± 2083 ab | 3309 ± 229 a | 3660 ± 511 a | 5.2 ± 0.1 b | 2592 ± 18 a |
| 4E | 87 ± 9 b | 90 ± 10 | 7.8 ± 0.8 | 9250 ± 876 abc | 3412 ± 379 a | 2491 ± 429 abc | 6.1 ± 0.1 a | 2355 ± 67 bc | |
| 80 | 3 | 59 ± 8 b | 106 ± 11 | 8.6 ± 0.7 | 6616 ± 784 bc | 1604 ± 156 b | 1594 ± 136 bc | 5.5 ± 0.4 ab | 2586 ± 36 ab |
| 4E | 35 ± 7 b | 99 ± 6 | 9.4 ± 0.4 | 5574 ± 769 c | 1719 ± 95 b | 1212 ± 180 c | 6 ± 0.3 ab | 2493 ± 78 abc | |
| F-test | Accession | *** | ns | ns | ns | ns | ns | * | ** |
| Row spacing | *** | ns | ns | *** | *** | *** | ns | * |
| Row Spacing (cm) | Accession | Plant Density at Harvest (Plant m−2) | Plant Height (cm) | Stem Diameter (mm) | Hay Yield (kg DM ha−1) | Grain Yield (kg ha−1) | Straw Yield (kg DM ha−1) | Grain Protein Content (%) | Thousand Grain Weight (mg) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | 3 | 238 ± 34 a | 111 ± 8 | 6.6 ± 0.6 | 17,979 ± 1488 a | 3088 ± 388 ab | 2369 ± 501 ab | 8.2 ± 1.1 b | 2553 ± 54 a |
| 4E | 256 ± 30 a | 113 ± 4 | 6.7 ± 0.4 | 14,011 ± 1334 a | 3299 ± 203 a | 3187 ± 315 a | 9.7 ± 0.7 ab | 2313 ± 147 ab | |
| 26 | 3 | 165 ± 37 ab | 112 ± 10 | 6.3 ± 0.7 | 14,731 ± 1368 a | 2437 ± 56 abc | 2022 ± 360 ab | 7.2 ± 0.6 b | 2530 ± 55 a |
| 4E | 154 ± 7 ab | 116 ± 9 | 7.1 ± 0.4 | 13,514 ± 1800 a | 2987 ± 283 ab | 2864 ± 244 ab | 9.8 ± 0.7 ab | 2224 ± 129 ab | |
| 80 | 3 | 50 ± 6 b | 123 ± 5 | 7.6 ± 0.3 | 6773 ± 655 b | 1704 ± 68 c | 1496 ± 159 b | 10.6 ± 0.4 ab | 2588 ± 61 a |
| 4E | 57 ± 8 b | 126 ± 5 | 8.4 ± 0.9 | 7651 ± 388 b | 2195 ± 179 bc | 2106 ± 97 ab | 12.6 ± 1.3 a | 2033 ± 67 b | |
| F-test | Accession | ns | ns | ns | ns | * | * | * | *** |
| Row spacing | *** | ns | ns | *** | *** | * | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, A.; Dagan, R.; Galili, S.; Rubinovich, L. Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate. Agriculture 2022, 12, 1298. https://doi.org/10.3390/agriculture12091298
Asher A, Dagan R, Galili S, Rubinovich L. Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate. Agriculture. 2022; 12(9):1298. https://doi.org/10.3390/agriculture12091298
Chicago/Turabian StyleAsher, Aviv, Reut Dagan, Shmuel Galili, and Lior Rubinovich. 2022. "Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate" Agriculture 12, no. 9: 1298. https://doi.org/10.3390/agriculture12091298
APA StyleAsher, A., Dagan, R., Galili, S., & Rubinovich, L. (2022). Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate. Agriculture, 12(9), 1298. https://doi.org/10.3390/agriculture12091298






