Long-Term Impact of Genomic Selection on Genetic Gain Using Different SNP Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation of Founder Population
2.2. Simulation of Selection Strategy
2.2.1. GBLUP
2.2.2. BayesA
2.2.3. PS
2.2.4. RS
2.3. Statistics Analysis for Long-Term Simulation
2.3.1. Genetic Gain
2.3.2. Average Kinship Coefficient
2.3.3. Accuracy of the GBLUP and BayesA Approach
3. Results
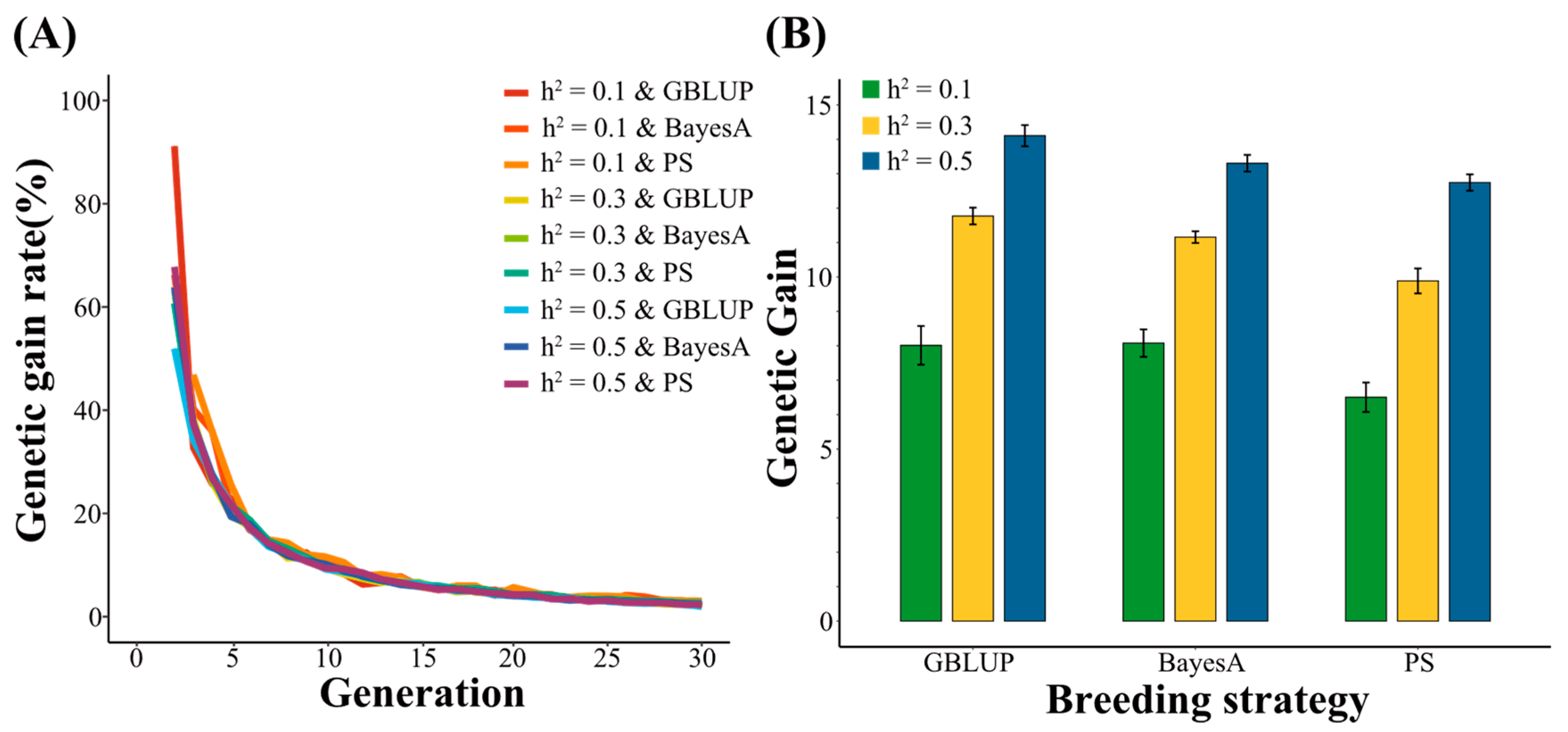
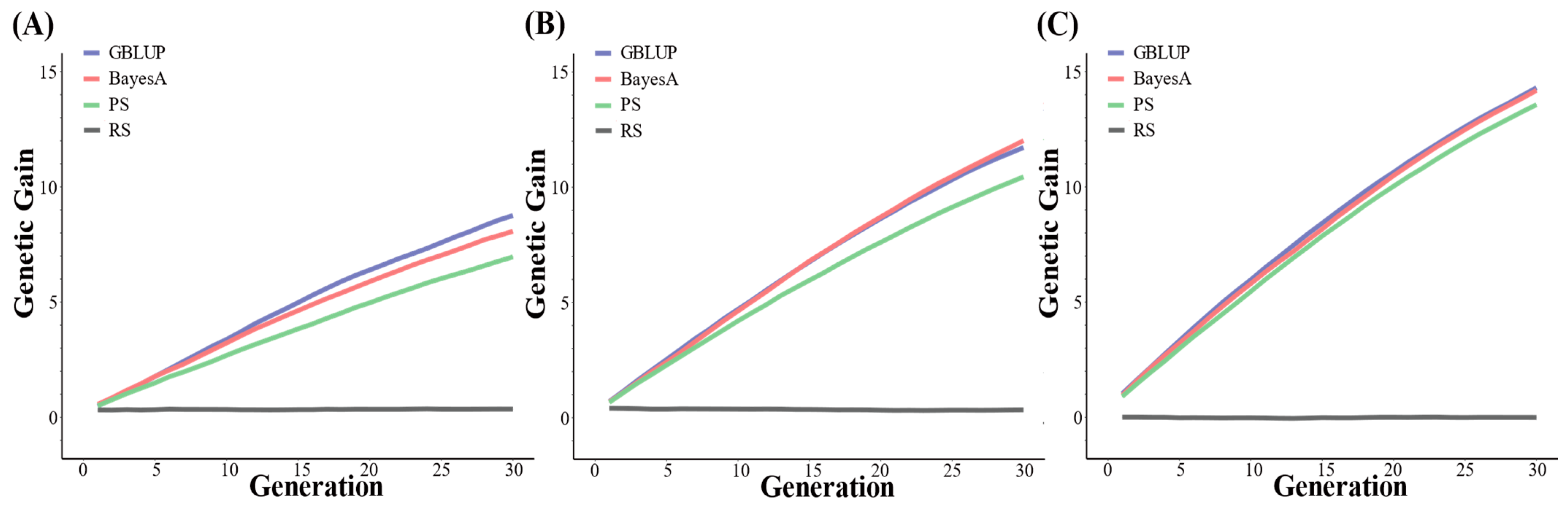
3.1. Genetic Gain Using 54 K SNPs
3.2. Genetic Gains Using 100 K SNPs
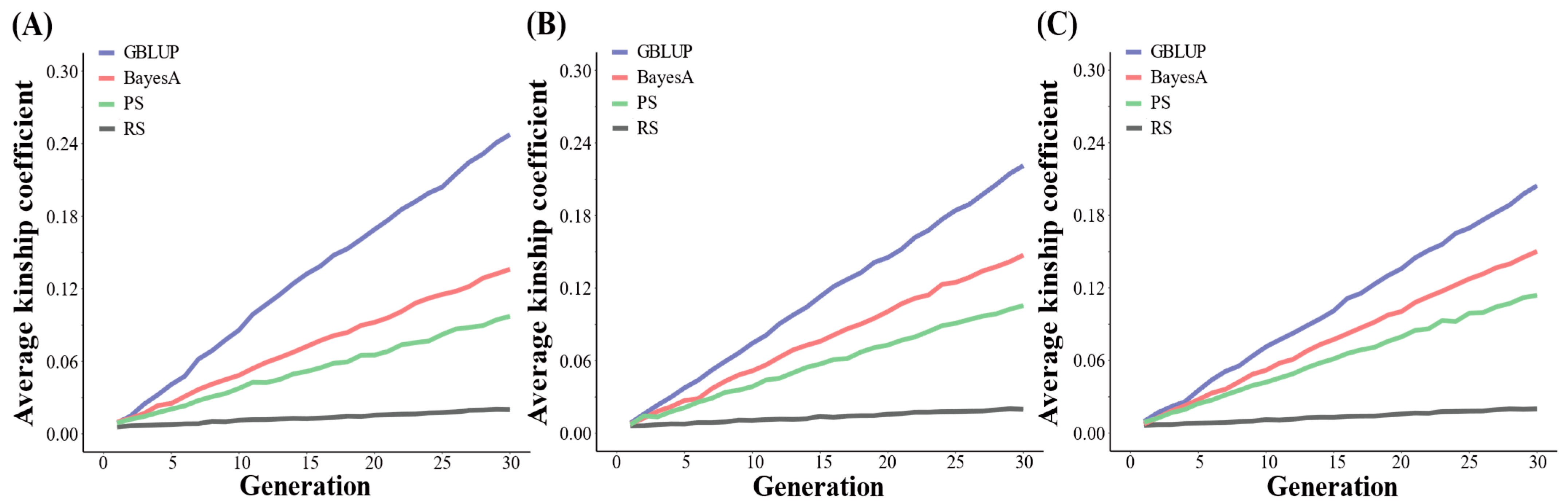
3.3. Average Kinship Coefficients Using 54 K SNPs
3.4. Average Kinship Coefficients Using 100 K SNPs
3.5. Accuracy of GBLUP and BayesA Approach
4. Discussion
4.1. The Changes in Genetic Gain
4.2. Evaluating Effects on Average Kinship Coefficients
4.3. Limitation and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- VanRaden, P. Symposium review: How to implement genomic selection. J. Dairy Sci. 2020, 103, 5291–5301. [Google Scholar] [CrossRef]
- Schierenbeck, S.; Pimentel, E.C.G.; Tietze, M.; Körte, J.; Reents, R.; Reinhardt, F.; Simianer, H.; König, S. Controlling inbreeding and maximizing genetic gain using semi-definite programming with pedigree-based and genomic relationships. J. Dairy Sci. 2011, 94, 6143–6152. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Calus, M.P.L.; Pong-Wong, R.; de los Campos, G.; Hickey, J.M. Genomic Prediction in Animals and Plants: Simulation of Data, Validation, Reporting, and Benchmarking. Genetics 2013, 193, 347–365. [Google Scholar] [CrossRef]
- Hickey, J.M.; Gorjanc, G. Simulated Data for Genomic Selection and Genome-Wide Association Studies Using a Combination of Coalescent and Gene Drop Methods. G3 Genes Genomes Genet. 2012, 2, 425–427. [Google Scholar] [CrossRef]
- Seno, L.D.O.; Guidolin, D.G.F.; Aspilcueta-Borquis, R.R.; Nascimento, G.B.D.; da Silva, T.B.R.; de Oliveira, H.N.; Munari, D.P. Genomic selection in dairy cattle simulated populations. J. Dairy Res. 2018, 85, 125–132. [Google Scholar] [CrossRef]
- de Rezende Neves, H.H.; Carvalheiro, R.; de Queiroz, S.A. Trait-specific long-term consequences of genomic selection in beef cattle. Genetica 2017, 146, 85–99. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Zhu, B.; Liu, Y.; Li, H.; Bordbar, F.; Chen, Y.; Zhang, L.; Gao, X.; Gao, H. Theoretical Evaluation of Multi-Breed Genomic Prediction in Chinese Indigenous Cattle. Animals 2019, 9, 789. [Google Scholar] [CrossRef]
- Akanno, E.; Schenkel, F.; Sargolzaei, M.; Friendship, R.; Robinson, J. Persistency of accuracy of genomic breeding values for different simulated pig breeding programs in developing countries. J. Anim. Breed. Genet. 2014, 131, 367–378. [Google Scholar] [CrossRef]
- da Silva, E.D.B.; Xavier, A.; Faria, M.V. Impact of Genomic Prediction Model, Selection Intensity, and Breeding Strategy on the Long-Term Genetic Gain and Genetic Erosion in Soybean Breeding. Front. Genet. 2021, 12, 637133. [Google Scholar] [CrossRef]
- Burrow, H.M.; Mrode, R.; Mwai, A.O.; Coffey, M.P.; Hayes, B.J. Challenges and Opportunities in Applying Genomic Selection to Ruminants Owned by Smallholder Farmers. Agriculture 2021, 11, 1172. [Google Scholar] [CrossRef]
- de Roos, A.; Schrooten, C.; Veerkamp, R.; van Arendonk, J. Effects of genomic selection on genetic improvement, inbreeding, and merit of young versus proven bulls. J. Dairy Sci. 2011, 94, 1559–1567. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Cole, J.B.; VanRaden, P.M.; Wiggans, G.R.; Ruiz-López, F.J.; Van Tassell, C.P. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. USA 2016, 113, E3995–E4004. [Google Scholar] [CrossRef]
- Samorè, A.B.; Fontanesi, L. Genomic selection in pigs: State of the art and perspectives. Ital. J. Anim. Sci. 2016, 15, 211–232. [Google Scholar] [CrossRef]
- Weller, J.; Ezra, E.; Ron, M. Invited review: A perspective on the future of genomic selection in dairy cattle. J. Dairy Sci. 2017, 100, 8633–8644. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Cole, J.B.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327. [Google Scholar] [CrossRef]
- Rutkoski, J.E. Chapter Four—A practical guide to genetic gain. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 157, pp. 217–249. [Google Scholar]
- Xu, Y.; Boddupalli, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Commun. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Vanraden, P.M.; Van Tassell, C.P.; Wiggans, G.R.; Sonstegard, T.S.; Schnabel, R.D.; Taylor, J.F.; Schenkel, F.S. Invited Review: Reliability of genomic predictions for North American Holstein bulls. J. Dairy Sci. 2009, 92, 16–24. [Google Scholar] [CrossRef]
- Dekkers, J.C.M.; Su, H.; Cheng, J. Correction to: Predicting the accuracy of genomic predictions. Genet. Sel. Evol. 2021, 53, 81. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, P.; Wang, Z.; Zhang, W.; Chen, Y.; Zhang, L.; Gao, H.; Gao, X.; Xu, L.; Li, J. Accuracies of genomic prediction for twenty economically important traits in Chinese Simmental beef cattle. Anim. Genet. 2019, 50, 634–643. [Google Scholar] [CrossRef]
- Forutan, M.; Mahyari, S.A.; Baes, C.; Melzer, N.; Schenkel, F.S.; Sargolzaei, M. Inbreeding and runs of homozygosity before and after genomic selection in North American Holstein cattle. BMC Genom. 2018, 19, 98. [Google Scholar] [CrossRef]
- Howard, J.T.; Pryce, J.E.; Baes, C.; Maltecca, C. Invited review: Inbreeding in the genomics era: Inbreeding, inbreeding depression, and management of genomic variability. J. Dairy Sci. 2017, 100, 6009–6024. [Google Scholar] [CrossRef]
- Pook, T.; Schlather, M.; Simianer, H. MoBPS-Modular Breeding Program Simulator. G3 Genes Genomes Genet. 2020, 10, 1915–1918. [Google Scholar] [CrossRef]
- Bastiaansen, J.W.; Coster, A.; Calus, M.P.; van Arendonk, J.A.; Bovenhuis, H. Long-term response to genomic selection: Effects of estimation method and reference population structure for different genetic architectures. Genet. Sel. Evol. 2012, 44, 3. [Google Scholar] [CrossRef]
- Brito, F.V.; Neto, J.B.; Sargolzaei, M.; Cobuci, J.A.; Schenkel, F.S. Accuracy of genomic selection in simulated populations mimicking the extent of linkage disequilibrium in beef cattle. BMC Genet. 2011, 12, 80. [Google Scholar] [CrossRef]
- Wientjes, Y.C.J.; Bijma, P.; Calus, M.P.L.; Zwaan, B.J.; Vitezica, Z.G.; van den Heuvel, J. The long-term effects of genomic selection: 1. Response to selection, additive genetic variance, and genetic architecture. Genet. Sel. Evol. 2022, 54, 19. [Google Scholar] [CrossRef]
- VanRaden, P. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Guidelines to MoBPS.pdf. Available online: https://github.com/tpook92/MoBPS/blob/master/Guidelines%20to%20MoBPS.pdf (accessed on 26 June 2022).
- He, J.; Wu, X.-L.; Zeng, Q.; Li, H.; Ma, H.; Jiang, J.; Rosa, G.J.M.; Gianola, D.; Tait, R.G.; Bauck, S. Genomic mating as sustainable breeding for Chinese indigenous Ningxiang pigs. PLoS ONE 2020, 15, e0236629. [Google Scholar] [CrossRef]
- Seno, L.; Fernández, J.; Cardoso, V.; García-Cortés, L.; Toro, M.A.; Santos, D.; Albuquerque, L.; De Camargo, G.; Tonhati, H. Selection strategies for dairy buffaloes: Economic and genetic consequences. J. Anim. Breed. Genet. 2012, 129, 488–500. [Google Scholar] [CrossRef]
- Schaeffer, L.R. Strategy for applying genome-wide selection in dairy cattle. J. Anim. Breed. Genet. 2006, 123, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bolormaa, S.; Pryce, J.E.; Kemper, K.; Savin, K.; Hayes, B.J.; Barendse, W.; Zhang, Y.; Reich, C.M.; Mason, B.A.; Bunch, R.J.; et al. Accuracy of prediction of genomic breeding values for residual feed intake and carcass and meat quality traits in Bos taurus, Bos indicus, and composite beef cattle1. J. Anim. Sci. 2013, 91, 3088–3104. [Google Scholar] [CrossRef] [PubMed]
- Daetwyler, H.D.; Pong-Wong, R.; Villanueva, B.; Woolliams, J.A. The Impact of Genetic Architecture on Genome-Wide Evaluation Methods. Genetics 2010, 185, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M. Genomic selection: Prediction of accuracy and maximisation of long term response. Genetica 2009, 136, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Weigel, K.; Campos, G.D.L.; González-Recio, O.; Naya, H.; Wu, X.; Long, N.; Rosa, G.; Gianola, D. Predictive ability of direct genomic values for lifetime net merit of Holstein sires using selected subsets of single nucleotide polymorphism markers. J. Dairy Sci. 2009, 92, 5248–5257. [Google Scholar] [CrossRef]
- Su, G.; Brøndum, R.; Ma, P.; Guldbrandtsen, B.; Aamand, G.P.; Lund, M. Comparison of genomic predictions using medium-density (∼54,000) and high-density (∼777,000) single nucleotide polymorphism marker panels in Nordic Holstein and Red Dairy Cattle populations. J. Dairy Sci. 2012, 95, 4657–4665. [Google Scholar] [CrossRef]
- Maynard, J.; Haigh, J. The hitch-hiking effect of a favourable gene. Genet. Res. 2007, 89, 391–403. [Google Scholar] [CrossRef]
- Kim, E.-S.; Cole, J.B.; Huson, H.; Wiggans, G.R.; Van Tassell, C.P.; Crooker, B.A.; Liu, G.; Da, Y.; Sonstegard, T.S. Effect of Artificial Selection on Runs of Homozygosity in U.S. Holstein Cattle. PLoS ONE 2013, 8, e80813. [Google Scholar] [CrossRef]
- Kim, E.-S.; Sonstegard, T.S.; Van Tassell, C.P.; Wiggans, G.; Rothschild, M.F. The Relationship between Runs of Homozygosity and Inbreeding in Jersey Cattle under Selection. PLoS ONE 2015, 10, e0129967. [Google Scholar] [CrossRef]
- Bosse, M.; Megens, H.-J.; Madsen, O.; Paudel, Y.; Frantz, L.A.F.; Schook, L.B.; Crooijmans, R.P.M.A.; Groenen, M.A.M. Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape. PLoS Genet. 2012, 8, e1003100. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.; Haile-Mariam, M.; Cocks, B.; Pryce, J. How genomic selection has increased rates of genetic gain and inbreeding in the Australian national herd, genomic information nucleus, and bulls. J. Dairy Sci. 2021, 104, 11832–11849. [Google Scholar] [CrossRef] [PubMed]
- Curik, I.; Ferenčaković, M.; Sölkner, J. Inbreeding and runs of homozygosity: A possible solution to an old problem. Livest. Sci. 2014, 166, 26–34. [Google Scholar] [CrossRef]
- De Beukelaer, H.; Badke, Y.; Fack, V.; De Meyer, G. Moving Beyond Managing Realized Genomic Relationship in Long-Term Genomic Selection. Genetics 2017, 206, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, D.; Beavis, W.; Fritsche-Neto, R.; Singh, A.K.; Isidro-Sánchez, J. Multi-objective optimized genomic breeding strategies for sustainable food improvement. Heredity 2018, 122, 672–683. [Google Scholar] [CrossRef]
- Makanjuola, B.O.; Miglior, F.; Abdalla, E.A.; Maltecca, C.; Schenkel, F.S.; Baes, C.F. Effect of genomic selection on rate of inbreeding and coancestry and effective population size of Holstein and Jersey cattle populations. J. Dairy Sci. 2020, 103, 5183–5199. [Google Scholar] [CrossRef]
- Carthy, T.; McCarthy, J.; Berry, D. A mating advice system in dairy cattle incorporating genomic information. J. Dairy Sci. 2019, 102, 8210–8220. [Google Scholar] [CrossRef]
- Meuwissen, T.; Sonesson, A.K. Maximizing the response of selection with a predefined rate of inbreeding: Overlapping generations. J. Anim. Sci. 1998, 76, 2575–2583. [Google Scholar] [CrossRef]



| Scenario | Trait Heritability | SNP Density |
|---|---|---|
| 1 | 0.1 | 54 K |
| 2 | 0.1 | 100 K |
| 3 | 0.3 | 54 K |
| 4 | 0.3 | 100 K |
| 5 | 0.5 | 54 K |
| 6 | 0.5 | 100 K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhang, T.; Wang, T.; Niu, Q.; Wu, J.; Wang, Z.; Gao, H.; Li, J.; Xu, L. Long-Term Impact of Genomic Selection on Genetic Gain Using Different SNP Density. Agriculture 2022, 12, 1463. https://doi.org/10.3390/agriculture12091463
Zheng X, Zhang T, Wang T, Niu Q, Wu J, Wang Z, Gao H, Li J, Xu L. Long-Term Impact of Genomic Selection on Genetic Gain Using Different SNP Density. Agriculture. 2022; 12(9):1463. https://doi.org/10.3390/agriculture12091463
Chicago/Turabian StyleZheng, Xu, Tianliu Zhang, Tianzhen Wang, Qunhao Niu, Jiayuan Wu, Zezhao Wang, Huijiang Gao, Junya Li, and Lingyang Xu. 2022. "Long-Term Impact of Genomic Selection on Genetic Gain Using Different SNP Density" Agriculture 12, no. 9: 1463. https://doi.org/10.3390/agriculture12091463





