Effect of Shade Screen on Sap Flow, Chlorophyll Fluorescence, NDVI, Plant Growth and Fruit Characteristics of Cultivated Paprika in Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Treatment
2.3. Sap Flow Measurement
2.4. Chlorophyll Fluorescence Measurements
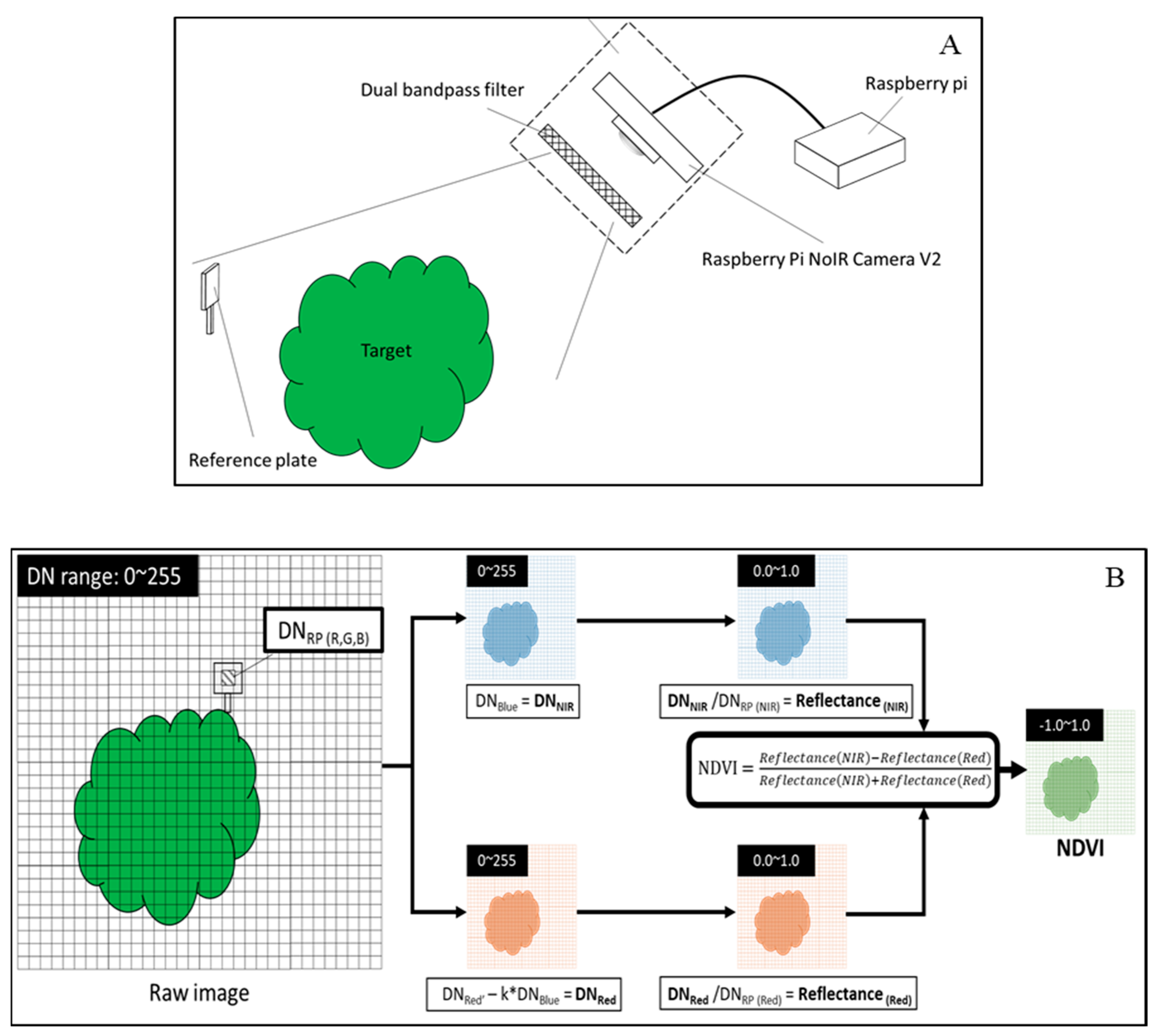
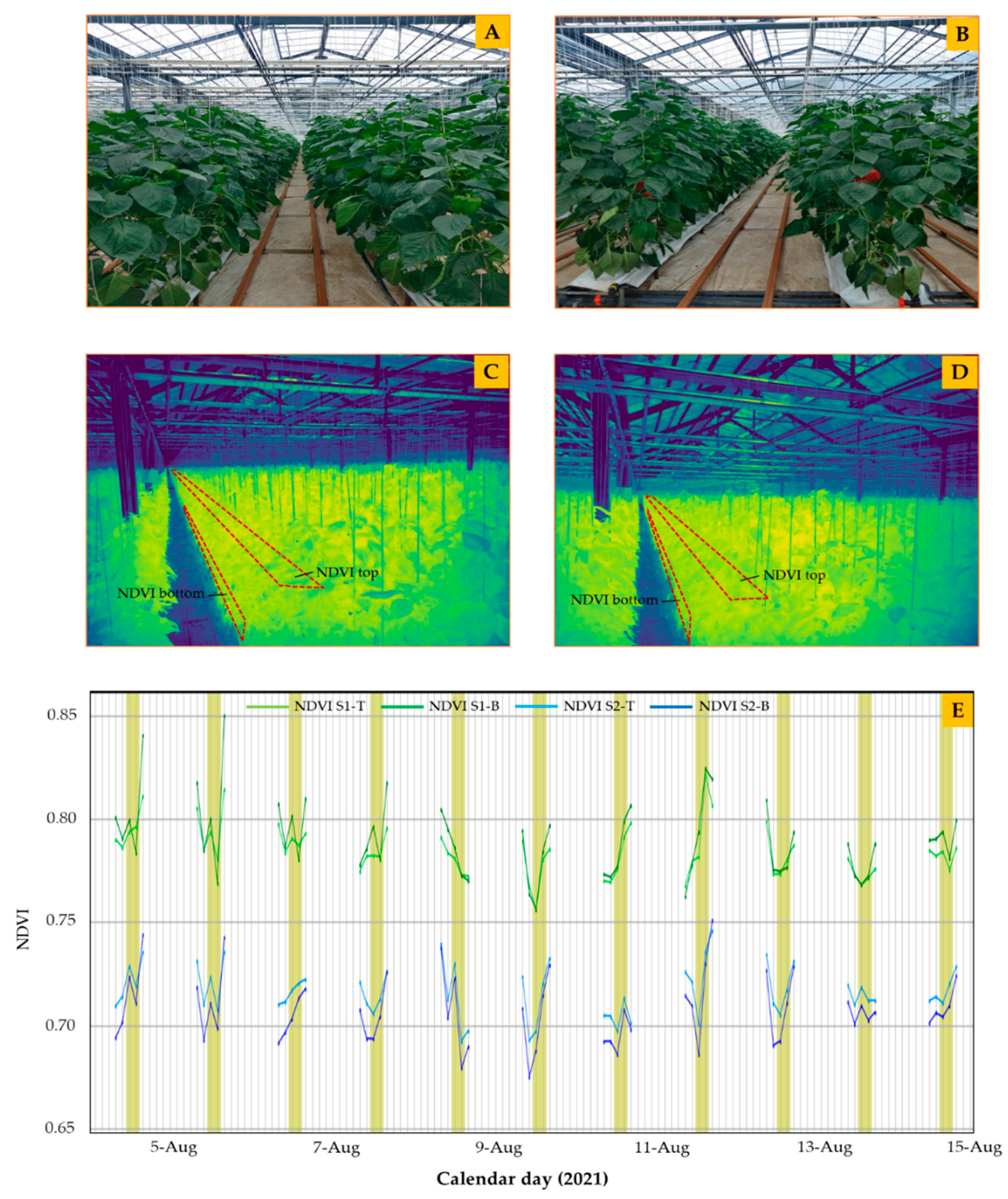
2.5. Measurement of NDVI
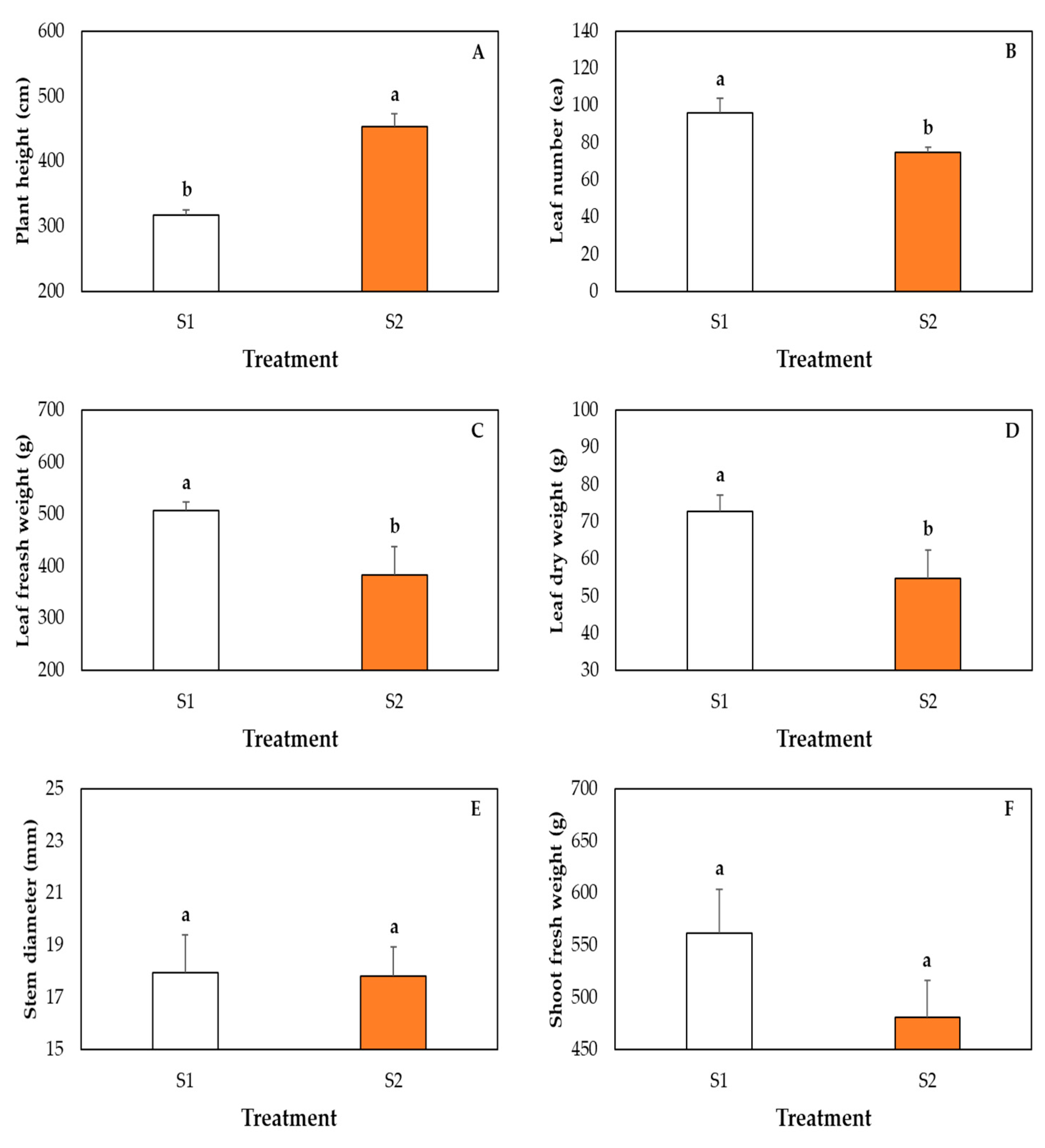
2.6. Measurement of Plant Growth Parameters
2.7. Measurement of Fruit Growth Parameters
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; An, C.G.; Park, J.S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chem. 2016, 201, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.W.; Choi, S.I.; Han, X.; Men, X.; Kwon, H.Y.; Choi, Y.E.; Park, M.H.; Lee, O.H. Method validation and measurement uncertainty determination of ethoxyquin and antioxidant activity in paprika seasonings and paprika sauces frequently consumed in South Korea. Separations 2020, 7, 50. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Moon, B. Changes in carotenoids, ascorbic acids, and quality characteristics by the pickling of paprika (Capsicum annuum L.) cultivated in Korea. J. Food Sci. 2011, 76, 1075–1080. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N.; Fujiwara, Y.; Hashimoto, K. Structure of new carotenoids with the 6-Oxo-κ end group from the fruits of paprika, Capsicum annuum. J. Nat. Prod. 2004, 67, 115–117. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.; Lee, S.J.; Moon, B.; Ha, T.Y.; Kim, S. Phytochemicals and antioxidant activity of fruits and leaves of paprika (Capsicum annuum L., var. Special) cultivated in Korea. J. Food Sci. 2011, 76, 193–198. [Google Scholar]
- Liu, X.; Fan, Y.; Chang, C.P.; Lo, C.K.; Wang, X. High-throughput screening and quantification of pesticides in paprika by uhplc-q-tof/ms. Food Anal. Methods. 2021, 14, 2186–2198. [Google Scholar] [CrossRef]
- Go, S.M.; Park, M.R.; Kim, H.S.; Choi, W.S.; Jeong, R.D. Antifungal effect of non-thermal atmospheric plasma and its application for control of postharvest Fusarium oxysporum decay of paprika. Food Control 2019, 98, 245–252. [Google Scholar] [CrossRef]
- Jeong, E.M.; Kim, W.T.; Kim, S.R.; Yun, S.H. The Actual Condition and Subjects of Paprika in Korea; Munwonsa: Seoul, Korea, 2008; pp. 2008–2022. [Google Scholar]
- Moon, H.; Seok, J.H.; Lee, S.; Reed, M.R. Who has price leadership in paprika trade between Korea and Japan? evidence from threshold vector autoregressive model approach. Singap. Econ. Rev. 2022, 1–18. [Google Scholar] [CrossRef]
- Lee, J.W.; Shin, C.; Yoe, H. An implementation of paprika greenhouse system using wireless sensor networks. Int. J. Smart Home 2010, 4, 57–68. [Google Scholar]
- Yamada, Y.; Nakayama, M.; Shibata, H.; Kishimoto, S.; Ikeda, T. Anthocyanin production and enzymatic degradation during the development of dark purple and lilac paprika fruit. J. Am. Soc. Hortic. Sci. 2019, 144, 329–338. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, K.S.; Goo, H.W.; Park, G.E.; Myung, D.J.; Jeon, Y.H.; Na, H. Effect of cooling in a semi-closed greenhouse at high temperature on the growth and photosynthesis characteristics in paprika. J. Bio-Environ. Control 2021, 30, 335–341. [Google Scholar] [CrossRef]
- Raju, S.; Shah, S.; Gajbhiye, N. Effect of light intensity on photosynthesis and accumulation of sennosides in plant parts of senna (Cassia angustifolia Vahl.). Indian J. Plant Physiol. 2013, 18, 285–289. [Google Scholar] [CrossRef]
- De Wit, M.; Galvao, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Yang, X.; Xiao, J.; Zhang, H.; Zhang, Z.; Wang, Y.; Jiang, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria × ananassa) fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef]
- Cruz-Tejada, D.M.; Acosta-Rojas, D.C.; Stevenson, P.R. Are seeds able to germinate before fruit color ripening? Evidence from six Neotropical bird-dispersed plant species. Ecosphere 2018, 9, e02174. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef]
- Zavala, J.A.; Ravetta, D.A. Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crop. Res. 2001, 69, 143–149. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Krupinska, K.; Shimakawa, G. The impact of photosynthesis on initiation of leaf senescence. Physiol. Plant. 2019, 166, 148–164. [Google Scholar] [CrossRef]
- Venturin, A.Z.; Guimarães, C.M.; de Sousa, E.F.; Machado Filho, J.A.; Rodrigues, W.P.; de Araujo Serrazine, Í.; Bressan-Smith, R.; Marciano, C.R.; Campostrini, E. Using a crop water stress index based on a sap flow method to estimate water status in conilon coffee plants. Agric. Water Manag. 2020, 241, 106343. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-based protein hydrolysate improves salinity tolerance in hemp: Agronomical and physiological aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Beisel, N.S.; Callaham, J.B.; Sng, N.J.; Taylor, D.J.; Paul, A.L.; Ferl, R.J. Utilization of single-image normalized difference vegetation index (SI-NDVI) for early plant stress detection. Appl. Plant Sci. 2018, 6, e01186. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Shin, J.H.; Ahn, T.I.; Son, J.E. Modeling of transpiration of paprika (Capsicum annuum L.) plants based on radiation and leaf area index in soilless culture. Hortic. Environ. Biotechnol. 2011, 52, 265–269. [Google Scholar]
- Park, K.S.; Kwon, D.Y.; Lee, J.W.; Son, J.E. Comparing photosynthesis, growth, and yield of paprika (Capsicum annuum L. ‘Cupra’) under supplemental sulfur plasma and high-pressure sodium lamps in growth chambers and greenhouses. Prot. Hortic. Plant Fact. 2018, 27, 332–340. [Google Scholar] [CrossRef]
- Nam, D.S.; Moon, T.; Lee, J.W.; Son, J.E. Estimating transpiration rates of hydroponically-grown paprika via an artificial neural network using aerial and root-zone environments and growth factors in greenhouses. Hortic. Environ. Biotechnol. 2019, 60, 913–923. [Google Scholar] [CrossRef]
- Somnuek, S.; Hong, Y.; Kim, M.; Lee, S.; Baek, J.; Kwak, K.; Lee, H.; Lee, J. Assessment of water control model for tomato and paprika in the greenhouse using the penman-monteith model. Prot. Hortic. Plant Fact. 2020, 29, 209–218. [Google Scholar] [CrossRef]
- Aloni, B.; Karni, L.; Zaidman, Z.; Schaffer, A.A. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann. Bot. 1996, 78, 163–168. [Google Scholar] [CrossRef]
- Jung, D.I.; Farha, W.; AM, A.E.A.; Kim, S.W.; Rahman, M.; Choi, J.H.; Kabir, M.; Im, S.J.; Lee, Y.J.; Truong, L.; et al. Effects of light shading and climatic conditions on the metabolic behavior of flonicamid in red bell pepper. Environ. Monit. Assess. 2016, 188, 1–9. [Google Scholar] [CrossRef]
- Stirbet, A. On the relation between the kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Nadezhdina, N. Sap flow index as an indicator of plant water status. Tree Physiol. 1999, 19, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C.O. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81–89. [Google Scholar] [CrossRef]
- Strachan, S. Precipitation and conifer response in semiarid mountains: A case from the 2012–2015 drought in the Great Basin, USA. Dev. Earth Surf. Process. 2016, 21, 193–238. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Rossini, M.; Nedbal, L.; Guanter, L.; Ač, A.; Alonso, L.; Burkart, A.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M.; et al. Red and far red sun-induced chlorophyll fluorescence as a measure of plant photosynthesis. Geophys. Res. Lett. 2015, 42, 1632–1639. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Lin, M.J.; Hsu, B.D. Photosynthetic plasticity of Phalaenopsis in response to different light environments. J. Plant Physiol. 2004, 161, 1259–1268. [Google Scholar] [CrossRef]
- Tucker, R.; Callaham, J.A.; Zeidler, C.; Paul, A.L.; Ferl, R.J. NDVI imaging within space exploration plant growth modules—A case study from EDEN ISS Antarctica. Life Sci. Space Res. 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Calera, A.; Martínez, C.; Meliá, J. A procedure for obtaining green plant cover: Relation to NDVI in a case study for barley. Int. J. Remote Sens. 2001, 22, 3357–3362. [Google Scholar]
- Fiorani, F.; Rascher, U.; Jahnke, S.; Schurr, U. Imaging plants dynamics in heterogenic environments. Curr. Opin. Biotechnol. 2012, 23, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.B.; Lim, C.S.; Kang, H.Y.; Kang, Y.S.; Hwang, S.J.; Mun, H.S.; An, C.G. Effect of shading methods on growth and fruit quality of paprika in summer season. J. Bio-Environ. Control 2012, 21, 419–427. [Google Scholar] [CrossRef]
- Rylski, I.; Spigelman, M. Effect of shading on plant development, yield and fruit quality of sweet pepper grown under conditions of high temperature and radlation. Sci. Hortic. 1986, 29, 31–35. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Microenvironment, plant growth, leaf gas exchange, and leaf mineral nutrient concentration. HortScience 2013, 48, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Gálvez, A.; Albacete, A.; del Amor, F.M.; López-Marín, J. The Use of red shade nets improves growth in salinized pepper (Capsicum annuum L.) plants by regulating their ion homeostasis and hormone balance. Agronomy 2020, 10, 1766. [Google Scholar] [CrossRef]
- Kesumawati, E.; Apriyatna, D.; Rahmawati, M. The effect of shading levels and varieties on the growth and yield of chili plants (Capsicum annuum L.). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changchun, China, 21–23 August 2020; IOP Publishing: Bristol, UK, 2020; Volume 425, p. 012080. [Google Scholar]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Tinyane, P.P.; Sivakumar, D.; Soundy, P. Influence of photo-selective netting on fruit quality parameters and bioactive compounds in selected tomato cultivars. Sci. Hortic. 2013, 161, 340–349. [Google Scholar] [CrossRef]
- Song, U.; Mun, S.; Ho, C.H.; Lee, E.J. Responses of two invasive plants under various microclimate conditions in the Seoul metropolitan region. Environ Manag. 2012, 49, 1238–1246. [Google Scholar] [CrossRef]
- Shawon, R.A.; Ha, S.Y.; Lee, T.H.; Cao, T.L.; Kim, H.C.; Bae, J.H.; Ku, Y.G. Influence of shade treatment on plant growth characteristics and spear production in five asparagus (Asparagus officinalis L.) Cultivars. Hortic. Sci. Technol. 2021, 39, 37–48. [Google Scholar]
- King, D.A. The adaptive significance of tree height. Am. Nat. 1990, 135, 809–828. [Google Scholar] [CrossRef]
- Wu, N.B.; Tan, F. Studies of water regimes of Cinnamomum pauciflorum seedlings grown under different light intensity conditions. J. Southwest Agri. Univ. 2002, 27, 755–758. [Google Scholar]
- Larcher, W. Photosynthesis as a tool for indicating temperature stress events. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 261–277. [Google Scholar]
- Kittas, C.; Katsoulas, N.; Bartzanas, T. Characterization and analysis of the effects of greenhouse climate control equipment on greenhouse microclimate and crop response. Acta Hortic. 2009, 893, 117–132. [Google Scholar] [CrossRef]
- Zhu, J.J.; Qiang, P.E.N.G.; Liang, Y.L.; Xing, W.U.; Hao, W.L. Leaf gas exchange, chlorophyll fluorescence, and fruit yield in hot pepper (Capsicum anmuum L.) grown under different shade and soil moisture during the fruit growth stage. J. Integr. Agric. 2012, 11, 927–937. [Google Scholar] [CrossRef]
- Castronuovo, D.; Statuto, D.; Muro, N.; Picuno, P.; Candido, V. The use of shading nets for the greenhouse cultivation of sweet pepper in the Mediterranean area. In Proceedings of the International Symposium on New Technologies and Management for Greenhouses-GreenSys2015, Evora, Portugal, 19–23 July 2015; pp. 373–380. [Google Scholar]
- Stevens, R.; Page, D.; Gouble, B.; Garchery, C.; Zamir, D.; Causse, M. Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 2008, 31, 1086–1096. [Google Scholar] [CrossRef]
- Choi, I.L.; Yoo, T.J.; Kang, H.M. UV-C treatments enhance antioxidant activity, retain quality and microbial safety of fresh-cut paprika in MA storage. Hortic. Environ. Biotechnol. 2015, 56, 324–329. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z.; Abbasi, N.A. Pre-storage putrescine application suppresses ethylene biosynthesis and retards fruit softening during low temperature storage in ‘Angelino’ plum. Postharvest Biol. Technol. 2007, 46, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Papageorge, L.M.; McFeeters, R.F.; Fleming, H.P. Factors influencing texture retention of salt-free, acidified, red bell peppers during storage. J. Agric. Food Chem. 2003, 51, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- McFeeters, R.F.; Barrangou, L.M.; Barish, A.O.; Morrison, S.S. Rapid softening of acidified peppers: Effect of oxygen and sulfite. J. Agric. Food Chem. 2004, 52, 4554–4557. [Google Scholar] [CrossRef]
- Almela, L.; Nieto-Sandoval, J.M.; Fernández López, J.A. Microbial inactivation of paprika by a high-temperature short-X time treatment. Influence on color properties. J. Agric. Food Chem. 2002, 50, 1435–1440. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Mathematical Equation | Description |
|---|---|---|
| Fluorescence transient OJIP | ||
| Fo | F50μs | First reliable fluorescence value after the onset of actinic illumination; used as initial value of the fluorescence |
| Fj | F2ms | Fluorescence value at 2 ms (J-level) |
| Fi | F30ms | Fluorescence value at 30 ms (I-level) |
| Fm (=Fp) | Fluorescence value at the peak of OJIP curve; maximum value under saturating illumination | |
| Technical fluorescence parameters | ||
| Fv | Fm − Fo | Maximum variable Chl fluorescence |
| Vj | (Fj − Fo)/(Fm − Fo) | Relative variable fluorescence at the J-level |
| Vi | (Fi − Fo)/(Fm − Fo) | Relative variable fluorescence at the I-level |
| Fm/Fo | Representing quantum yield of PSII photochemistry | |
| Fv/Fo | (Fm − Fo)/Fo | Maximum primary yield of photochemistry of PSII |
| Fv/Fm | (Fm − Fo)/Fm | Maximum quantum efficiency of PSII |
| Mo | (ΔV/Δt)o = 4 ms−1 × (F300μs − Fo)/(Fm − Fo) | Slope at the beginning of the transient Fo → Fm, maximal fractional rate of photochemistry |
| Quantum yields and efficiencies/probabilities | ||
| ΦPo | TRo/ABS = 1 − (Fo/Fm) (or Fv/Fm) | Maximum quantum yield of primary PSII photochemistry |
| Ψo | ETo/TRo = 1 − Vj | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA |
| Specific energy fluxes (per active PSII reaction center) | ||
| ABS/RC | (Mo/Vj) × (1/ΦPo) | Absorption flux per RC |
| TRo/RC | Mo/Vj | Trapped energy flux per RC (at t = 0) |
| ETo/RC | (Mo/Vj) × Ψo | Electron transport flux from QA to QB per RC (at t = 0) |
| DIo/RC | (ABS/RC) − (TRo/RC) | Dissipated energy flux per RC (at t = 0) |
| Performance index (combination of parameters) | ||
| PIABS | (RC/ABS) × [ΦPo/(1 − ΦPo)]× [Ψo/(1 − Ψo)] | Performance index (PI) on an absorption basis (= energy conservation from photons absorbed by PSII antenna to the reduction in QB) |
| Treatment | Length (cm) | Width (cm) | Diameter (cm) | Pericarp Thickness (mm) | Firmness (N/ϕ5 mm) | Hunter Value | Volume (cm3) | ||
|---|---|---|---|---|---|---|---|---|---|
| L | a | b | |||||||
| S1 | 10.2 ± 0.5 z a y | 8.6 ± 0.6 a | 27.1 ± 1.9 a | 7.6 ± 1.7 a | 17.65 ± 0.81 a | 33.8 ± 5.6 a | 30.7 ± 4 b | 17.18 ± 9.8 b | 763.5 ± 122.3 a |
| S2 | 9.5 ± 0.6 a | 8.0 ± 0.2 a | 25.1 ± 1.5 a | 5.8 ± 0.8 b | 13.28 ± 2.06 b | 30.7 ± 3.2 a | 42.5 ± 5.2 a | 28.12 ± 3.1 a | 603.6 ± 37.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Shawon, M.R.A.; An, J.H.; Lee, H.J.; Kwon, D.J.; Hwang, I.-C.; Bae, J.H.; Choi, K.Y. Effect of Shade Screen on Sap Flow, Chlorophyll Fluorescence, NDVI, Plant Growth and Fruit Characteristics of Cultivated Paprika in Greenhouse. Agriculture 2022, 12, 1405. https://doi.org/10.3390/agriculture12091405
Kim KH, Shawon MRA, An JH, Lee HJ, Kwon DJ, Hwang I-C, Bae JH, Choi KY. Effect of Shade Screen on Sap Flow, Chlorophyll Fluorescence, NDVI, Plant Growth and Fruit Characteristics of Cultivated Paprika in Greenhouse. Agriculture. 2022; 12(9):1405. https://doi.org/10.3390/agriculture12091405
Chicago/Turabian StyleKim, Kyeong Ho, Md Rayhan Ahmed Shawon, Jin Hee An, Hyoun Jin Lee, Dong Jae Kwon, In-Chul Hwang, Jong Hyang Bae, and Ki Young Choi. 2022. "Effect of Shade Screen on Sap Flow, Chlorophyll Fluorescence, NDVI, Plant Growth and Fruit Characteristics of Cultivated Paprika in Greenhouse" Agriculture 12, no. 9: 1405. https://doi.org/10.3390/agriculture12091405
APA StyleKim, K. H., Shawon, M. R. A., An, J. H., Lee, H. J., Kwon, D. J., Hwang, I.-C., Bae, J. H., & Choi, K. Y. (2022). Effect of Shade Screen on Sap Flow, Chlorophyll Fluorescence, NDVI, Plant Growth and Fruit Characteristics of Cultivated Paprika in Greenhouse. Agriculture, 12(9), 1405. https://doi.org/10.3390/agriculture12091405














