Measurement of Overlapping Leaf Area of Ice Plants Using Digital Image Processing Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
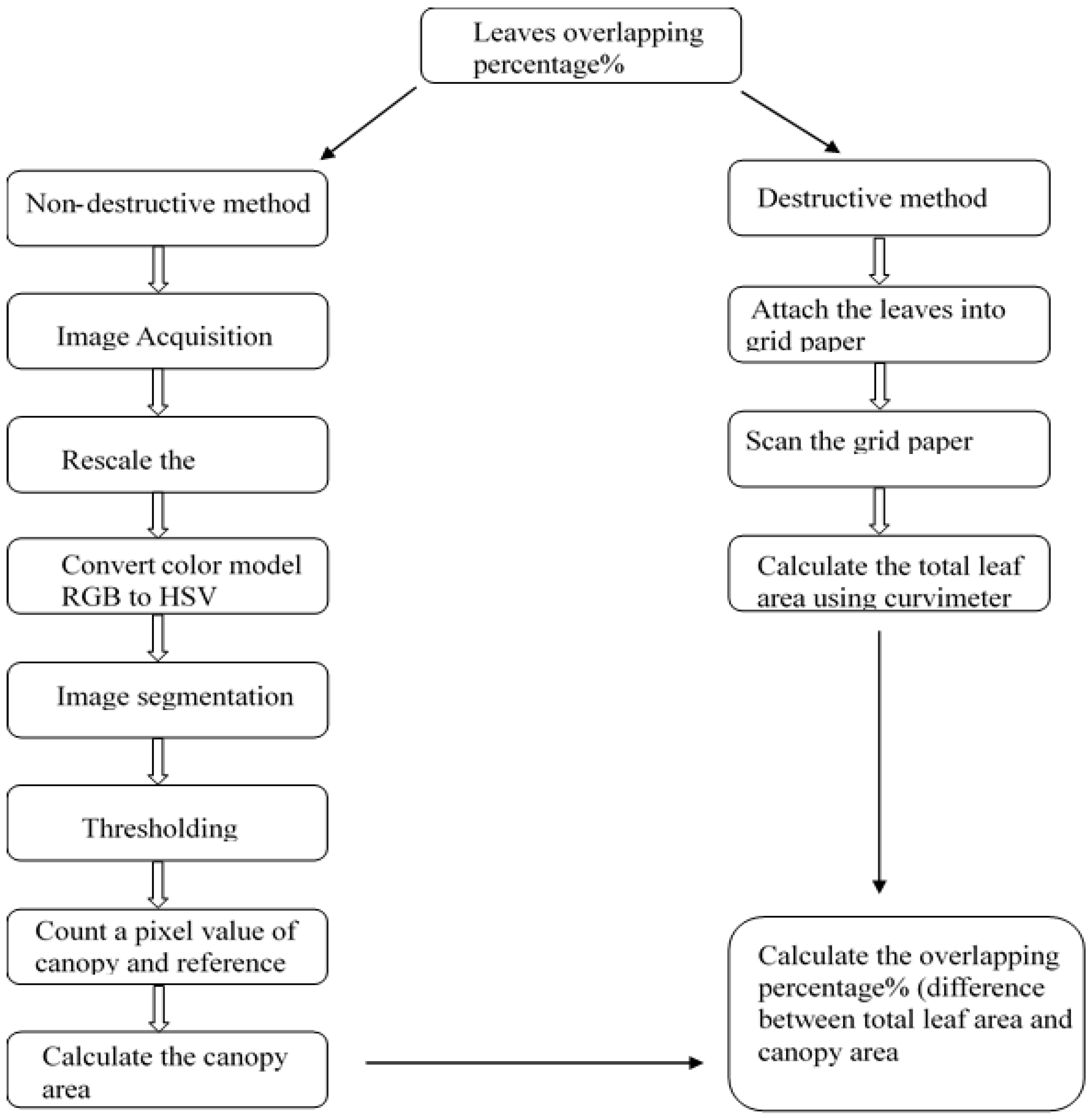
2.2. Calculation of Canopy Area and Total Leaf Area
2.3. Measurement of the Canopy Area
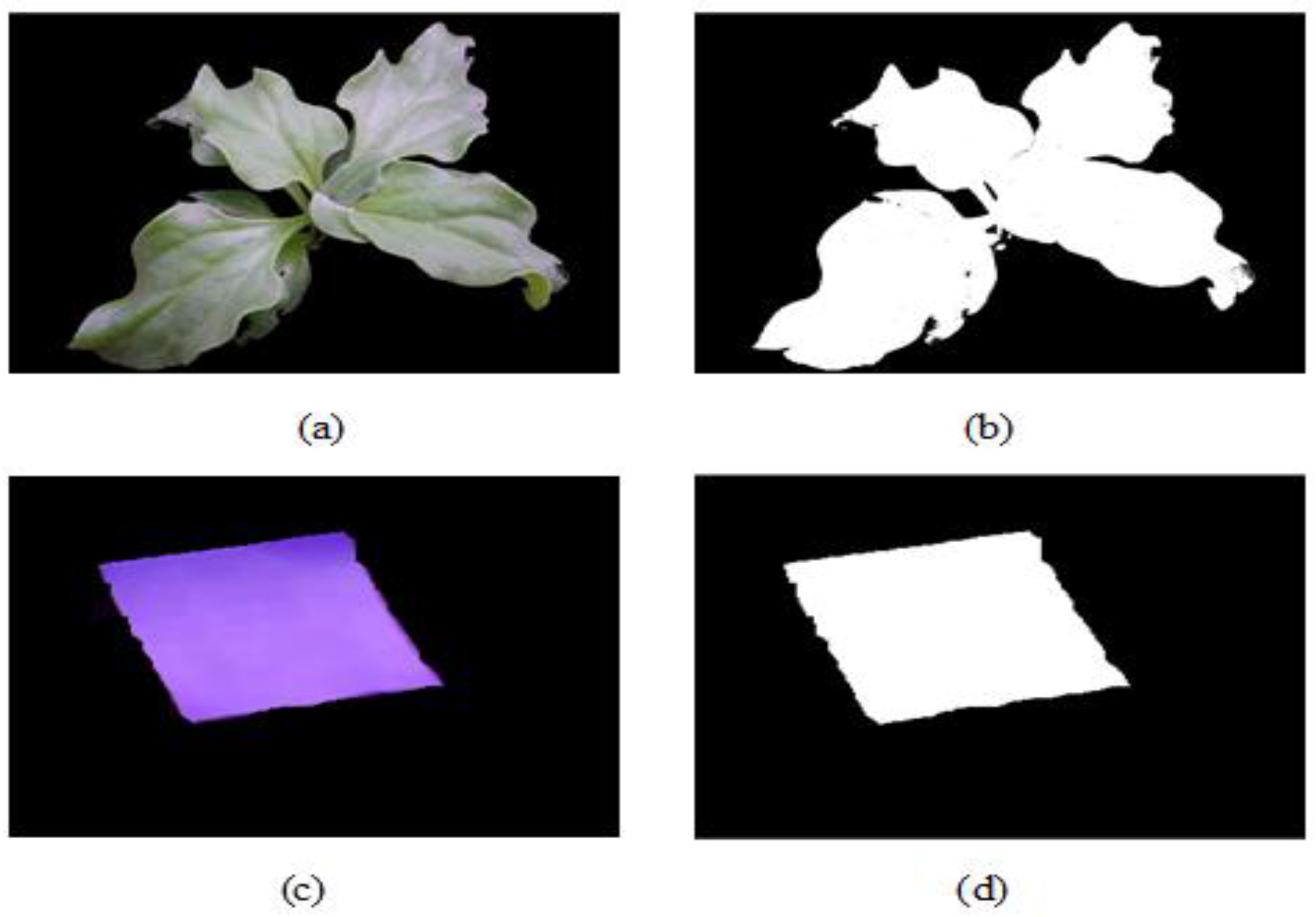
2.3.1. Image Acquisition
- Image colour transform
0 → of the opposite
- b.
- Image segmentation
2.3.2. Canopy Area Calculation
2.4. Total Leaf Area Calculation
2.5. Validation Procedure
3. Results and Discussion
3.1. Calculation of Canopy Area and Total Leaf Area
3.2. Measurement of the Canopy Area
3.3. Total Leaf Area Calculation
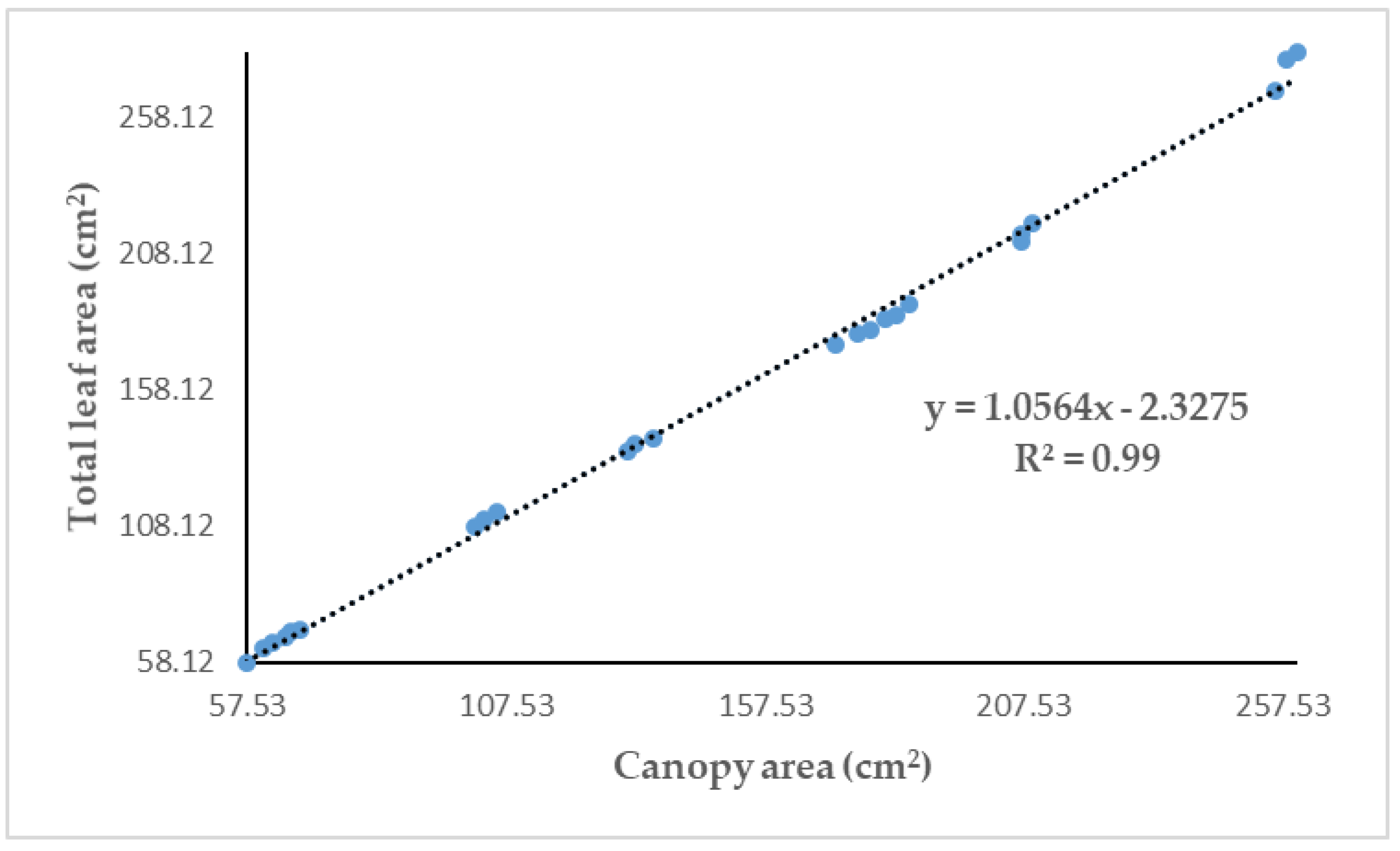
3.4. Validation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carvalho, J.O.; Toebe, M.; Tartaglia, F.L.; Bandeira, C.T.; Tambara, A.L. Leaf area estimation from linear measurements in different ages of Crotalaria juncea plants. An. Acad. Bras. Ciências 2017, 89, 1851–1868. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.J.; Yanagiwara, R.S.; Villas Bôas, R.L.; Backes, C.; Lima, C.P. Análise da imagem digital para estimativa da área foliar em plantas de laranja "Pêra". Rev. Bras. Frutic. 2007, 29, 420–424. [Google Scholar] [CrossRef]
- Madhavi, B.G.K.; Khan, F.; Bhujel, A.; Jaihuni, M.; Kim, N.E.; Moon, B.E.; Kim, H.T. Influence of different growing media on the growth and development of strawberry plants. Heliyon 2021, 7, e07170. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Godara, S.; Cheeran, A.N.; Chaudhari, A.K. Fast and accurate method for leaf area measurement. Int. J. Comput. Appl. 2012, 49, 22–25. [Google Scholar] [CrossRef]
- Li, Z.; Ji, C.; Liu, J. Leaf Area Calculating Based on Digital Image. In International Conference on Computer and Computing Technologies in Agriculture; Springer: Boston, MA, USA, 2007; pp. 1427–1433. [Google Scholar]
- Dey, A.K.; Guha, P.; Sharma, M.; Meshram, M.R. Comparison of different methods of in-situ leaf area measurement of betel leaf (Piper betle L.). Int. J. Recent Technol. Eng. 2019, 7, 1512–1516. [Google Scholar]
- Montgomery, E.G. Correlation studies in corn. Neb. Agric. Exp. Stn. Annu. Rep. 1911, 24, 108–159. [Google Scholar]
- Yan, G.; Hu, R.; Luo, J.; Weiss, M.; Jiang, H.; Mu, X.; Xie, D.; Zhang, W. Review of indirect optical measurements of leaf area index: Recent advances, challenges, and perspectives. Agric. Meteorol. 2019, 265, 390–411. [Google Scholar] [CrossRef]
- Ryu, Y.; Sonnentag, O.; Nilson, T.; Vargas, R.; Kobayashi, H.; Wenk, R.; Baldocchi, D.D. How to quantify tree leaf area index in an open savanna ecosystem: A multi-instrument and multi-model approach. Agric. Meteorol. 2010, 150, 63–76. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tanaka, Y.; Imachi, Y.; Yamashita, M.; Katsura, K. Feasibility of combining deep learning and rgb images obtained by unmanned aerial vehicle for leaf area index estimation in rice. Remote Sens. 2020, 13, 84. [Google Scholar] [CrossRef]
- Lu, C.; Ren, H.; Zhang, Y.; Shen, Y. Leaf area measurement based on image processing. In Proceedings of the 2010 International Conference on Measuring Technology and Mechatronics Automation, Changsha, China, 13–14 March 2010; Volume 2, pp. 580–582. [Google Scholar]
- Wang, Z.; Wang, K.; Yang, F.; Pan, S.; Han, Y. Image segmentation of overlapping leaves based on Chan–Vese model and Sobel operator. Inf. Processing Agric. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Han, Y.; Ouyang, C.; Li, D. An adaptive thresholding algorithm of field leaf image. Comput. Electron. Agric. 2013, 96, 23–39. [Google Scholar] [CrossRef]
- Cerutti, G.; Tougne, L.; Mille, J.; Vacavant, A.; Coquin, D. Understanding leaves in natural images—A model-based approach for tree species identification. Comput. Vis. Image Underst. 2013, 117, 1482–1501. [Google Scholar] [CrossRef]
- Grand-Brochier, M.; Vacavant, A.; Cerutti, G.; Kurtz, C.; Weber, J.; Tougne, L. Tree leaves extraction in natural images: Comparative study of preprocessing tools and segmentation methods. IEEE Trans. Image Processing 2015, 24, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Sandino, J.D.; Ramos-Sandoval, O.L.; Amaya-Hurtado, D. Method for estimating leaf coverage in strawberry plants using digital image processing. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 716–721. [Google Scholar] [CrossRef][Green Version]
- Apolo-Apolo, O.E.; Pérez-Ruiz, M.; Martínez-Guanter, J.; Egea, G. A mixed data-based deep neural network to estimate leaf area index in wheat breeding trials. Agronomy 2020, 10, 175. [Google Scholar] [CrossRef]
- Islam, S.; Reza, M.N.; Chowdhury, M.; Islam, M.N.; Ali, M.; Kiraga, S.; Chung, S.O. Image Processing Algorithm to Estimate Ice-Plant Leaf Area from RGB Images under Different Light Conditions. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 12013. [Google Scholar]
- Basak, J.K.; Qasim, W.; Okyere, F.G.; Khan, F.; Lee, Y.J.; Park, J.; Kim, H.T. Mitigating chilling stress of pepper plant (Capsicum annuum L.) using Dimethyl Ether combustion gas in controlled greenhouse. Aust. J. Crop Sci. 2019, 13, 1992–2002. [Google Scholar] [CrossRef]
- Madhavi, B.G.; Basak, J.K.; Paudel, B.; Kim, N.E.; Choi, G.M.; Kim, H.T. Prediction of Strawberry Leaf Color Using RGB Mean Values Based on Soil Physicochemical Parameters Using Machine Learning Models. Agronomy 2022, 12, 981. [Google Scholar] [CrossRef]
- Primer, S.; Molano-Flores, B.; Zaya, D.N.; Helm, C.; Coons, J. Effect of habitat structure on reproduction and prey capture of a rare carnivorous plant, Pinguicula lutea. Arthropod. Plant Interact. 2018, 12, 671–683. [Google Scholar] [CrossRef]
- Wu, X.; Xu, W.; Song, Y.; Cai, M. A detection method of weed in wheat field on machine vision. Procedia Eng. 2011, 15, 1998–2003. [Google Scholar] [CrossRef]
- Li, D.; Cao, Y.; Shi, G.; Cai, X.; Chen, Y.; Wang, S.; Yan, S. An overlapping-free leaf segmentation method for plant point clouds. IEEE Access 2019, 7, 129054–129070. [Google Scholar] [CrossRef]
- Damgaard, C. Hierarchical and spatially aggregated plant cover data. Ecol. Inform. 2013, 18, 35–39. [Google Scholar] [CrossRef]
- Demirsoy, H. Leaf area estimation in some species of fruit tree by using models as a non-destructive method. Fruits 2009, 64, 45–51. [Google Scholar] [CrossRef]
- Singh, M.C.; Singh, K.G.; Singh, J.P. Indirect method for measurement of leaf area and leaf area index of soilless cucumber crop. Adv. Plants Agric. Res. 2018, 8, 188–191. [Google Scholar] [CrossRef]
- Posse, R.P.; Sousa, E.F.; Bernardo, S.; Pereira, M.G.; Gottardo, R.D. Total leaf area of papaya trees estimated by a nondestructive method. Sci. Agric. 2009, 66, 462–466. [Google Scholar] [CrossRef]
- Raj, R.; Walker, J.P.; Pingale, R.; Nandan, R.; Naik, B.; Jagarlapudi, A. Leaf area index estimation using top-of-canopy airborne RGB images. Int. J. Appl. Earth Obs. Geoinf. 2021, 96, 102282. [Google Scholar] [CrossRef]



| Image | CA (cm2) | TA (cm2) | Overlapping Leaves Percentage (%) |
|---|---|---|---|
| 1 | 57.53 | 58.12 | 1.02 |
| 2 | 60.62 | 63.15 | 4.01 |
| 3 | 62.63 | 65.17 | 3.90 |
| 4 | 65 | 67.58 | 3.82 |
| 5 | 66.25 | 69.17 | 4.22 |
| 6 | 67.84 | 70.15 | 3.41 |
| 7 | 101.75 | 107.73 | 5.55 |
| 8 | 103.45 | 110.60 | 6.46 |
| 9 | 105.85 | 112.90 | 6.24 |
| 10 | 130.91 | 135.15 | 3.13 |
| 11 | 132.56 | 137.97 | 3.92 |
| 12 | 135.86 | 139.98 | 2.94 |
| 13 | 171.07 | 174.56 | 2.00 |
| 14 | 175.52 | 178.87 | 1.87 |
| 15 | 177.76 | 180.32 | 1.42 |
| 16 | 180.56 | 184.11 | 1.93 |
| 17 | 182.97 | 185.11 | 1.16 |
| 18 | 185.50 | 189.70 | 2.21 |
| 19 | 206.95 | 212.15 | 2.45 |
| 20 | 207.15 | 215.12 | 3.70 |
| 21 | 209.13 | 219.17 | 4.58 |
| 22 | 255.96 | 267.95 | 4.47 |
| 23 | 258.16 | 279.17 | 7.53 |
| 24 | 260.13 | 282.18 | 7.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushalya Madhavi, B.G.; Bhujel, A.; Kim, N.E.; Kim, H.T. Measurement of Overlapping Leaf Area of Ice Plants Using Digital Image Processing Technique. Agriculture 2022, 12, 1321. https://doi.org/10.3390/agriculture12091321
Kaushalya Madhavi BG, Bhujel A, Kim NE, Kim HT. Measurement of Overlapping Leaf Area of Ice Plants Using Digital Image Processing Technique. Agriculture. 2022; 12(9):1321. https://doi.org/10.3390/agriculture12091321
Chicago/Turabian StyleKaushalya Madhavi, Bolappa Gamage, Anil Bhujel, Na Eun Kim, and Hyeon Tae Kim. 2022. "Measurement of Overlapping Leaf Area of Ice Plants Using Digital Image Processing Technique" Agriculture 12, no. 9: 1321. https://doi.org/10.3390/agriculture12091321
APA StyleKaushalya Madhavi, B. G., Bhujel, A., Kim, N. E., & Kim, H. T. (2022). Measurement of Overlapping Leaf Area of Ice Plants Using Digital Image Processing Technique. Agriculture, 12(9), 1321. https://doi.org/10.3390/agriculture12091321








