Taif’s Rose (Rosa damascena Mill var. trigentipetala) Wastes Are a Potential Candidate for Heavy Metals Remediation from Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sampling
2.2. Plant Analysis
2.3. Soil Sampling and Analysis
2.4. Data Analysis
3. Results
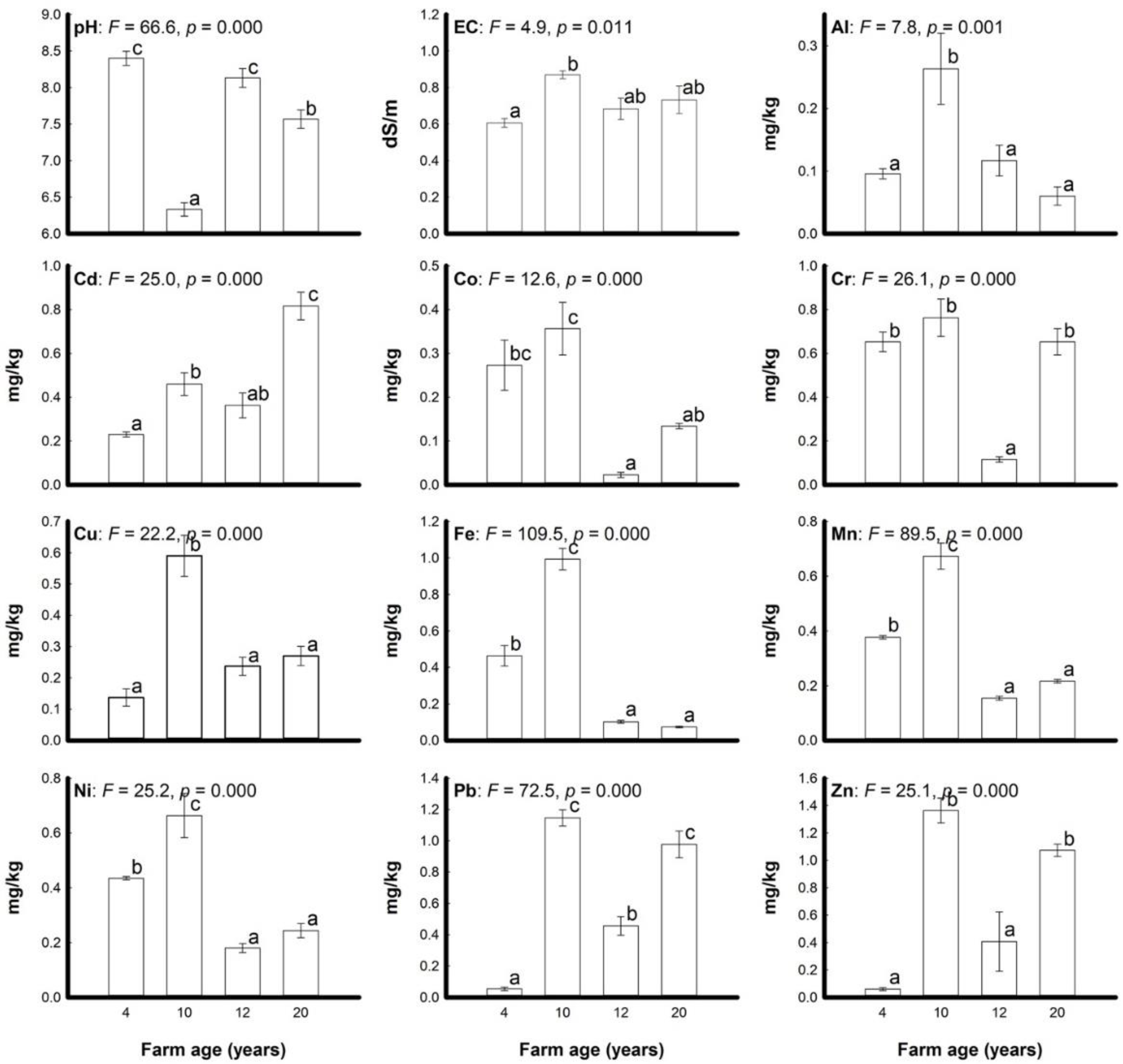
3.1. Soil Properties
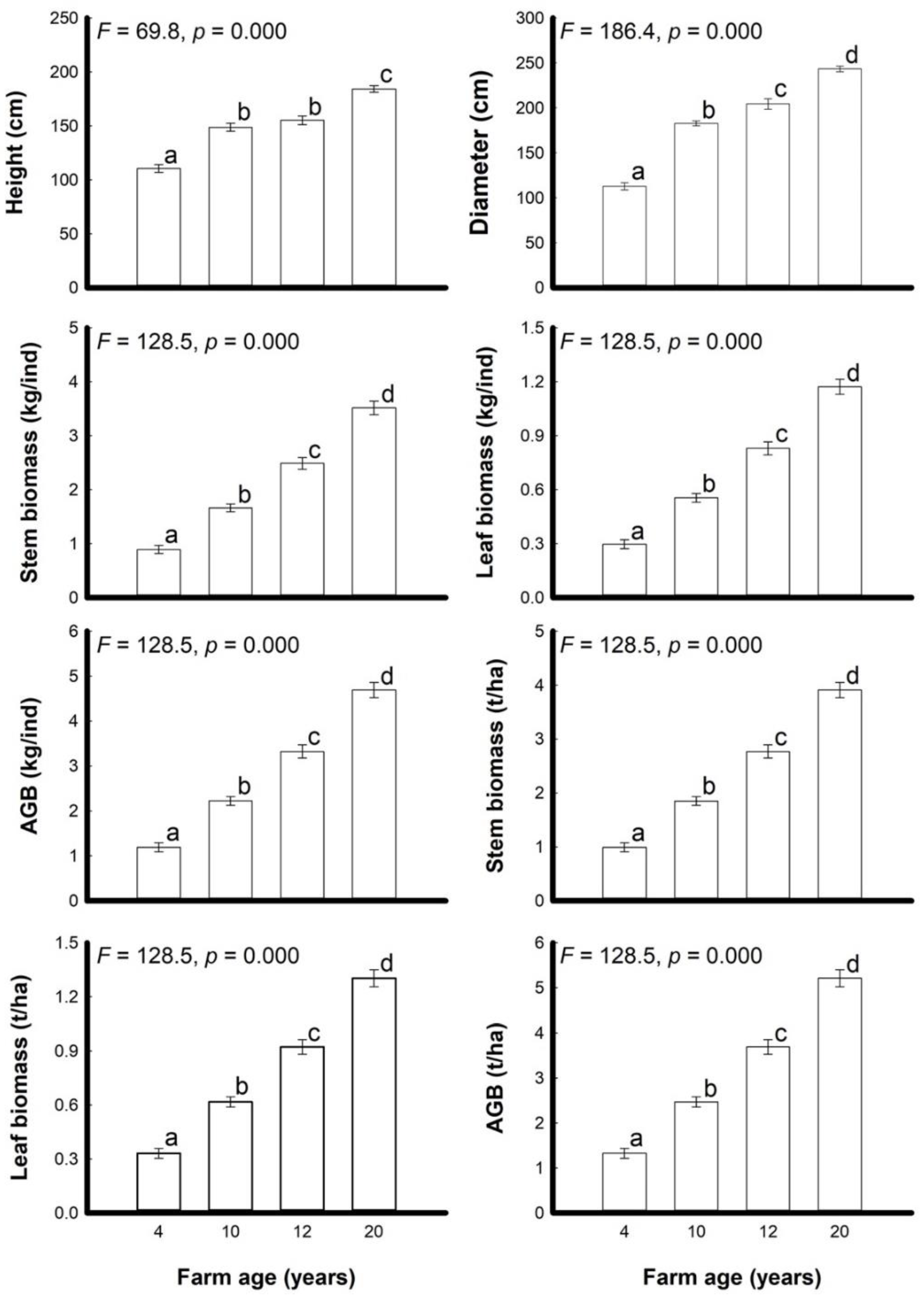
3.2. Plant Growth Properties
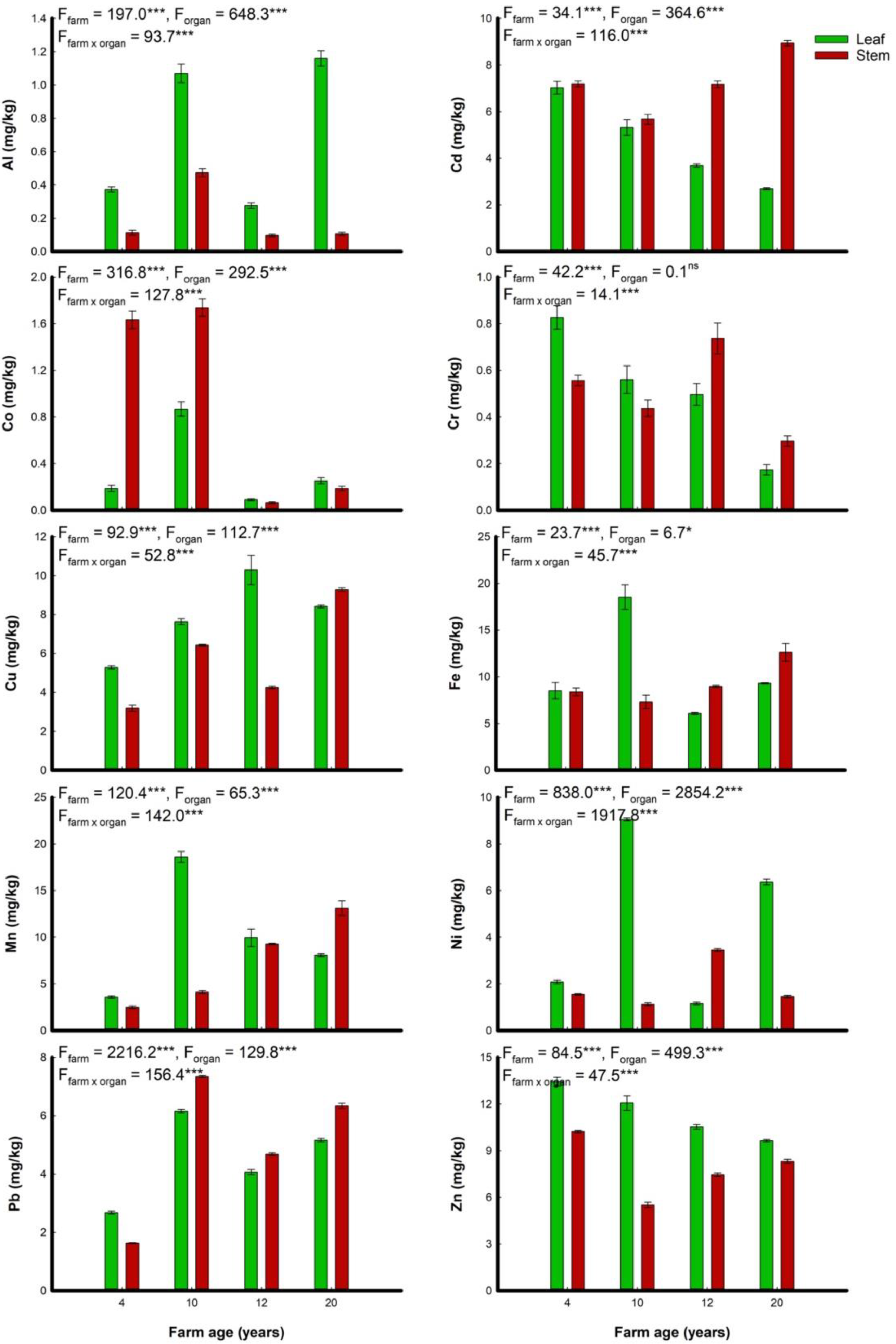
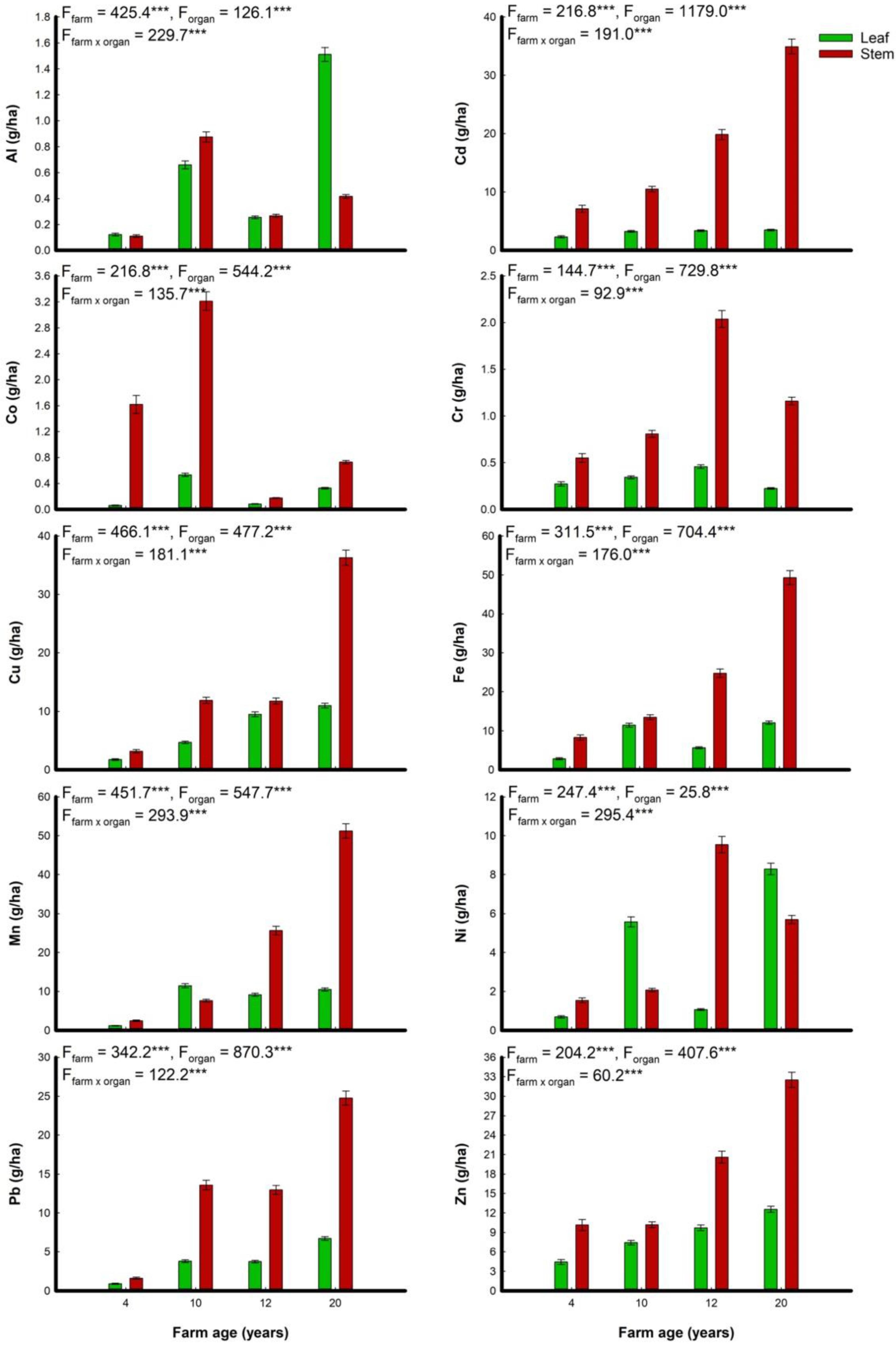
3.3. Heavy Metals Concentration
3.4. Heavy Metals Removal Efficiency
3.5. Bioaccumulation Factor (BAF)
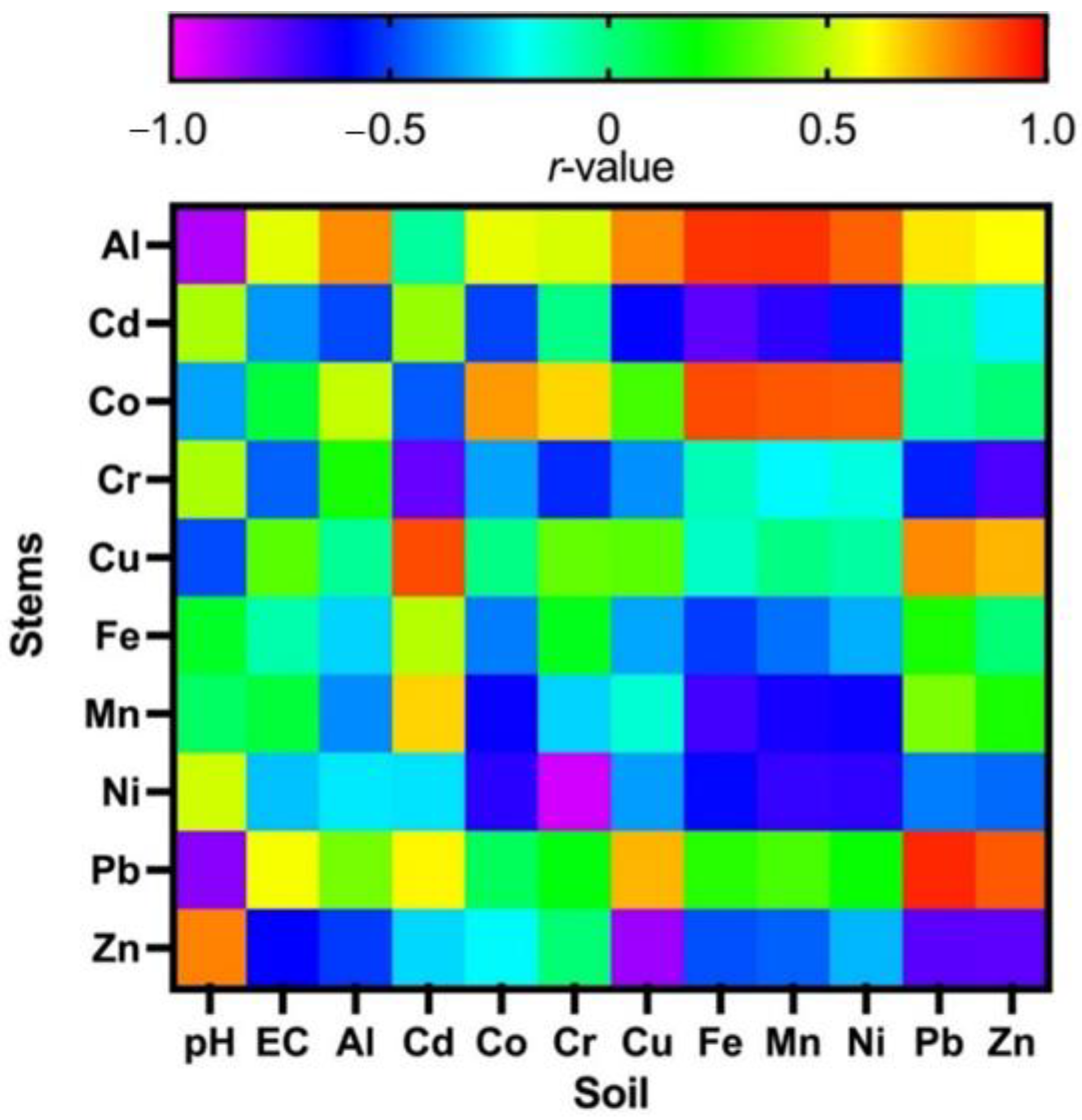
3.6. Plant Stem-Soil Correlations
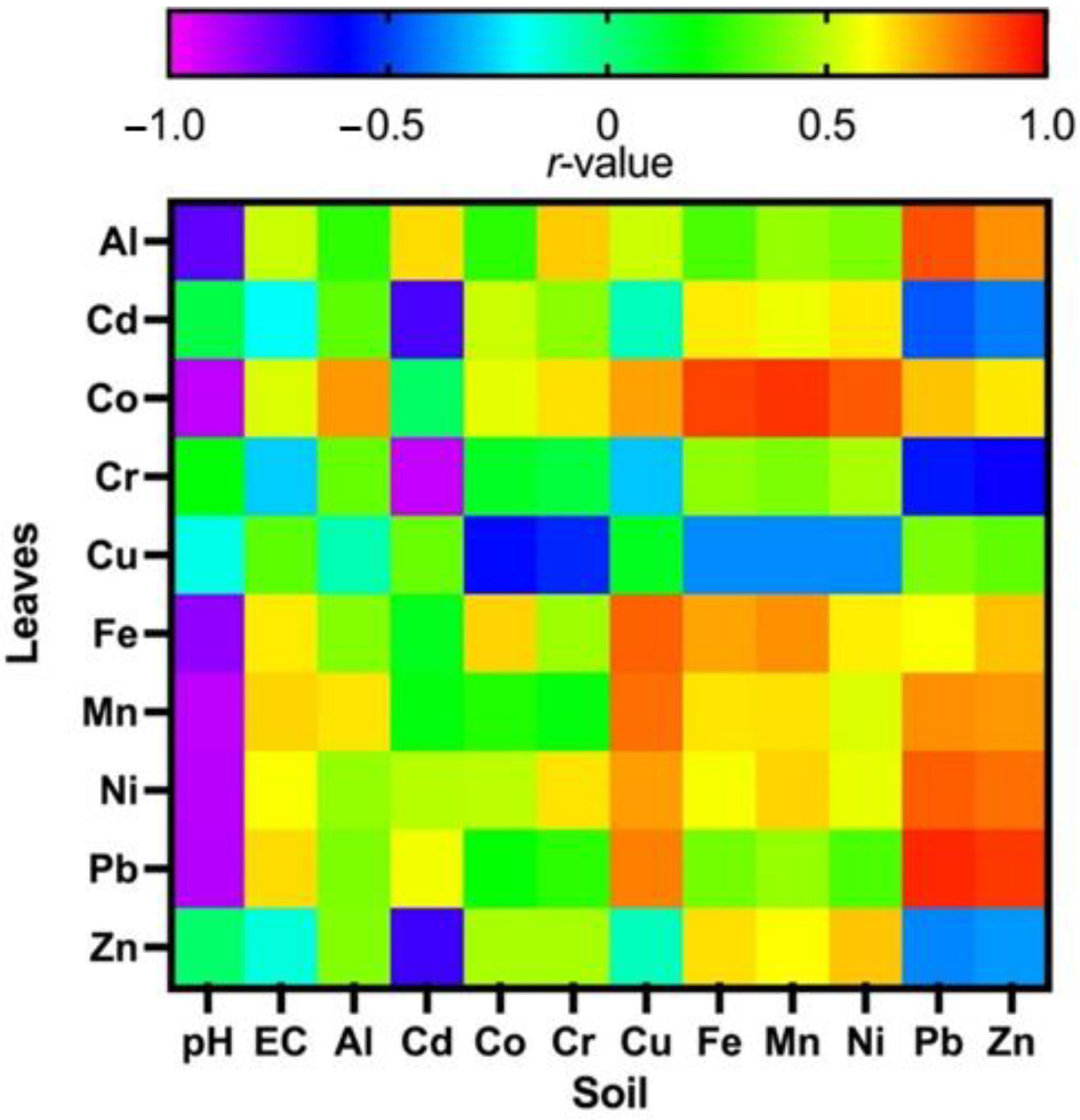
3.7. Plant Leaves-Soil Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deniz, F. Green purification of heavy metal pollution from aquatic environment by biorefinery waste biomass of Nigella sativa L.: A novel and effective treatment agent. Environ. Technol. Innov. 2022, 25, 102118. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Kolluru, S.S.; Agarwal, S.; Sireesha, S.; Sreedhar, I.; Kale, S.R. Heavy metal removal from wastewater using nanomaterials-process and engineering aspects. Process Saf. Environ. Prot. 2021, 150, 323–355. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Pandey, J.; Verma, R.K.; Singh, S. Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: A review. Int. J. Phytoremediation 2019, 21, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Singh, K.; Gupta, A.K.; Pandey, V.C.; Trivedi, P.; Verma, R.K.; Patra, D.D. Aromatic grasses for phytomanagement of coal fly ash hazards. Ecol. Eng. 2014, 73, 425–428. [Google Scholar] [CrossRef]
- Galal, T.M.; Al-Yasi, H.M.; Fawzy, M.A.; Abdelkader, T.G.; Hamza, R.Z.; Eid, E.M.; Ali, E.F. Evaluation of the Phytochemical and Pharmacological Potential of Taif’s Rose (Rosa damascena Mill var. trigintipetala) for Possible Recycling of Pruning Wastes. Life 2022, 12, 273. [Google Scholar] [CrossRef]
- Nasir, M.H.; Nadeem, R.; Akhtar, K.; Hanif, M.A.; Khalid, A.M. Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for lead(II) and zinc(II) removal from aqueous solutions. J. Hazard. Mater. 2007, 147, 1006–1014. [Google Scholar] [CrossRef]
- Nunes, H.S.; Miguel, M.G. Rosa damascena essential oils: A brief review about chemical composition and biological properties. Trends in Phytochemical Research (TPR). Trends Phytochem. Res. 2017, 1, 111–128. [Google Scholar]
- Najem, W.; Beyrouthy, M.E.; Wakim, L.H.; Neema, C.; Ouaini, N. Essential oil composition of Rosa damascene Mill. from different localities in Lebanon. Acta Bot. Gall. 2011, 158, 365–373. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Esmat, F.; Elshazly, S.; Siddiqued, K.H.M.; Hessiniae, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shawl, A.S.; Adams, R. Rose Oil in Kashmiri India, an emerging cash crop benefiting industry and local agribusiness. Perfum. Flavorist 2009, 34, 22–25. [Google Scholar]
- Pal, P.K. Evaluation, Genetic Diversity, Recent Development of Distillation Method, Challenges and Opportunities of Rosa damascena: A Review. J. Essent. Oil Bear. Plants 2013, 16, 1–10. [Google Scholar] [CrossRef]
- Rabbani, D.; Mahmoudkashi, N.; Mehdizad, F.; Shaterian, M. Green approach to wastewater treatment by application of Rosa damascena waste as nano-biosorbent. J. Environ. Sci. Technol. 2016, 9, 121–130. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Maham, S.G.; Rahimi, A.; Smith, D.L. Environmental assessment of the essential oils produced from dragonhead (Dracocephalum moldavica L.) in conventional and organic farms with different irrigation rates. J. Clean. Prod. 2018, 204, 1070–1086. [Google Scholar] [CrossRef]
- Chipomho, J.; Rugare, J.T.; Mabasa, S.; Zingore, S. Short-term impacts of soil nutrient management on maize (Zea mays L.) productivity and weed dynamics along a toposequence in Eastern Zimbabwe. Heliyon 2020, 6, e05223. [Google Scholar] [CrossRef]
- Wu, C.; Wu, G.; Wang, Z.; Zhang, Z.; Qian, Y.; Ju, L. Soil mercury speciation and accumulation in rice (Oryza sativa L.) grown in wastewater-irrigated farms. Appl. Geochem. 2018, 89, 202–209. [Google Scholar] [CrossRef]
- Eid, E.M.; Khedher, K.M.; Ayed, H.; Arshad, M.; Mouldi, A.; Shaltout, K.H.; Sewelam, N.A.; Galal, T.M.; El-Bebany, A.F.; Alshehri, A.M.A. Prediction models based on soil properties for evaluating the heavy metal uptake into Hordeum vulgare L. grown in agricultural soils amended with different rates of sewage sludge. Int. J. Environ. Health Res. 2022, 32, 106–120. [Google Scholar] [CrossRef]
- Wortman, S.E.; Drijber, R.A.; Francis, C.A.; Lindquist, J.L. Arable weeds, cover crops, and tillage drive soil microbial community composition in organic cropping systems. Appl. Soil Ecol. 2013, 72, 232–241. [Google Scholar] [CrossRef]
- Abd Elsalam, H.E.; El-Sharnouby, M.E.; Mohamed, A.E.; Raafat, B.M. Effect of sewage sludge compost usage on corn and faba bean growth, carbon and nitrogen forms in plants and soil. Agronomy 2021, 11, 628. [Google Scholar] [CrossRef]
- Mansoora, N.; Kausar, S.; Amjad, S.F.; Yaseen, S.; Id, M.E.; Mustafa, G.; Ali, S.A.; Id, S.D. Application of sewage sludge combined with thiourea improves the growth and yield attributes of wheat (Triticum aestivum L.) genotypes under arsenic-contaminated soil. PLoS ONE 2021, 16, e0259289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, B.; Liu, H.; Zhao, Y.; Li, L. Effects of pyrolysis temperature on biochar’s characteristics and speciation and environmental risks of heavy metals in sewage sludge biochars. Environ. Technol. Innov. 2022, 26, 102288. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Zheng, Q.; Lang, Q.; Xia, Y.; Peng, N.; Gai, C. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis. Bioresour. Technol. 2018, 247, 282–290. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Kaundal, M.; Sharma, S.; Thakur, M. Response of damask rose (Rosa damascena Mill.) to foliar application of magnesium (Mg), copper (Cu) and zinc (Zn) sulphate under western Himalayas. Ind. Crops Prod. 2016, 83, 596–602. [Google Scholar] [CrossRef]
- Anser, M.K.; Hanif, I.; Vo, X.V.; Alharthi, M. The long-run and short-run influence of environmental pollution, energy consumption, and economic activities on health quality in emerging countries. Environ. Sci. Pollut. Res. 2020, 27, 32518–32532. [Google Scholar] [CrossRef] [PubMed]
- Tokay, B.; Akpınar, I. A comparative study of heavy metals removal using agricultural waste biosorbents. Bioresour. Technol. Rep. 2021, 15, 100719. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Al-Yasi, H.M.; Galal, T.M.; Hamza, R.Z.; Abdelkader, T.G.; Ali, E.F.; Hassan, S.H.A. Statistical optimization, kinetic, equilibrium isotherm and thermodynamic studies of copper biosorption onto Rosa damascena leaves as a low-cost biosorbent. Sci. Rep. 2022, 12, 8583. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: London, UK, 1989. [Google Scholar]
- Wade, T.L.; Brooks, J.M.; Kennicutt, M.C.; McDonald, T.J.; Sericano, J.L.; Jackson, T.L. GERG Trace Metals and Organic Contaminants Analytical Techniques. In Sampling and Analytical Methods of the National Status and Trend Program; National Benthic Surveillance and Mussel Watch Projects 1984–1992, 121–139; NOAA Technical Memorandum NOS ORCA 71; Lauenstein, G.G., Cantillo, A.Y., Eds.; NOAA: Silver Spring, MD, USA, 1993. [Google Scholar]
- SPSS. IBM SPSS Statistics Version 21.0; IBM: New York, NY, USA, 2012. [Google Scholar]
- Pal, P.K.; Singh, R.D. Understanding crop- ecology and agronomy of Rosa damascena Mill for higher productivity. Aust. J. Crop Sci. 2013, 7, 196–205. [Google Scholar]
- Brichet, H. Distribution and Ecology: Continental Asia and Japan. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Eid, E.M.; Shaltout, K.H. Bioaccumulation and translocation of heavy metals by nine native plant species grown at a sewage sludge dump site. Int. J. Phytoremed. 2016, 18, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M. Plant biomass in the semi-arid zone of India. J. Arid Environ. 1982, 5, 29–33. [Google Scholar] [CrossRef]
- Karlik, J.F.; Becker, J.O.; Pemberton, H.B.; Schuch, U.K. Production and Marketing: Field Rose production. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Ali, E.F.; Al-Yasi, H.M.; Hassan, F.A.S.; Alamer, K.H.; Hessini, K.; Attia, H.; El-Shazly, S. Effect of the pruning system and P-Fertilizer on growth and productivity of Rosa damascena mill. var. trigentipetala plant. Egypt. J. Bot. 2021, 61, 565–578. [Google Scholar] [CrossRef]
- Atay, O.A.; Ekinci, K. Characterization of pellets made from rose oil processing solid wastes/coal powder/pine bark. Renew. Energy 2020, 149, 933–939. [Google Scholar] [CrossRef]
- Galal, T.M.; Gharib, F.A.; Al-Yasi, H.M.; Al-Mutairi, K.A.; Mansour, K.H.; Eid, E.M. Nutrient Remediation Efficiency of the Sedge Plant (Cyperus alopecuroides Rottb.) to Restore Eutrophic Freshwater Ecosystems. Sustainability 2022, 14, 2823. [Google Scholar] [CrossRef]
- Misra, S.G.; Mani, D. Soil Pollution; Ashish Publishing House: Punjabi Bagh, India, 1991. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Chiroma, T.M.; Ebewele, R.O.; Hymore, F.K. Comparative assessment of heavy metal levels in soil, vegetables and urban grey waste water used for irrigation in Yola and Kano. Int. Refereed J. Eng. Sci. 2014, 3, 1–9. [Google Scholar]
- Hassan, F.A.S.; Ali, E.F.; Mahfouz, S.A. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of coriander plant. Aust. J. Basic Appl. Sci. 2012, 6, 600–615. [Google Scholar]
- Gharib, F.A.; Mansour, K.H.; Ahmed, E.Z.; Galal, T.M. Heavy metals concentration, and antioxidant activity of the essential oil of the wild mint (Mentha longifolia L.) in the Egyptian watercourses. Int. J. Phytoremediation 2021, 23, 641–651. [Google Scholar] [CrossRef]
- Raklami, A.; Oufdou, K.; Tahiri, A.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Meddich, A.; Redondo-Gómez, S.; Pajuelom, E. Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat- and metallo-resistant PGPR. Microorganisms 2019, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Long-term (two annual cycles) phytoremediation of heavy metal-contaminated estuarine sediments by Phragmites australis. New Biotechnol. 2017, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Shaltout, K.H.; Al-Sodany, Y.M.; Haroun, S.A.; Galal, T.M.; Ayed, H.; Khedher, K.M.; Jensen, K. Seasonal potential of Phragmites australis in nutrient removal to eliminate the eutrophication in Lake Burullus, Egypt. J. Freshw. Ecol. 2020, 35, 135–155. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in constructed wetlands for wastewater treatment through plant harvesting—Biomass and load matter the most. Ecol. Eng. 2020, 155, 105962. [Google Scholar] [CrossRef]
- Galal, T.M.; Hassan, L.M.; Ahmed, D.A.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M. Heavy metals uptake by the global economic crop (Pisum sativum L.) grown in contaminated soils and its associated health risks. PLoS ONE 2021, 16, e0252229. [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; Galal, T.M.; El-Bebany, A.F. Regression models for monitoring trace metal accumulations by Faba sativa Bernh. plants grown in soils amended with different rates of sewage sludge. Sci. Rep. 2019, 9, 5443. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Evaluation of the invasive macrophyte Myriophyllum spicatum L. as a bioaccumulator for heavy metals in some watercourses of Egypt. Ecol. Indic. 2014, 41, 209–214. [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Fawy, K.F.; Taher, M.A.; Hesham, A.; El-Shaboury, G.A.; Ahmed, M.T. Evaluation of the potential of sewage slude as a valuable fertilizer for wheat (Triticum aestivum L.) crops. Environ. Sci. Pollut. Res. 2019, 26, 392–401. [Google Scholar] [CrossRef]
- Jung, M.C. Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu-W mine. Sensors 2008, 8, 2413–2423. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; El-Bebany, A.F. Prediction models for monitoring heavy metals accumulation by wheat (Triticum aestivum L.) plants grown in soil amended with different rates of sewage sludge. Int. J. Phytoremediation 2020, 22, 1000–1008. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Galal, T.M.; Gharib, F.A.; Ghazi, S.M.; Mansour, K.H. Metal uptake capability of Cyperus articulatus L. and its role in mitigating heavy metals from contaminated wetlands. Environ. Sci. Pollut. Res. 2017, 24, 21636–21648. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Astatkie, T. Effect of residual distillation water of 15 plants and three plant hormones on Scotch spearmint (Mentha × gracilis Sole). Ind. Crop Prod. 2011, 33, 704–709. [Google Scholar] [CrossRef]
- Siddiqui, F.; Krishna, S.K.; Tandon, P.K.; Srivastava, S. Arsenic accumulation in Ocimum spp. and its effect on growth and oil constituents. Acta Physiol. Plant. 2013, 35, 1071–1079. [Google Scholar] [CrossRef]
- Kunwar, G.; Pande, C.; Tewari, G.; Singh, C.; Kharkwal, G.C. Effect of heavy metals on terpenoid composition of Ocimum basilicum L. and Mentha spicata L. J. Essent. Oil Bear Plants 2015, 18, 818–825. [Google Scholar] [CrossRef]
- Parra, A.; Zornoza, R.; Conesa, E.; Gómez-López, M.D.; Faz, A. Seedling emergence, growth and trace elements tolerance and accumulation by Lamiaceae species in a mine soil. Chemosphere 2014, 113, 132–140. [Google Scholar] [CrossRef]
- Chand, S.; Pandey, A.; Patra, D.D. Influence of nickel and lead applied in combination with vermicompost on growth and accumulation of heavy metals by Mentha arvensis Linn. cv.‘Kosi’. Indian J. Nat. Prod. Resour. 2012, 3, 256–261. [Google Scholar]
- Zheljazkov, V.D.; Nielsen, N.E. Effect of heavy metals on peppermint and cornmint. Plant Soil. 1996, 178, 59–66. [Google Scholar] [CrossRef]
- Onursal, E.; Ekinci, K. Co-composting of rose oil processing waste with caged layer manure and straw or sawdust: Effects of carbon source and C/N ratio on decomposition. Waste Manag. Res. 2015, 33, 332–338. [Google Scholar] [CrossRef]
- Schnoor, J.L.; Licht, L.A.; McCutcheon, S.C.; Wolfe, N.L.; Carreira, L.H. Phytoremediation of organic and nutrient contaminants. Environ. Sci. Technol. 1995, 29, 318A–323A. [Google Scholar] [CrossRef]
- Meagher, R.B. Phytoremediation of toxic elemental and organic pollutants. Curr. Opin. Plant Biol. 2000, 3, 153–162. [Google Scholar] [CrossRef]
| Metal | Factor | Farm Age (Years) | F-Value | |||
|---|---|---|---|---|---|---|
| 4 | 10 | 12 | 20 | |||
| Al | BAFstem | 1.16 ± 0.06a | 2.27 ± 0.47a | 1.11 ± 0.30a | 2.82 ± 0.88a | 2.6 ns |
| BAFleaf | 3.98 ± 0.18a | 5.13 ± 1.05a | 3.12 ± 0.79a | 30.40 ± 9.57b | 7.5 ** | |
| Cd | BAFstem | 31.47 ± 1.06b | 13.41 ± 1.94a | 23.02 ± 4.30b | 11.33 ± 0.97a | 14.2 *** |
| BAFleaf | 30.58 ± 0.37c | 12.74 ± 2.17b | 11.45 ± 1.69b | 3.42 ± 0.29a | 67.4 *** | |
| Co | BAFstem | 7.24 ± 1.28c | 6.00 ± 1.40bc | 3.23 ± 0.41ab | 1.39 ± 0.14a | 7.4 ** |
| BAFleaf | 0.73 ± 0.05a | 3.06 ± 0.76bc | 4.93 ± 0.92c | 1.89 ± 0.19ab | 8.7 ** | |
| Cr | BAFstem | 0.86 ± 0.03a | 0.58 ± 0.02a | 6.88 ± 1.12b | 0.47 ± 0.04a | 31.0 *** |
| BAFleaf | 1.32 ± 0.17a | 0.74 ± 0.01a | 4.65 ± 0.77b | 0.27 ± 0.03a | 25.4 *** | |
| Cu | BAFstem | 27.49 ± 4.22bc | 11.69 ± 1.44a | 19.25 ± 2.16ab | 36.93 ± 4.53c | 10.5 *** |
| BAFleaf | 48.59 ± 10.39b | 13.97 ± 1.89a | 44.94 ± 2.35b | 33.27 ± 3.63ab | 7.5 ** | |
| Fe | BAFstem | 18.93 ± 1.43a | 7.26 ± 0.30a | 90.46 ± 6.77b | 175.64 ± 15.37c | 84.8 *** |
| BAFleaf | 21.15 ± 4.76a | 19.39 ± 2.48a | 61.34 ± 3.52b | 129.71 ± 7.70c | 106.2 *** | |
| Mn | BAFstem | 6.61 ± 0.43a | 6.18 ± 0.20a | 60.82 ± 2.62b | 60.60 ± 3.30b | 218.2 *** |
| BAFleaf | 9.54 ± 0.46a | 28.11 ± 1.59b | 63.81 ± 3.12d | 37.51 ± 1.58c | 136.5 *** | |
| Ni | BAFstem | 3.57 ± 0.14ab | 1.91 ± 0.37a | 20.14 ± 2.13c | 6.41 ± 0.86b | 50.9 *** |
| BAFleaf | 4.81 ± 0.25a | 14.92 ± 2.14b | 6.82 ± 0.91a | 27.52 ± 2.54c | 35.6 *** | |
| Pb | BAFstem | 43.36 ± 12.61b | 6.46 ± 0.25a | 11.22 ± 1.52a | 6.78 ± 0.69a | 7.8 ** |
| BAFleaf | 68.99 ± 18.43b | 5.43 ± 0.29a | 9.67 ± 1.20a | 5.52 ± 0.55a | 11.3 *** | |
| Zn | BAFstem | 206.81 ± 41.54b | 4.17 ± 0.40a | 94.38 ± 34.32a | 7.83 ± 0.40a | 12.5 *** |
| BAFleaf | 279.50 ± 63.27b | 9.16 ± 0.93a | 126.40 ± 44.41a | 9.07 ± 0.41a | 11.0 *** | |
| F-valueBAFstem | 20.3 *** | 19.9 *** | 10.0 *** | 108.9 *** | ||
| F-valueBAFleaf | 16.1 *** | 28.9 *** | 8.2 *** | 85.7 *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal, T.M.; Majrashi, A.; Al-Yasi, H.M.; Farahat, E.A.; Eid, E.M.; Ali, E.F. Taif’s Rose (Rosa damascena Mill var. trigentipetala) Wastes Are a Potential Candidate for Heavy Metals Remediation from Agricultural Soil. Agriculture 2022, 12, 1319. https://doi.org/10.3390/agriculture12091319
Galal TM, Majrashi A, Al-Yasi HM, Farahat EA, Eid EM, Ali EF. Taif’s Rose (Rosa damascena Mill var. trigentipetala) Wastes Are a Potential Candidate for Heavy Metals Remediation from Agricultural Soil. Agriculture. 2022; 12(9):1319. https://doi.org/10.3390/agriculture12091319
Chicago/Turabian StyleGalal, Tarek M., Ali Majrashi, Hatim M. Al-Yasi, Emad A. Farahat, Ebrahem M. Eid, and Esmat F. Ali. 2022. "Taif’s Rose (Rosa damascena Mill var. trigentipetala) Wastes Are a Potential Candidate for Heavy Metals Remediation from Agricultural Soil" Agriculture 12, no. 9: 1319. https://doi.org/10.3390/agriculture12091319






