Blue-Light-Dependent Stomatal Density and Specific Leaf Weight Coordinate to Promote Gas Exchange of Soybean Leaves
Abstract
:1. Introduction
2. Materials and Methods
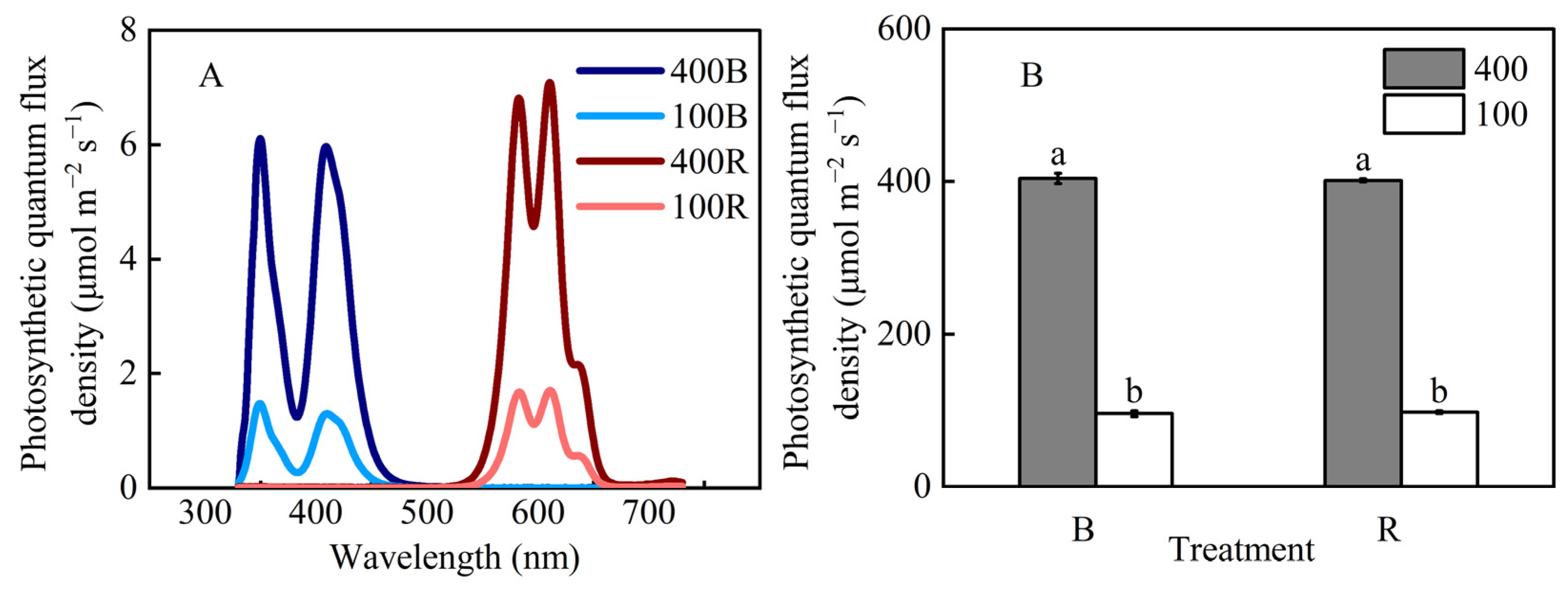
2.1. Plant Material and Growth Conditions
2.2. Measurement of Morphological Characteristics
2.3. Leaf Anatomical Feature Measurements
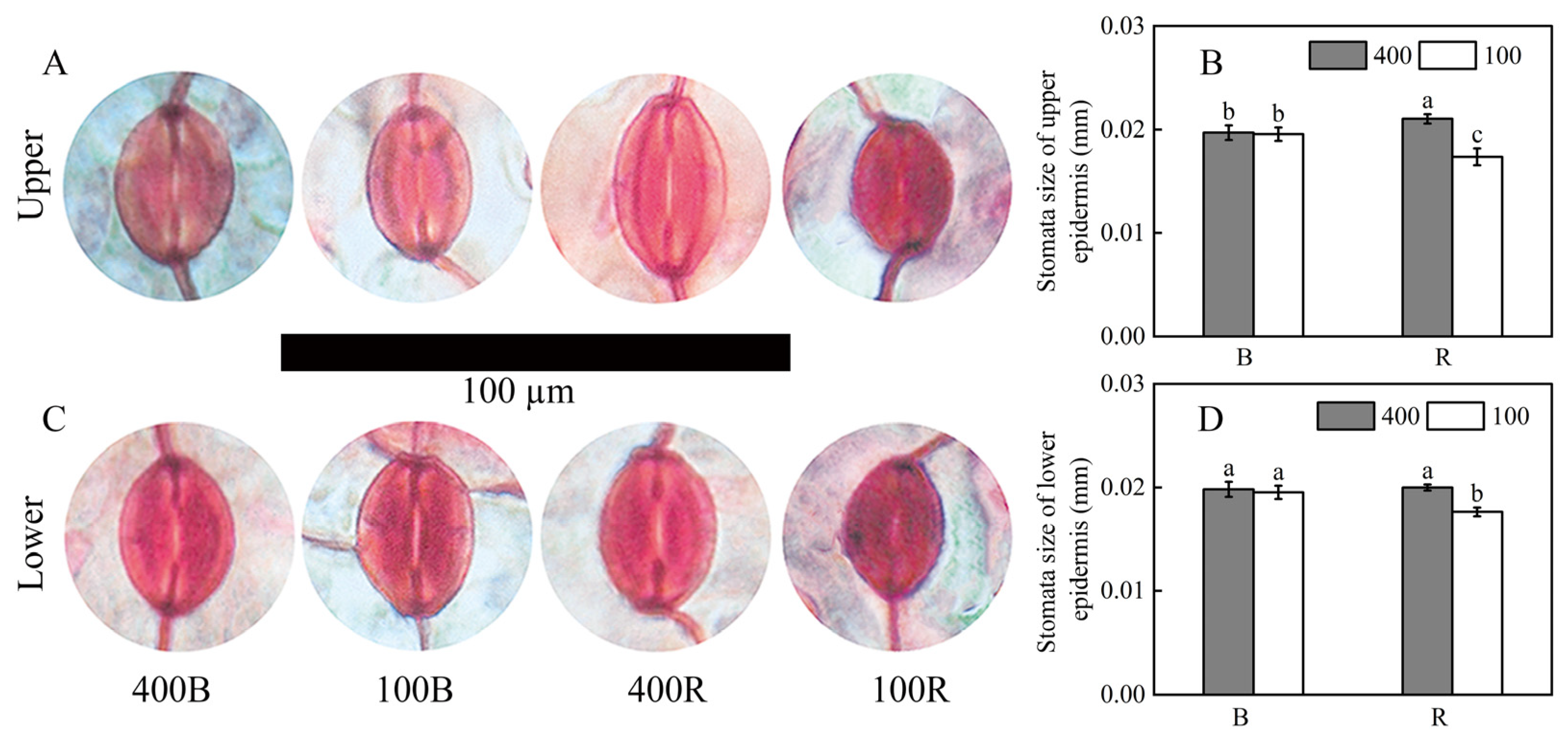
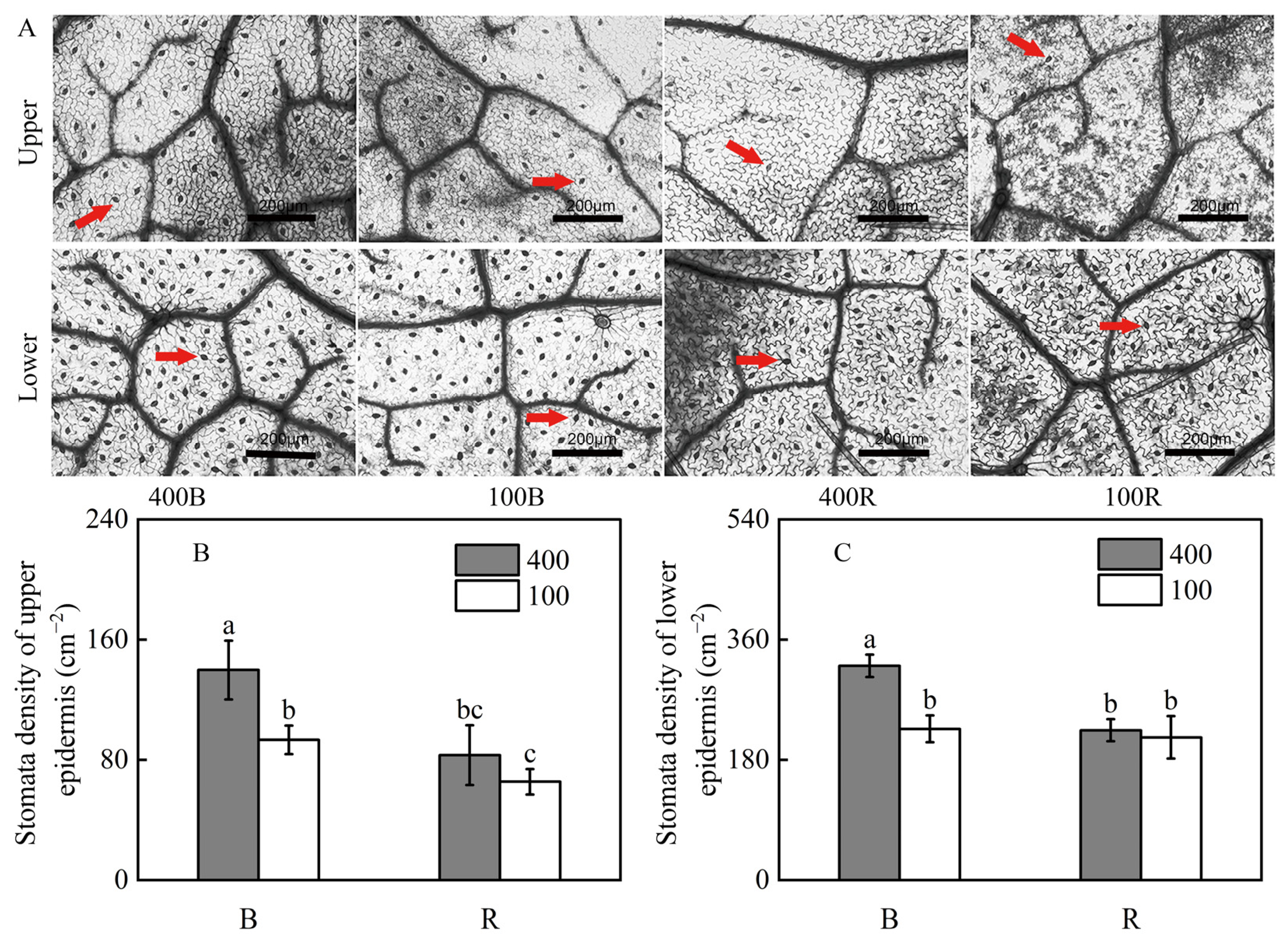
2.4. Determination of Stomatal Structure Characteristics
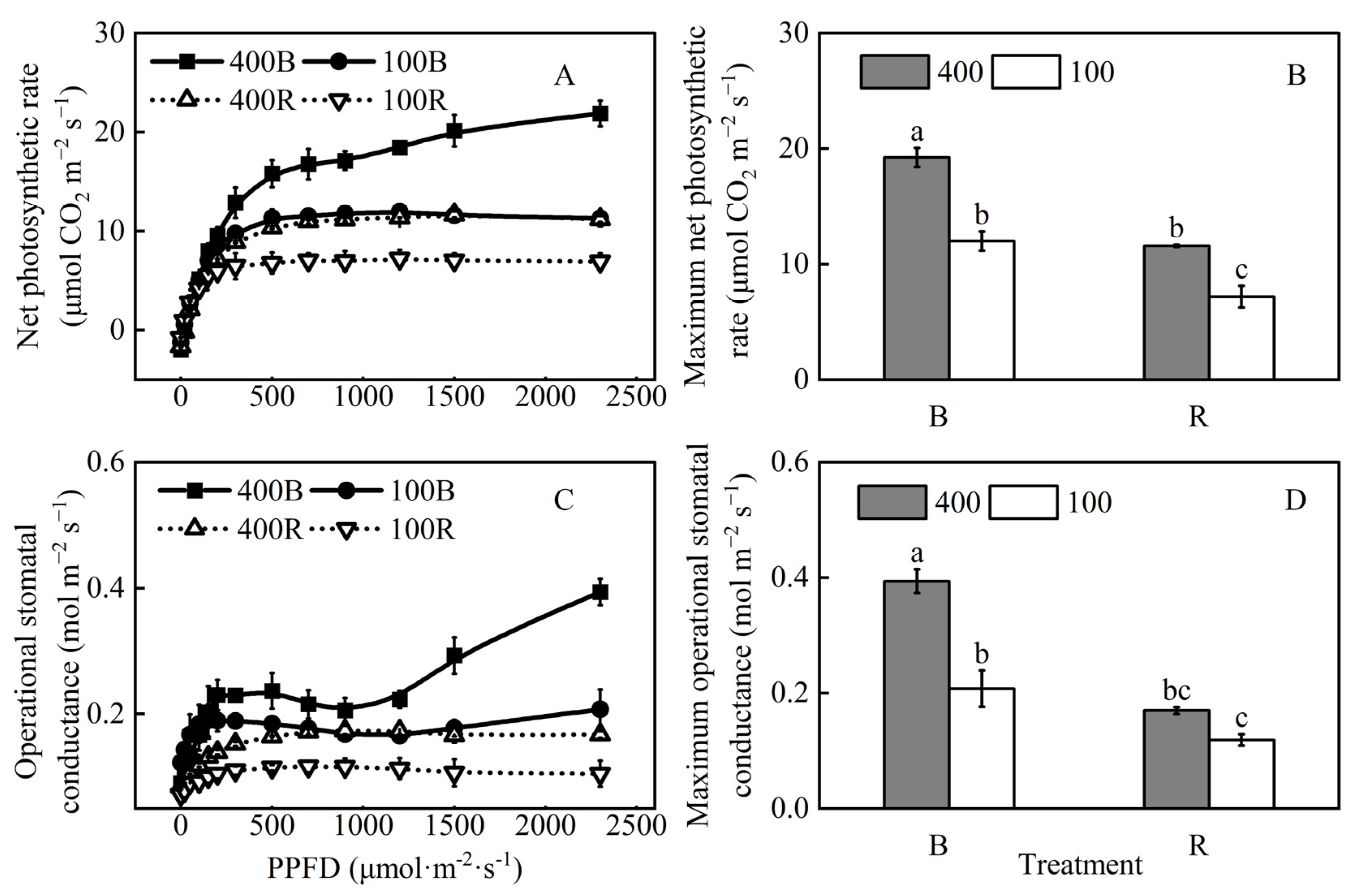
2.5. Photosynthesis Measurements
2.6. Statistical Analysis
3. Results
3.1. Leaf Structure Response to Blue and Red Light
3.2. Stomatal Size of the Two Sides
3.3. Stomatal Density of the Two Sides
3.4. The Response Curves of Light Intensities
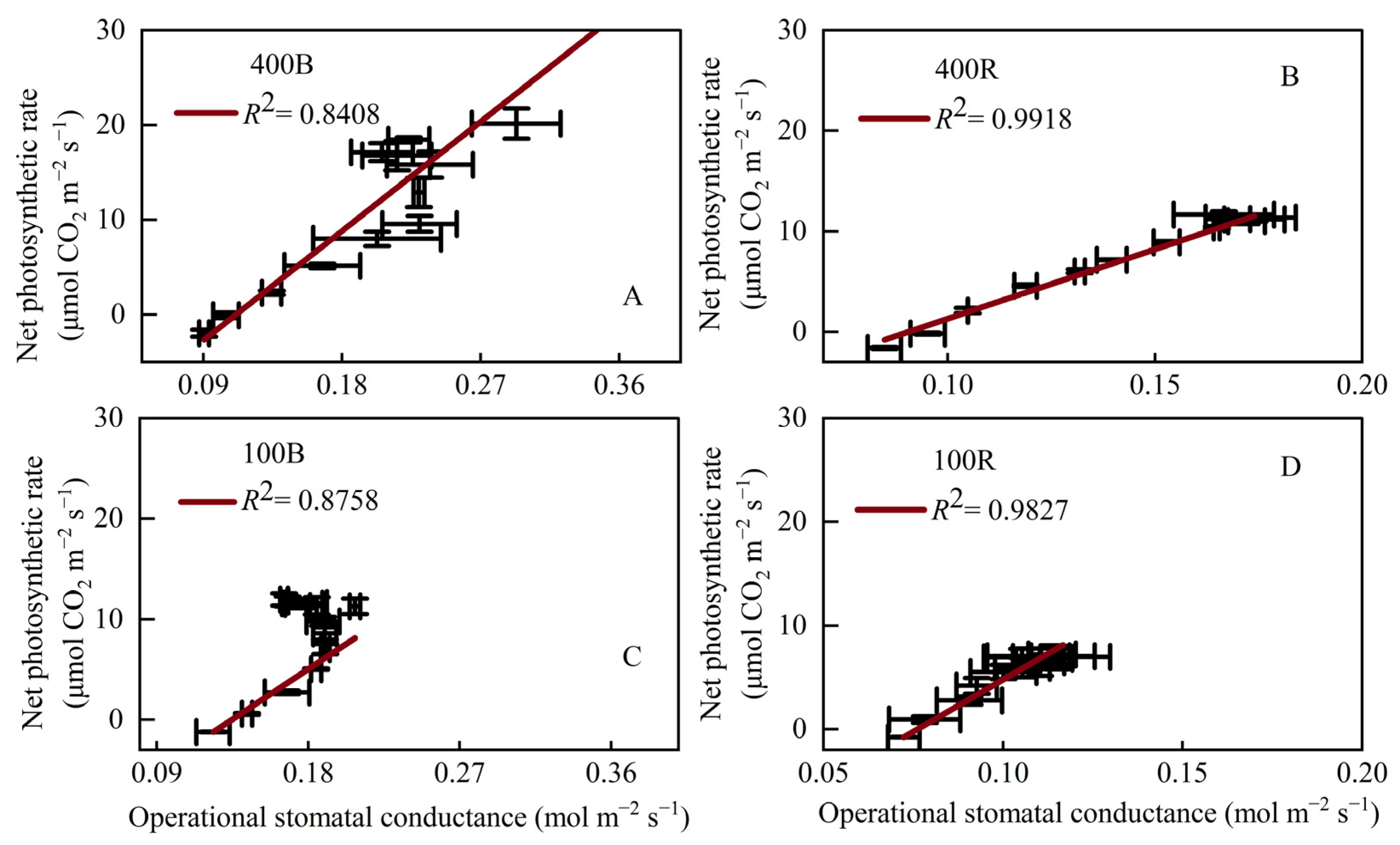
3.5. Relationship between Net Photosynthetic Rate and Operational Stomatal Conductance
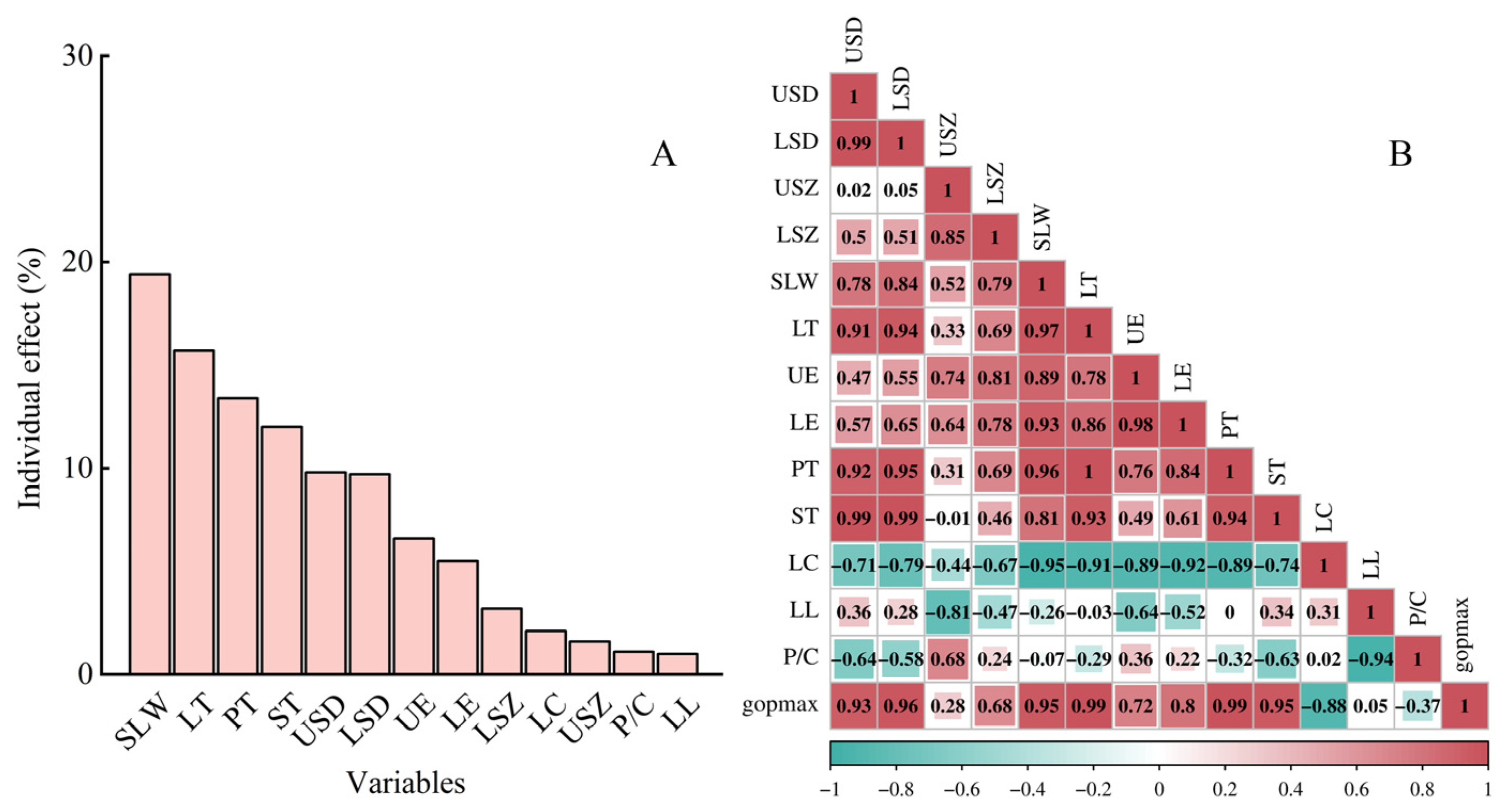
3.6. Contribution of Leaf Characteristics and Stomatal Traits to Maximum Operational Stomatal Conductance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [Green Version]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef] [Green Version]
- von Caemmerer, S.; Evans, J. Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 Plants. Funct. Plant Biol. 1991, 18, 287–305. [Google Scholar] [CrossRef]
- Lloyd, J.; Syvertsen, J.P.; Kriedemann, P.E.; Farquhar, G.D. Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ. 1992, 15, 873–899. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, K.; Maruyama, A.; Kuwagata, T.; Mano, M.; Takimoto, T.; Hayashi, K.; Hasegawa, T.; Miyata, A. Canopy-scale relationships between stomatal conductance and photosynthesis in irrigated rice. Glob. Chang. Biol. 2013, 19, 2209–2220. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. From one side to two sides: The effects of stomatal distribution on photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Doheny Adams, T.W.; Britton Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef]
- Franks, P.J.; Leitch, I.J.; Ruszala, E.M.; Hetherington, A.M.; Beerling, D.J. Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Dow, G.J.; Bergmann, D.C.; Berry, J.A. An integrated model of stomatal development and leaf physiology. New Phytol. 2014, 201, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- McElwain, J.C.; Yiotis, C.; Lawson, T. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol. 2016, 209, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, S.E.; Cannon, W.L.; Elowsky, C.; Tan, S.; Davies, S.J. Variation in leaf stomatal traits of 28 tree species in relation to gas exchange along an edaphic gradient in a Bornean rain forest. Am. J. Bot. 2010, 97, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgren, M.R.; Mathers, A.; Baillie, A.L.; Dunn, J.; Wilson, M.J.; Hunt, L.; Pajor, R.; Fradera-Soler, M.; Rolfe, S.; Osborne, C.P.; et al. Mesophyll porosity is modulated by the presence of functional stomata. Nat. Commun. 2019, 10, 2825. [Google Scholar] [CrossRef] [Green Version]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. Disruption of stomatal lineage signaling or transcriptional regulators has differential effects on mesophyll development, but maintains coordination of gas exchange. New Phytol. 2017, 216, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Wild, A.; Wolf, G. The effect of different light intensities on the frequency and size of stomata, the size of cells, the number, size and chlorophyll content of chloroplasts in the mesophyll and the guard cells during the ontogeny of primary leaves of Sinapis alba. Z. Pflanzenphysiol. 1980, 97, 325–342. [Google Scholar] [CrossRef]
- Dongchen, N.A. Effect of water stress on the structure and chlorophyll content of Sedum tatarinowii Maxim leaves. J. Med. Plant 2011, 2, 40–42. [Google Scholar]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:far-red ratio. Field Crops Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, W. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J.; et al. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crops Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Wang, Z.; Tan, T.; Li, S.; Li, J.; Wang, B.; Zhang, J.; Cheng, Y.; Wu, X.; et al. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant Biol. 2019, 19, 34. [Google Scholar] [CrossRef] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Kuiper, P.J. Dependence upon Wavelength of Stomatal Movement in Epidermal Tissue of Senecio odoris. Plant Physiol. 1964, 39, 952–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D.; Raschke, K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiol. 1981, 68, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Shimazaki, K. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol. 2001, 42, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, M.; Kinoshita, T.; Shimazaki, K. Guard-cell chloroplasts provide ATP required for H(+) pumping in the plasma membrane and stomatal opening. Plant Cell Physiol. 2001, 42, 795–802. [Google Scholar] [CrossRef]
- Olsen, R.L.; Pratt, R.B.; Gump, P.; Kemper, A.; Tallman, G. Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO(2) concentrations in Vicia spp. New Phytol. 2002, 153, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, M.; Shigenaga, A.; Emi, T.; Kinoshita, T.; Shimazaki, K. A transgene encoding a blue-light receptor, phot1, restores blue-light responses in the Arabidopsis phot1 phot2 double mutant. J. Exp. Bot. 2004, 55, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messinger, S.M.; Buckley, T.N.; Mott, K.A. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 2006, 140, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Li, L.L.; Jiang, X.Q.; Liu, Q.C.; Liu, Q.H.; Sun, Y.K.; Wang, K.L. Adaptability of Camellia sasanqua leaf morphology during natural changes in temperature. J. Appl. Ecol. 2016, 27, 2815–2822. [Google Scholar]
- Xie, Z.S.; Du, H.R.; Li, J.B.; Bondada, B. Morphological and structural changes of stomata and leaf veins during growth of grape leaves using tissue clearing technique. Plant Physiol. J. 2018, 54, 237–246. [Google Scholar]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef] [PubMed]
- López-Juez, E.; Bowyer, J.R.; Sakai, T. Distinct leaf developmental and gene expression responses to light quantity depend on blue-photoreceptor or plastid-derived signals, and can occur in the absence of phototropins. Planta 2007, 227, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Fujita, M.; Ohta, Y.; Sase, S.; Nishimura, S.; Ezura, H. Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci. Hortic. 2008, 115, 176–182. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. FPB 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Harbinson, J. An artificial solar spectrum substantially alters plant development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of Lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [Green Version]
- Ando, E.; Kinoshita, T. Red Light-Induced Phosphorylation of Plasma Membrane H(+)-ATPase in Stomatal Guard Cells. Plant Physiol. 2018, 178, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Lampard, G.R.; Macalister, C.A.; Bergmann, D.C. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 2008, 322, 1113–1116. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Kingston, C.; Haworth, M.; Yearsley, J.M.; Batke, S.P.; Lawson, T.; McElwain, J.C. Does size matter? atmospheric CO2 may be a stronger driver of stomatal closing rate than stomatal size in taxa that diversified under low CO2. Front. Plant Sci. 2016, 7, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, M.; Scutt, C.P.; Douthe, C.; Marino, G.; Gomes, M.T.G.; Loreto, F.; Flexas, J.; Centritto, M. Allocation of the epidermis to stomata relates to stomatal physiological control: Stomatal factors involved in the evolutionary diversification of the angiosperms and development of amphistomaty. Environ. Exp. Bot. 2018, 151, 55–63. [Google Scholar] [CrossRef]
- Scoffoni, C.; Chatelet, D.S.; Pasquet-Kok, J.; Rawls, M.; Donoghue, M.J.; Edwards, E.J.; Sack, L. Hydraulic basis for the evolution of photosynthetic productivity. Nat. Plants 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Tosens, T.; Nishida, K.; Gago, J.; Coopman, R.E.; Cabrera, H.M.; Carriquí, M.; Laanisto, L.; Morales, L.; Nadal, M.; Rojas, R.; et al. The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 2016, 209, 1576–1590. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef]
- Jordan, G.J.; Carpenter, R.J.; Koutoulis, A.; Price, A.; Brodribb, T.J. Environmental adaptation in stomatal size independent of the effects of genome size. New Phytol. 2015, 205, 608–617. [Google Scholar] [CrossRef] [Green Version]
- de Boer, H.J.; Price, C.A.; Wagner-Cremer, F.; Dekker, S.C.; Franks, P.J.; Veneklaas, E.J. Optimal allocation of leaf epidermal area for gas exchange. New Phytol. 2016, 210, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Carriquí, M.; Cabrera, H.M.; Conesa, M.; Coopman, R.E.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Tomás, M.; et al. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 2015, 38, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- de Boer, H.J.; Eppinga, M.B.; Wassen, M.J.; Dekker, S.C. A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat. Commun. 2012, 3, 1221. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.J.; Drake, P.L.; Wendt, E.; Price, C.A.; Schulze, E.D.; Turner, N.C.; Nicolle, D.; Veneklaas, E.J. Apparent overinvestment in leaf venation relaxes leaf morphological constraints on photosynthesis in Arid Habitats. Plant Physiol. 2016, 172, 2286–2299. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; de Boer, H.J.; Zixiao, Z.; Chen, X.; Shi, Y.; Peng, S.; Wang, F. The coordinated increase in stomatal density and vein dimensions during genetic improvement in rice. Agron. J. 2020, 112, 2791–2804. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
| Treatments | 400 B | 100 B | 400 R | 100 R |
|---|---|---|---|---|
| SLW (mg/cm2) | 0.223 ± 0.04 a | 0.185 ± 0.0 b | 0.14 ± 0.01 c | 0.125 ± 0.01 c |
| LT (µm) | 115.678 ± 4.4 a | 63.252 ± 4.5 c | 80.334 ± 6.2 b | 55.401 ± 2.9 d |
| UET (µm) | 12.346 ± 1.7 a | 5.813 ± 0.9 b | 12.976 ± 2.1 a | 6.581 ± 1.1 b |
| LET (µm) | 7.161 ± 1.2 a | 4.081 ± 1 b | 6.979 ± 1.2 a | 4.453 ± 0.7 b |
| PT (µm) | 68.541 ± 4.4 a | 40.826 ± 3.3 b | 49.209 ± 5.3 c | 24.386 ± 2.2 d |
| ST (µm) | 33.485 ± 3.8 a | 19.014 ± 2.8 b | 17.873 ± 2.1 b | 14.923 ± 1.5 c |
| LC | 0.593 ± 0.04 b | 0.649 ± 0.08 a | 0.614 ± 0.07 ab | 0.622 ± 0.04 ab |
| LL | 0.289 ± 0.03 ab | 0.298 ± 0.04 a | 0.221 ± 0.02 c | 0.268 ± 0.03 b |
| P/C | 2.07 ± 0.25 c | 2.19 b ± 0.35 bc | 2.805 ± 0.3 a | 2.35 ± 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, J.; Wang, Q.; Tan, X.; Li, S.; Chen, P.; Yong, T.; Wang, X.; Wu, Y.; Yang, F.; et al. Blue-Light-Dependent Stomatal Density and Specific Leaf Weight Coordinate to Promote Gas Exchange of Soybean Leaves. Agriculture 2023, 13, 119. https://doi.org/10.3390/agriculture13010119
Chen J, Gao J, Wang Q, Tan X, Li S, Chen P, Yong T, Wang X, Wu Y, Yang F, et al. Blue-Light-Dependent Stomatal Density and Specific Leaf Weight Coordinate to Promote Gas Exchange of Soybean Leaves. Agriculture. 2023; 13(1):119. https://doi.org/10.3390/agriculture13010119
Chicago/Turabian StyleChen, Jiyu, Jing Gao, Qi Wang, Xianming Tan, Shenglan Li, Ping Chen, Taiwen Yong, Xiaochun Wang, Yushan Wu, Feng Yang, and et al. 2023. "Blue-Light-Dependent Stomatal Density and Specific Leaf Weight Coordinate to Promote Gas Exchange of Soybean Leaves" Agriculture 13, no. 1: 119. https://doi.org/10.3390/agriculture13010119
APA StyleChen, J., Gao, J., Wang, Q., Tan, X., Li, S., Chen, P., Yong, T., Wang, X., Wu, Y., Yang, F., & Yang, W. (2023). Blue-Light-Dependent Stomatal Density and Specific Leaf Weight Coordinate to Promote Gas Exchange of Soybean Leaves. Agriculture, 13(1), 119. https://doi.org/10.3390/agriculture13010119








