Human Activity Played a Key Role in Rice Stripe Disease Epidemics: From an Empirical Evaluation of over a 10-Year Period
Abstract
:1. Introduction
2. Materials and Methods
3. Results
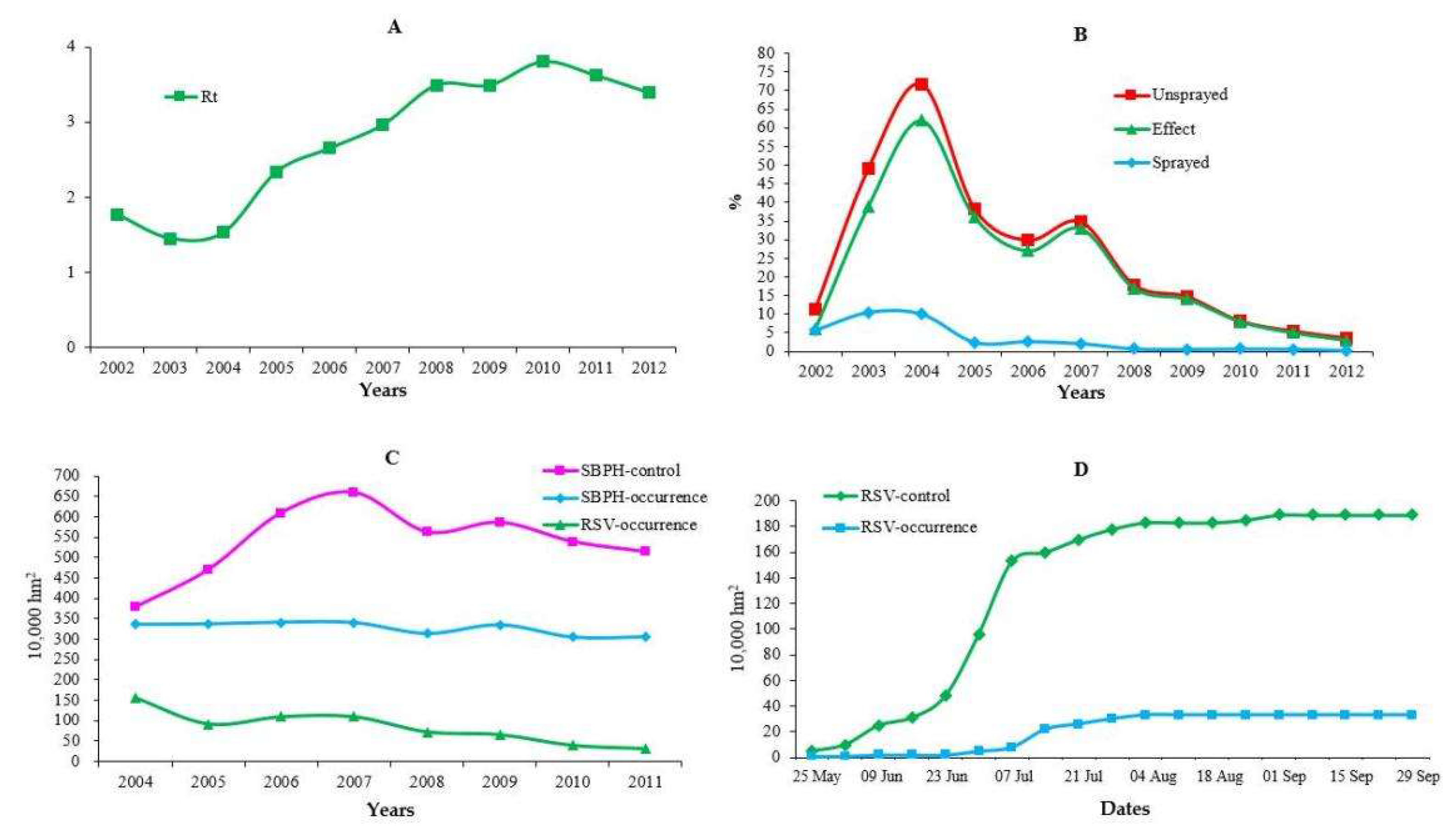
3.1. Associations of RSV Disease Severity with Human Factors
3.2. Effects of Human Factors and Their Interaction on RSV Disease Severity
3.3. Associations of SBPH Traits with Human Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, D.C.; Ma, Y.L.; Li, Z.Z.; Zhong, C.S.; Cheng, Z.B.; Zhan, J. Crop rotation enhances agricultural sustainability: From an empirical evaluation of eco-economic benefifits in rice production. Agriculture 2021, 11, 91. [Google Scholar] [CrossRef]
- Kisimoto, R. Genetic variation in the ability of a planthopper vector, Laodelphax striatellus (Fallen) to acquire the rice stripe virus. Virology 1967, 32, 144–152. [Google Scholar] [CrossRef]
- Kisimoto, R. Biology and monitoring of vectors in rice stripe epidemiology. ASPAC Food Fertilzer Technol. Cent. 1993, 373, 1–8. [Google Scholar]
- Kiyosawa, S. Genetics and epidemiological modeling of breakdown of plant disease resistance. Annu. Rev. Phytopathol. 1982, 20, 93–117. [Google Scholar] [CrossRef]
- Shinkai, A. Present situation of rice stripe disease. Plant Prot. Jpn. 1985, 11, 503–507. [Google Scholar]
- Wei, T.Y.; Yang, J.; Liao, F.R.; Gao, F.; Lu, L.M.; Zhang, X.T.; Li, F.; Wu, Z.J.; Lin, Q.Y.; Xie, L.H.; et al. Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 2009, 90, 1548. [Google Scholar] [CrossRef]
- Otuka, A. Migration of rice planthoppers and their vectored re-emerging and novel rice virus in East Asia. Front. Microbiol. 2013, 4, 309. [Google Scholar] [CrossRef]
- Lee, J.A.; Halbert, S.E.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singer, B.H. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 24, 7605–7610. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; Zhan, J.; Xie, L.H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 154, 60345–60352. [Google Scholar] [CrossRef]
- Khatun, M.T.; Nessa, B.; Salam, M.U.; Kabir, M.S. Strategy for rice disease management in Bangladesh. Bangladesh Rice J. 2021, 25, 23–36. [Google Scholar] [CrossRef]
- He, D.C.; Burdon, J.; Xie, L.H.; Zhan, J. Triple bottom-line consideration of sustainable plant disease management: From economic, sociological and ecological perspectives. J. Integr. Agric. 2021, 20, 2–12. [Google Scholar] [CrossRef]
- He, D.C.; He, M.H.; Amalin, D.; Liu, W.; Alvindia, D.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Liu, L.; Cui, X.; Shen, Z.; Liu, J.; Yu, X.G.; Wang, Z.; Zhang, S.; Mu, Q.Q. Prevalence characteristics of rice black-streaked dwarf virus disease and continuous control strategies. Asian Agric. Res. 2021, 13, 5. [Google Scholar]
- Kiritani, K.; Plumb, R.T.; Thresh, J.M. Changes in cropping practices and the incidence of hopper-borne diseases of rice in Japan. Plant Virus Epidemiol. 1983, 9, 239–247. [Google Scholar]
- Kisimoto, R. Planthopper-rice virus epidemiology model: Rice stripe and small brown planthopper, Laodelphax striatellus fallen. In Plant Virus Epidemics Monitoring Modelling & Predicting Outbreaks; Academic Press: Cambridge, MA, USA, 1986; pp. 327–344. [Google Scholar]
- Kiritani, K.; Nakasuji, F.; Miyai, S.I. Systems approaches for management of insect-borne rice diseases. Curr. Top. Vector Res. 1987, 3, 57–80. [Google Scholar]
- Hayakawa, T.; Zhu, Y.; Itoh, K.; Kimura, Y.; Izawa, T.; Shimamoto, K.; Toriyama, S. Genetically engineered rice resistant to rice stripe virus, an insect-transmitted virus. Proc. Natl. Acad. Sci. USA 1992, 89, 9865–9869. [Google Scholar] [CrossRef]
- Bae, S.D.; Kim, D.K. Occurrence of small brown planthopper (Laodelphax striatellus Fallen) and incidence of rice virus disease by different seeding date in dry seeded rice. Korean J. Appl. Entomol. 1994, 3, 173–177. [Google Scholar]
- Endo, S.; Tsurumachi, M. Insecticide resistance and insensitive acetylcholinesterase in small brown planthopper, Laodelphax striatellus. J. Pestic. Sci. 2000, 25, 395–397. [Google Scholar] [CrossRef]
- Endo, S.; Tsurumachi, M. Insecticide susceptibility of the brown planthopper and the white-backed planthopper collected from Southeast Asia. J. Pestic. Sci. 2001, 26, 82–86. [Google Scholar] [CrossRef]
- Xiao, D.; Li, W.; Wei, T.; Wu, Z.; Xie, L. Advances in the studies of rice stripe virus. Front. Agric. China 2010, 4, 287–292. [Google Scholar] [CrossRef]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.X.; Yang, P.; Barbetti, M.; et al. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; Zhan, J.; Cheng, Z.B.; Xie, L.H. Viruliferous rate of small brown planthopper is a good indicator of rice stripe disease epidemics. Sci. Rep. 2016, 6, 21376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Shen, J.; Hu, R. Productivity effect and overuse of pesticide in crop production in China. J. Integr. Agric. 2015, 14, 1903–1910. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, H.; Zhuang, X.; Guo, X.; He, Z.; Xu, K.; Liu, F. Sensitive and high-throughput polyclonal antibody-based serological methods for rice stripe virus detection in both rice and brown planthopper. Crop. Prot. 2021, 144, 105599. [Google Scholar] [CrossRef]
- Murakami, M.K.T. Occurrence of insect pests in rice stripe disease resistant cultivar. Proc. Kanto-Tosan Plant Prot. Soc. 1986, 33, 186–187. [Google Scholar]
- Zhu, J.L.; Zhu, Z.R.; Zhou, Y.; Lu, Q.; Sun, X.L.; Tao, X.G.; Chen, Y.; Wang, H.D.; Cheng, J.A. Effect of rice sowing date on occurrence of small brown planthopper and epidemics of planthopper-transmitted rice stripe viral disease. Agric. Sci. China 2009, 8, 332–341. [Google Scholar] [CrossRef]
- Arata, G.; Martínez, M.; Elguezábal, C.; Rojas, D.E.; Cristos, D.; Dinolfo, M.; Arata, A. Effects of sowing date, nitrogen fertilization, and Fusarium graminearum in an argentinean bread wheat: Integrated analysis of disease parameters, mycotoxin contamination, grain quality, and seed deterioration. J. Food Compost. Anal. 2021, 107, 104364. [Google Scholar] [CrossRef]
- Ahn, E.K.; Hyun, U.J.; Jung, K.H.; Won, Y.J.; Hong, H.C.; Park, H.M.; Chang, J.K.; Lee, J.H.; Sung, N.S.; Suh, J.P.; et al. ‘Keunpum’: A mid-late maturing, high yielding, giant embryo rice cultivar with resistance to multiple diseases and used as germinated brown rice. Korean J. Breed. Sci. 2021, 53, 515–525. [Google Scholar] [CrossRef]
- Naseri, B.; Hemmati, R. Bean root rot management: Recommendations based on an integrated approach for plant disease control. Rhizosphere 2017, 4, 48–53. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, J.; Lee, Y.; Lee, W.S. Predicting potential epidemics of rice leaf blast and sheath blight in South Korea under the RCP 4.5 and RCP 8.5 climate change scenarios using a rice disease epidemiology model, epirice. Agric. For. Meteorol. 2015, 203, 191–207. [Google Scholar] [CrossRef]
- Barnett, O.W.; Main, C.E. Plant virus disease—Economic aspects. Encycl. Virol. 1999, 16, 1318–1326. [Google Scholar]
- Hao, Z.; Wang, L.; He, Y.; Liang, J.; Tao, R. Expression of defense genes and activities of antioxidant enzymes in rice resistance to rice stripe virus and small brown planthopper. Plant Physiol. Biochem. PPB 2011, 49, 744–751. [Google Scholar] [CrossRef] [PubMed]
| Disease Severity in Sprayed Fields | ||
|---|---|---|
| Current Year | Following Year | |
| Rt in current year | −0.700 ** | −0.552 ** |
| Preceding Year | Current Year | ||||||
|---|---|---|---|---|---|---|---|
| Wheat Planting Way | Rice Harvest Date | Wheat Sowing Date | Wheat Harvest Date | Rice Planting Way | Rice Sowing Date | Rice Transplanting Date | |
| Severity in sprayed fields | −0.133 | −0.121 | −0.597 ** | 0.142 | 0 | −0.358 ** | −0.248 * |
| Severity in unsprayed fields | 0.325 ** | −0.243 | −0.608 ** | 0.403 ** | −0.026 | −0.559 ** | −0.309 ** |
| Preceding Year | Current Year | ||
|---|---|---|---|
| Interval of Wheat Sowing and Rice Harvest (WSRH) | Interval of Wheat Harvest and Rice Sowing (WHRS) | Interval of Rice Transplanting and Sowing (RTRS) | |
| Severity in sprayed fields | −0.354 ** | 0.384 ** | 0.181 |
| Severity in unsprayed fields | −0.182 | 0.556 ** | 0.355 ** |
| P | F | DF | |
|---|---|---|---|
| Corrected Model | 0.003 ** | 4.350 | 61 |
| Intercept | 0.000 ** | 127.135 | 1 |
| WSRH | 0.873 | 0.561 | 18 |
| WHRS | 0.024 * | 3.191 | 12 |
| Rt | 0.001 ** | 9.648 | 3 |
| WSRH × WHRS | 0.172 | 1.893 | 4 |
| WSRH × Rt | 0.986 | 0.047 | 3 |
| WHRS × Rt | 0.842 | 0.174 | 2 |
| Error | 13 | ||
| Current Year | Following Year | |||
|---|---|---|---|---|
| Q0 | V0 | Q0 | V0 | |
| Rt in preceding year | −0.139 | −0.639 ** | −0.219 | −0.616 ** |
| Wheat Planting Way | Rice Harvest Date | Wheat Sowing Date | Wheat Harvest Date | Rice Planting Way | Rice Sowing Date | Rice Transplanting Date | |
|---|---|---|---|---|---|---|---|
| Q0 | −0.442 ** | 0.254 * | 0.171 | 0.116 | 0.227 | 0.029 | −0.401 ** |
| V0 | 0.119 | −0.143 | −0.690 ** | −0.040 | −0.098 | −0.269 * | −0.106 |
| Interval of Wheat Sowing and Rice Harvest (WSRH) | Interval of Wheat Harvest and Rice Sowing (WHRS) | Interval of Rice Transplanting and Sowing (RTRS) | |
|---|---|---|---|
| Q0 | −0.11 | 0.069 | −0.323 ** |
| V0 | −0.361 ** | 0.224 | 0.214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.-L.; Lin, W.-W.; Guo, S.-S.; Xie, L.-H.; He, D.-C.; Cheng, Z.-B. Human Activity Played a Key Role in Rice Stripe Disease Epidemics: From an Empirical Evaluation of over a 10-Year Period. Agriculture 2022, 12, 1484. https://doi.org/10.3390/agriculture12091484
Ma Y-L, Lin W-W, Guo S-S, Xie L-H, He D-C, Cheng Z-B. Human Activity Played a Key Role in Rice Stripe Disease Epidemics: From an Empirical Evaluation of over a 10-Year Period. Agriculture. 2022; 12(9):1484. https://doi.org/10.3390/agriculture12091484
Chicago/Turabian StyleMa, Yan-Li, Wen-Wu Lin, Si-Si Guo, Lian-Hui Xie, Dun-Chun He, and Zhao-Bang Cheng. 2022. "Human Activity Played a Key Role in Rice Stripe Disease Epidemics: From an Empirical Evaluation of over a 10-Year Period" Agriculture 12, no. 9: 1484. https://doi.org/10.3390/agriculture12091484






