Geraniol: A Potential Defense-Related Volatile in “Baiye No. 1” Induced by Colletotrichum camelliae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Processing and Testing
2.3. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericide Concentration (MBC)
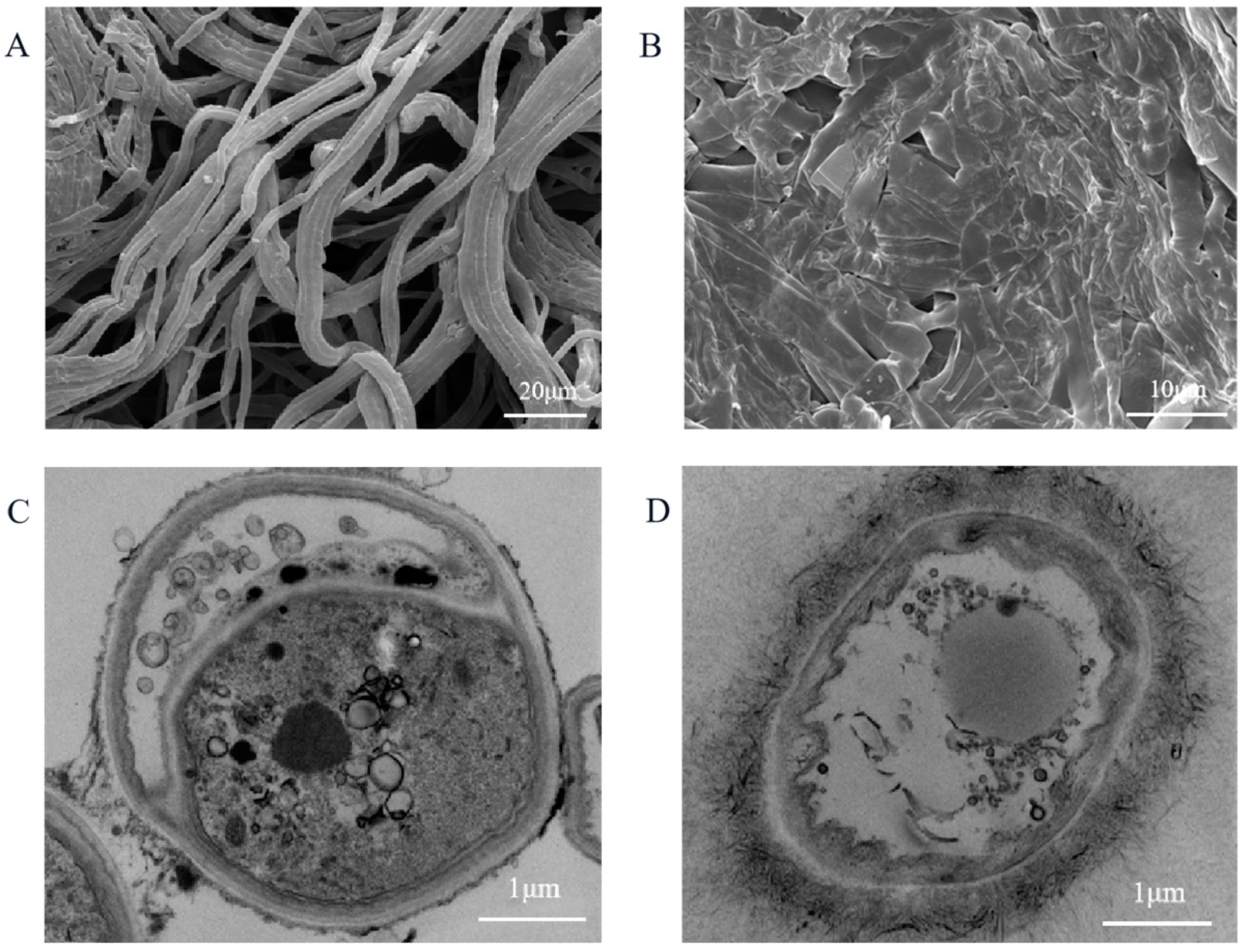
2.4. Mycelial Morphology and Structure of Mycelial Cell
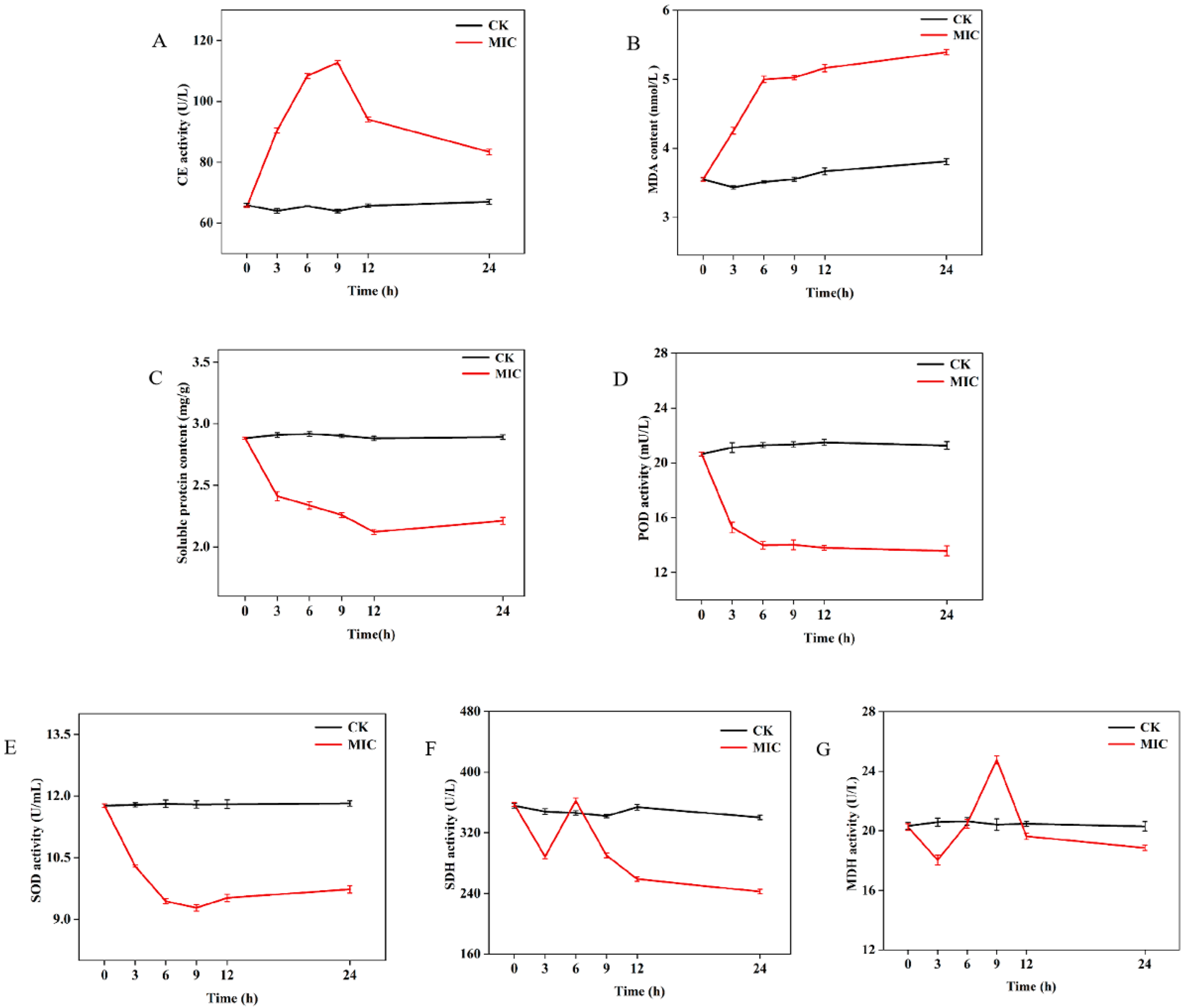
2.5. Determination of Enzymatic Activity and Content of C. camelliae
3. Results and Discussion
3.1. Difference Analysis of Volatiles
3.2. Determination of MIC and MBC
3.3. Effect of Geraniol on Mycelial Morphology and Cell Structures of C. camelliae
3.4. Analysis of the Activity and Content of C. camelliae-Related Enzymes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavijo Mccormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Erb, M. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant Cell Environ. 2019, 42, 959–971. [Google Scholar] [CrossRef] [Green Version]
- Song, G.C.; Ryu, C.M. Evidence for volatile memory in plants: Boosting defence priming through the recurrent application of plant volatiles. Mol. Cells 2018, 41, 724–732. [Google Scholar] [PubMed]
- Arimura, G.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Fang, T.; Zhao, W.L.; Gao, X.W. Communication between plants: Induced resistance in poplar seedlings following herbivore infestation, mechanical wounding, and volatile treatment of the neighbors. Entomol. Exp. Appl. 2013, 149, 110–117. [Google Scholar]
- Sharifi, R.; Lee, S.M.; Ryu, C.M. Microbe-induced plant volatiles. New Phytol. 2018, 220, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef] [Green Version]
- Kaori, S.; Kyutaro, K.; Rika, O.; Soichi, K.; Soichi, U.; Genichiro, A.; Junichiro, H.; Takaaki, N.; Kenji, M.; Junji, T. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.; Mclean, A.; Godfray HC, J.; Gols, R.; Dicke, M. Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, L.; Wang, L.; Wang, Q. Indole primes plant defense against necrotrophic fungal pathogen infection. PLoS ONE 2018, 13, e207607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Hamberg, M.; Fournier, J. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.S.; Greene-McDowelle, D.M.; Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. Effects of volatile aldehydes from Aspergillus-resistant varieties of corn on Aspergillus parasiticus growth and aflatoxin biosynthesis. Toxicon 2000, 38, 1215–1223. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Li, Y.B.; Qi, L.; Wan, X.C. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae massea. J. Agric. Food Chem. 2006, 54, 3936–3940. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Morales-Vargas, A.T.; Molina-Torres, J.; ádame-Alvarez, R.M.; Acosta-Gallegos, J.A.; Heil, M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.M.; Yang, Y.J. The Classic of Tea in China; Shanghai Culture Press: Shanghai, China, 2011; p. 390. [Google Scholar]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. Gloeosporioides complex. Persoonia 2015, 35, 63–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Pan, Y.; Dai, Y.; Gao, Z. First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui province, China. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Ai, Q. Inhibitory effect and antimicrobial mechanism of pyrolin on Monilinia fructicola in peach. Sci. Agric. Sin. 2009, 42, 2784–2792. [Google Scholar]
- Zhang, J.; Du, C.; Li, Q.; Hu, A.; Peng, R.; Sun, F.; Zhang, W. Inhibition mechanism and antibacterial activity of natural antibacterial agent citral on bamboo mould and its anti-mildew effect on bamboo. R. Soc. Open Sci. 2021, 8, 202244. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Brown, R.L.; Neucere, J.N.; Cleveland, T.E. Relationships between C-6-C-l 2 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin production. J. Agric. Food Chem. 1996, 44, 403–407. [Google Scholar] [CrossRef]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Mo, F.X.; Hu, X.F.; Ding, Y.; Li, R.Y.; Long, Y.H.; Wu, X.M.; Li, M. Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Phys. 2021, 178, 104942. [Google Scholar] [CrossRef]
- Chen, C.J.; Li, Q.Q.; Ma, Y.N.; Wang, W.; Cheng, Y.X.; Xu, F.R.; Dong, X. Antifungal effect of essential oils from five kinds of rutaceae Plants-Avoiding pesticide residue and resistance. Chem. Biodivers. 2019, 16, e1800688. [Google Scholar] [CrossRef]
- Miller, S.S.; Driscoll, B.T.; Gregerson, R.G.; Gantt, J.S.; Vance, C.P. Alfalfa malate dehydrogenase (MDH): Molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998, 15, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Yamamoto, Y.; Kamio, Y. Molecular biology of oxygen tolerance in lactic acid bacteria: Functions of NADH oxidases and Dpr in oxidative stress. J. Biosci. Bioeng. 2000, 90, 484–493. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Y.; Song, X.; Liu, S.; Li, M.; Li, R.; Ruan, H. Proteomic analysis reveals that naturally produced citral can significantly disturb physiological and metabolic processes in the rice blast fungus Magnaporthe oryzae. Pest. Biochem. Physiol. 2021, 175, 104835. [Google Scholar] [CrossRef] [PubMed]
- Kujur, A.; Kumar, A.; Yadav, A.; Prakash, B. Elucidation of antifungal and aflatoxin B1 inhibitory mode of action of Eugenia caryophyllata L. Essential oil loaded chitosan nanomatrix against Aspergillus flavus. Pest. Biochem. Physiol. 2021, 172, 104755. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Kim, Y.S.; Shi, Y.R.; Choi, G.J.; Yong, H.C.; Jang, K.S.; Cha, B.; Han, S.S.; Kim, J.C. In vitro and in vivo antifungal activities of decursin and decursinol angelate isolated from Angelica gigas against Magnaporthe oryzae, the causal agent of rice blast. Pestic. Biochem. Physiol. 2011, 101, 118–124. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; de Freitas, M.A.; Gondim, C.N.F.L.; de Albuquerque, R.S.; de Alencar Ferreira, J.V.; Andrade, J.C. In vitro antimicrobial activity of Geraniol and Cariophyllene against Staphylococcus aureus. Rev. Cuba. Plantas Med. 2015, 20, 98–105. [Google Scholar]
- Sharma, Y.; Khan, L.A.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Médicale 2016, 26, 244–254. [Google Scholar] [CrossRef]
- Bard, M.; Albrecht, M.R.; Gupta, N.; Guynn, C.J.; Stlllwell, W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids 1988, 23, 534–538. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Liu, H.; Chen, Y.; Liu, Y.; Ma, C.; Cheng, Y.; Yang, W. Geraniol: A Potential Defense-Related Volatile in “Baiye No. 1” Induced by Colletotrichum camelliae. Agriculture 2023, 13, 15. https://doi.org/10.3390/agriculture13010015
Chen W, Liu H, Chen Y, Liu Y, Ma C, Cheng Y, Yang W. Geraniol: A Potential Defense-Related Volatile in “Baiye No. 1” Induced by Colletotrichum camelliae. Agriculture. 2023; 13(1):15. https://doi.org/10.3390/agriculture13010015
Chicago/Turabian StyleChen, Wei, Huifang Liu, Yao Chen, Yaoguo Liu, Chiyu Ma, Yongjia Cheng, and Wen Yang. 2023. "Geraniol: A Potential Defense-Related Volatile in “Baiye No. 1” Induced by Colletotrichum camelliae" Agriculture 13, no. 1: 15. https://doi.org/10.3390/agriculture13010015



