Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production
Abstract
:1. Introduction
2. Case History
3. Clinical Analysis and Sampling
4. Chemical Investigation
4.1. Apparatus and Chromatographic Conditions
4.2. Immunoaffinity Clean-Up
4.3. Sample Extraction and Clean-Up of Animal Feeds
4.4. Sample Extraction and Clean-Up of Tissues and Organs
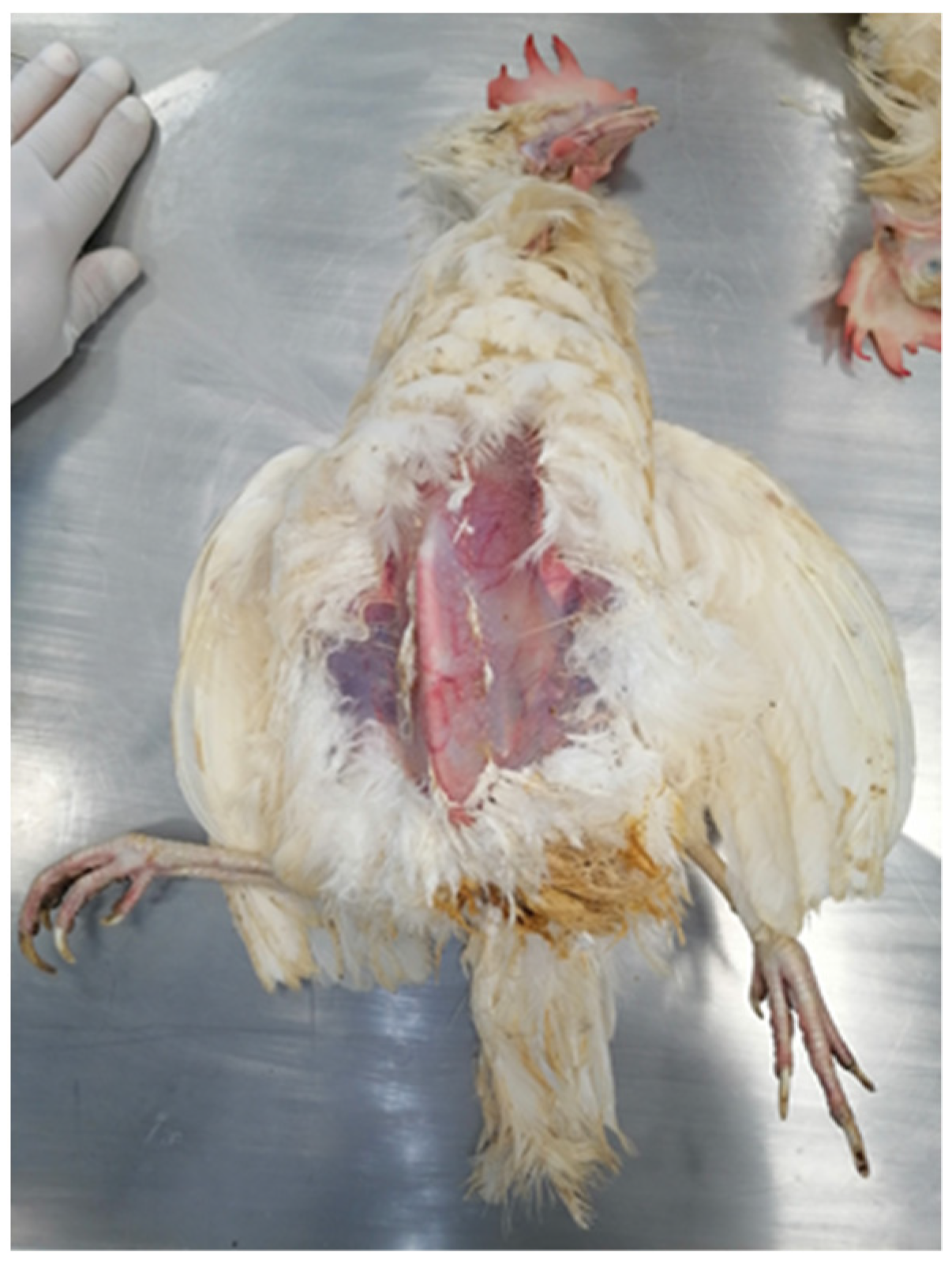
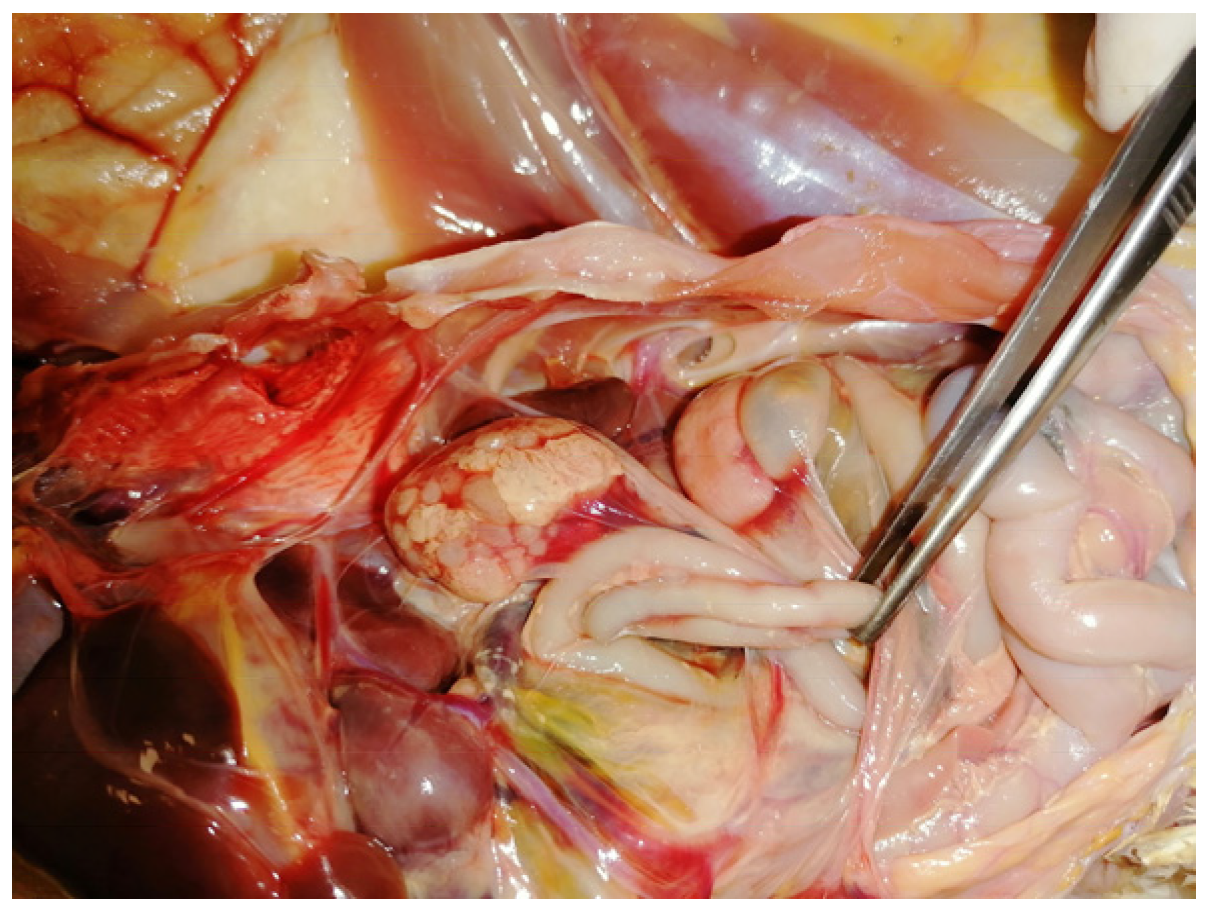
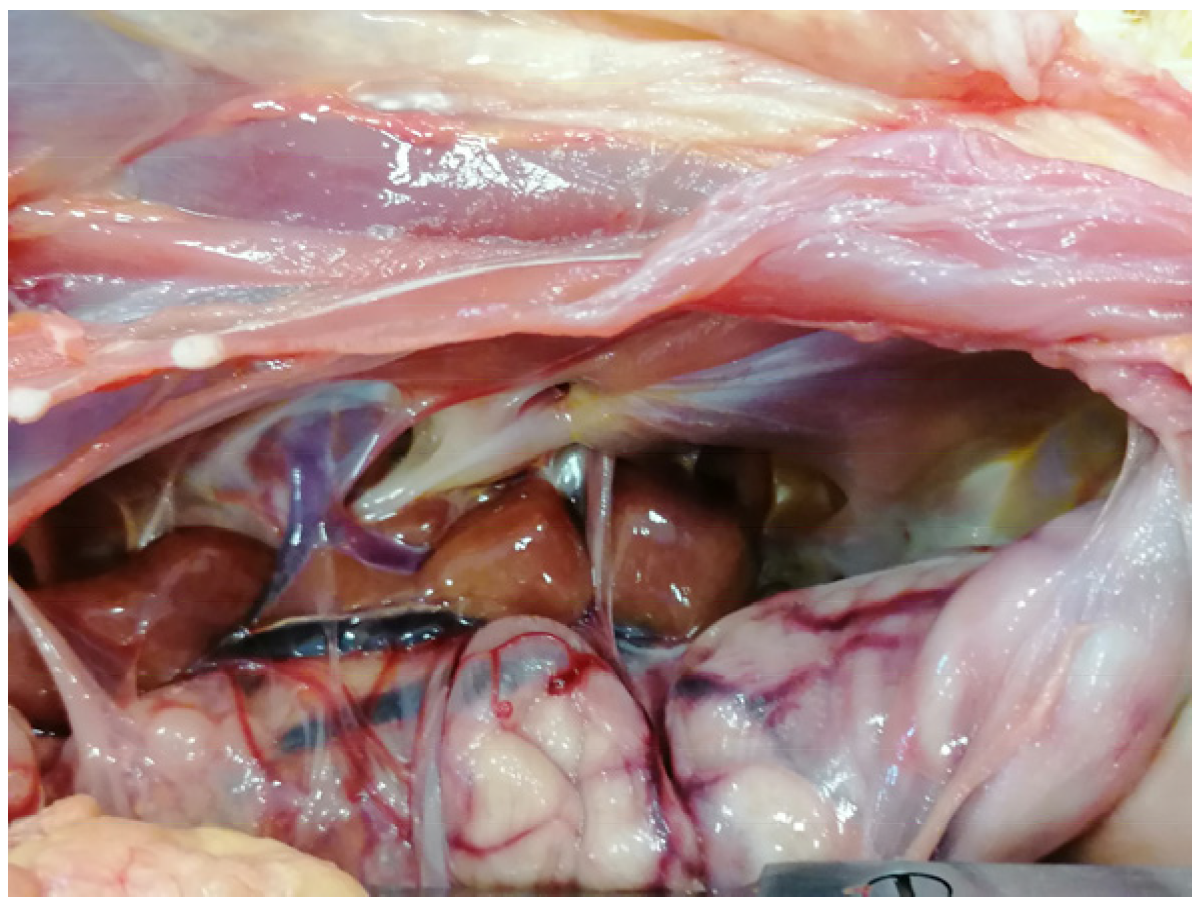
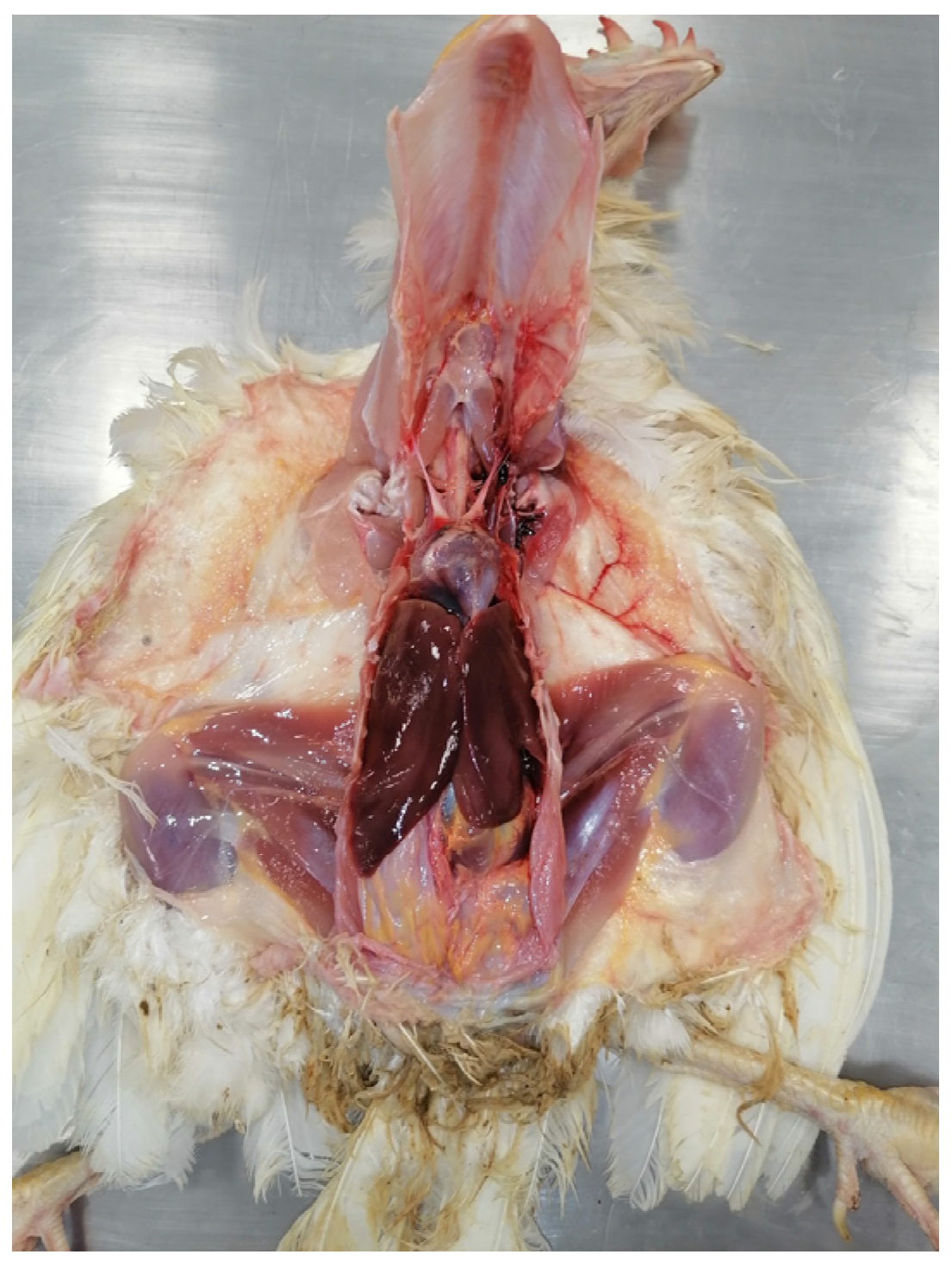
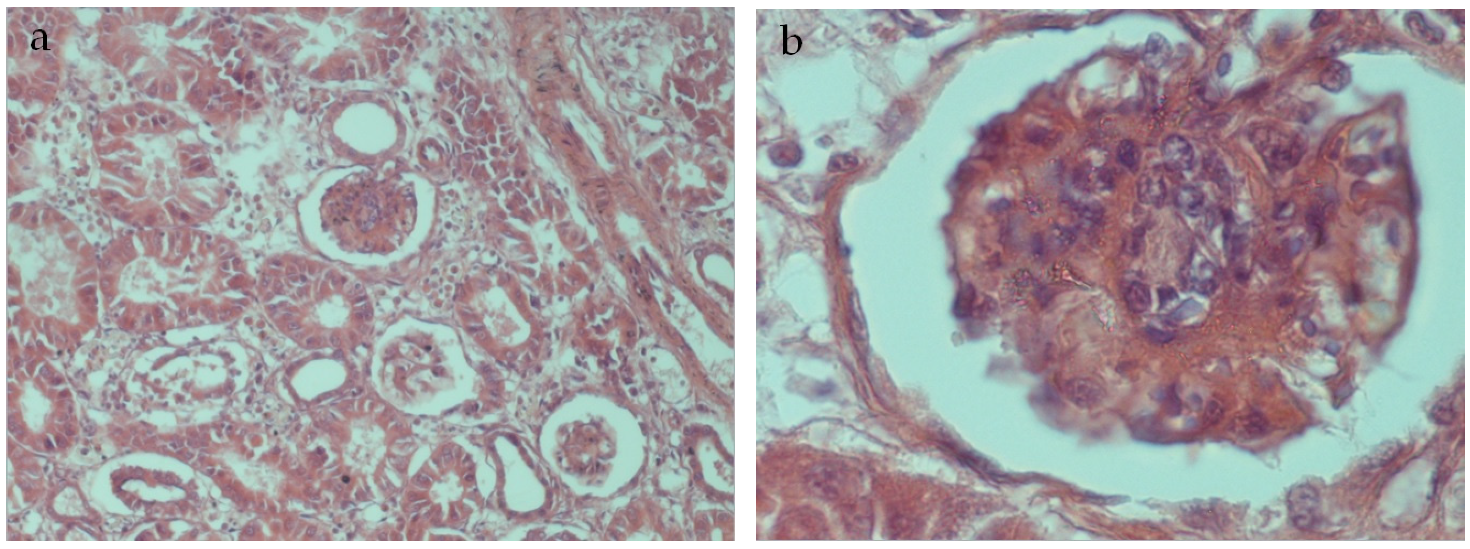
5. Post Mortem Examination
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feijó Corrêa, J.A.; Orso, P.B.; Bordin, K.; Vaz Hara, R.; Luciano, F.B. Toxicological effects of fumonisin B1 in combination with other Fusarium toxins. Food Chem. Toxicol. 2018, 121, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blanco, M.; Marín, S.; Sanchis, V.; Ramos, A.J. Fusarium mycotoxins in total mixed rations for dairy cows. Mycotoxin Res. 2020, 36, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodríguez, A.; Magan, N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Current Opinion in Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.; Focker, M.; De Rijk, T.; Liu, C. Mycotoxins in wheat cultivated in the Netherlands: Results from eight years of field surveys. Mycotoxin Res. 2021, 37, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Corrente, M.; Testa, G.; Casalino, G.; Dimuccio, M.M.; Circella, E.; Brescia, N.; Barrasso, R.; Celentano, F.E. Animal Welfare, Health and the Fight against Climate Change: One Solution for Global Objectives. Agriculture 2021, 11, 1248. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [Green Version]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and de-rived products. EFSA J. 2007, 446, 1–127. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the risk as-sessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A Review on Mycotoxins in Food and Feed: Malaysia Case Study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Bozzo, G.; Ceci, E.; Pinto, P.; Bonerba, E.; Martella, V.; Terio, E.; Tantillo, G. Ochratoxin A in avicultural meat production: Chemical and histological effects. World Mycotoxin J. 2009, 2, 61–69. [Google Scholar] [CrossRef]
- IARC. Agents Classified by the IARC Monographs. 2022, Volumes 1–132. Available online: http://monographs.iarc.fr/ENG/Classification/ (accessed on 20 May 2022).
- Bozzo, G.; Ceci, E.; Bonerba, E.; Desantis, S.; Tantillo, G. Ochratoxin a in laying hens: High-performance liquid chromatography detection and cytological and histological analysis of target tissues. J. Appl. Poult. Res. 2008, 17, 151–156. [Google Scholar] [CrossRef]
- Armorini, S.; Al-Qudah, K.M.; Altafini, A.; Zaghini, A.; Roncada, P. Biliary ochratoxin A as a biomarker of ochratoxin exposure in laying hens: An experimental study after administration of contaminated diets. Res. Vet. Sci. 2015, 100, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Galtier, P.; Alvinerie, M.; Charpenteau, J. The pharmacokinetic profiles of ochratoxin A in pigs, rabbits, and chickens. Food Cosmet. Toxicol. 1981, 19, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hamilton, P.B. Increased severity and new symptoms of infectious bursal disease during aflatoxicosis in broiler chickens. Poult. Sci. 1982, 61, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.M.; Bailey, C.A.; Kubena, L.F.; Huff, W.E.; Harvey, R.B. Ochratoxin A and dietary protein. 1. Effects on body weight, feed conversion, relative organ weight, and mortality in three-week-old broilers. Poult. Sci. 1989, 68, 658–663. [Google Scholar] [CrossRef]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Doerr, J.A. Mycotoxin interactions in poultry and swine. J. Anim. Sci. 1988, 66, 2351–2355. [Google Scholar] [CrossRef] [Green Version]
- Huff, W.E.; Wyatt, R.D.; Hamilton, P.B. Nephrotoxicity of dietary ochratoxin A in broiler chickens. Appl. Microbiol. 1975, 30, 48–51. [Google Scholar] [CrossRef]
- Glahn, R.P.; Wideman, R.F.J.; Evangelisti, J.W.; Huff, W.E. Effects of ochratoxin A alone and in combination with citrinin on kidney function. Of single comb white leghorn. Poult. Sci. 1988, 67, 1034–1042. [Google Scholar] [CrossRef]
- Glahn, R.P.; Shapiro, R.S.; Vena, V.E.; Wideman, R.F., Jr.; Huff, W.E. Effects of chronic ochratoxin A and citrinin toxicosis on kidney function of single comb White Leghorn pullets. Poult. Sci. 1989, 68, 1205–1212. [Google Scholar] [CrossRef]
- Ochieng, P.E.; Scippo, M.; Kemboi, D.C.; Croubels, S.; Okoth, S.; Kang’ethe, E.K.; Doupovec, B.; Gathumbi, J.K.; Lindahl, J.F.; Antonissen, G. Mycotoxins in Poultry Feed and Feed Ingredients from Sub-Saharan Africa and Their Impact on the Production of Broiler and Layer Chickens: A Review. Toxins 2021, 13, 633. [Google Scholar] [CrossRef]
- Rawal, S.; Kim, J.E.; Coulombe, R., Jr. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Voss, K.A.; Beasley, V.A. Selected Mycotoxins Affecting Animal and Human Health. Handb. Toxicol. Pathol. 2002, 1, 645–699. [Google Scholar] [CrossRef]
- Klein, P.J.; Buckner, R.; Kelly, J.; Coulombe, R.A., Jr. Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B-1. Toxicol. Appl. Pharmacol. 2000, 165, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Quist, C.F.; Bounous, D.I.; Kilburn, J.V.; Nettles, V.F.; Wyatt, R.D. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 2000, 36, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casteel, S.W.; Rottinghouse, G.E. Mycotoxicoses. Encycl. Microbiol. 2000, 3, 337–348. [Google Scholar]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Leeson, S.; Diaz, G.J.; Summers, J.D. Poultry Metabolic Disorders and Mycotoxins; University Books: Guelph, SO, Canada, 1995. [Google Scholar]
- Devegowda, G.; Murthy, T.N.K. Mycotoxins: Their effects in poultry and some practical solutions. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 25–56. [Google Scholar]
- Reed, K.M.; Mendoza, K.M.; Coulombe, R.A., Jr. Differential Transcriptome Responses to Aflatoxin B1 in the Cecal Tonsil of Susceptible and Resistant Turkeys. Toxins 2019, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Emmanuel, K.T.; Els, V.P.; Bart, H.; Evelyne, D.; Els, V.H.; Els, D. Carry-over of some Fusarium mycotoxins in tissues and eggs of chickens fed experimentally mycotoxin-contaminated diets. Food Chem. Toxicol. 2020, 145, 111715. [Google Scholar] [CrossRef]
- Stefanaki, I.; Foufa, E.; Tsatsou-Dritsa, A.; Dais, P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 2003, 20, 74–83. [Google Scholar] [CrossRef]
- Commission Recommendation 2006/576/EC of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. O J L 229, 23.8. 2006, pp. 7–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006H0576 (accessed on 13 October 2022).
- Commission Regulation (EU) 2011/574 of 16 June 2011 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as Regards Maximum Levels for Nitrite, Melamine, Ambrosia spp. and Carry-Over of Certain Coccidiostats and Histomonostats and Consolidating. O J L 159, 17.6. 2011, pp. 7–24. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0574 (accessed on 13 October 2022).
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Lohmann Breeders. Available online: https://lohmann-breeders.com/strains/lohmann-lsl-classic-cage-housing/ (accessed on 15 May 2022).
- Monaci, L.; Tantillo, G.; Palmisano, F. Determination of Ochratoxin A in Portuguese Rice Samples by High Performance Liquid Chromatography with Fluorescence Detection. Anal. Bioanal. Chem. 2004, 378, 1777–1782. [Google Scholar] [CrossRef]
- Losito, I.; Monaci, L.; Palmisano, F.; Tantillo, G. Determination of ochratoxin A in meat products by high-performance liquid chromatography coupled to electrospray ionisation sequential mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F.; Tantillo, M.G. Ochratoxin A determination in paired kidneys and muscle samples from swines slaughtered in southern Italy. Food Control 2006, 17, 114–117. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Stoloff, L.; Young, K.; Wyatt, R.D.; Miller, B.L. Aflatoxicol and aflatoxins B1 and M1 in eggs and tissues of laying hens consuming aflatoxin-contaminated feed. Poult. Sci. 1983, 62, 2176–2182. [Google Scholar] [CrossRef]
- Bozzo, G.; Bonerba, E.; Ceci, E.; Colao, V.; Tantillo, G. Determination of ochratoxin A in eggs and target tissues of ex-perimentally drugged hens using HPLC–FLD. Food Chem. 2011, 126, 1278–1282. [Google Scholar] [CrossRef]
- Dwivedi, P.; Burns, R.B. Pathology of ochratoxicosis in young broiler chicks. Res. Vet. Sci. 1984, 36, 92–103. [Google Scholar] [CrossRef]
- Kozaczyński, W. Experimental ochratoxicosis A in chickens. Histopathological and histochemical study. Arch. Vet. Pol. 1994, 34, 205–219. [Google Scholar] [PubMed]
- Ali, A.; Khatoon, A.; Almohaimeed, H.M.; Al-Sarraj, F.; Albiheyri, R.; Alotibi, I.; Abidin, Z.U. Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period. Toxins 2022, 14, 689. [Google Scholar] [CrossRef]
- Lötzsch, R.; Leistner, L. Transmission of aflatoxins into eggs and egg products. Ann. Nutr. L’alimenta-Tion 1977, 31, 499–508. [Google Scholar]
- Oliveira, C.A.; Kobashigawa, E.; Reis, T.A.; Mestieri, L.; Albuquerque, R.; Corrêa, B. Aflatoxin B1 residues in eggs of laying hens fed a diet containing different levels of the mycotoxin. Food Addit. Contam. 2000, 17, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Exarchos, C.C.; Gentry, R.F. Effect of aflatoxin B1 on egg production. Avian Dis. 1982, 26, 191–195. [Google Scholar] [CrossRef]
- Song, B.; Zeb, J.; Hussain, S.; Aziz, M.U.; Circella, E.; Casalino, G.; Camarda, A.; Yang, G.; Buchon, N.; Sparagano, O. A Review on the Marek’s Disease Outbreak and Its Virulence-Related meq Genovariation in Asia between 2011 and 2021. Animals 2022, 12, 540. [Google Scholar] [CrossRef]
- Dunn, J. Lymphoid Leukosis in Poultry. MSD Veterinary Manual 2022. Available online: https://www.msdvetmanual.com/veterinary/poultry/neoplasms/lymphoid-leukosis-in-poultry (accessed on 28 December 2022).
- Schat, K.A.; van Santen, V.L. Chicken infectious anemia and circovirus infections in commercial flocks. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 284–320. [Google Scholar] [CrossRef]
- Jackwood, M.W.; de Wit, S. Infectious bronchitis. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 167–188. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Graziani, G.; Gaspari, A.; Izzo, L.; Tolosa, J.; Rodríguez-Carrasco, Y.; Ritieni, A. Target analysis and ret-rospective screening of multiple mycotoxins in pet food using UHPLC-Q-orbitrap HRMS. Toxins 2019, 11, 434. [Google Scholar] [CrossRef]
- Pozzo, L.; Cavallarin, L.; Antoniazzi, S.; Guerre, P.; Biasibetti, E.; Capucchio, M.T.; Schiavone, A. Feeding a diet con-taminated with ochratoxin A for broiler chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 2. Effects on meat quality, oxidative stress, residues and histological traits. J. Anim. Physiol. Anim. Nutr. 2013, 97 (Suppl. 1), 23–31. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. O J L 95, 7.4.2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0625 (accessed on 24 October 2022).
- Bozzo, G.; Dimuccio, M.M.; Casalino, G.; Ceci, E.; Corrente, M. New approaches for risk assessment and management of bovine protothecosis. Saudi J. Biol. Sci. 2022, 29, 103368. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzo, G.; Pugliese, N.; Samarelli, R.; Schiavone, A.; Dimuccio, M.M.; Circella, E.; Bonerba, E.; Ceci, E.; Camarda, A. Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production. Agriculture 2023, 13, 138. https://doi.org/10.3390/agriculture13010138
Bozzo G, Pugliese N, Samarelli R, Schiavone A, Dimuccio MM, Circella E, Bonerba E, Ceci E, Camarda A. Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production. Agriculture. 2023; 13(1):138. https://doi.org/10.3390/agriculture13010138
Chicago/Turabian StyleBozzo, Giancarlo, Nicola Pugliese, Rossella Samarelli, Antonella Schiavone, Michela Maria Dimuccio, Elena Circella, Elisabetta Bonerba, Edmondo Ceci, and Antonio Camarda. 2023. "Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production" Agriculture 13, no. 1: 138. https://doi.org/10.3390/agriculture13010138
APA StyleBozzo, G., Pugliese, N., Samarelli, R., Schiavone, A., Dimuccio, M. M., Circella, E., Bonerba, E., Ceci, E., & Camarda, A. (2023). Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production. Agriculture, 13(1), 138. https://doi.org/10.3390/agriculture13010138






