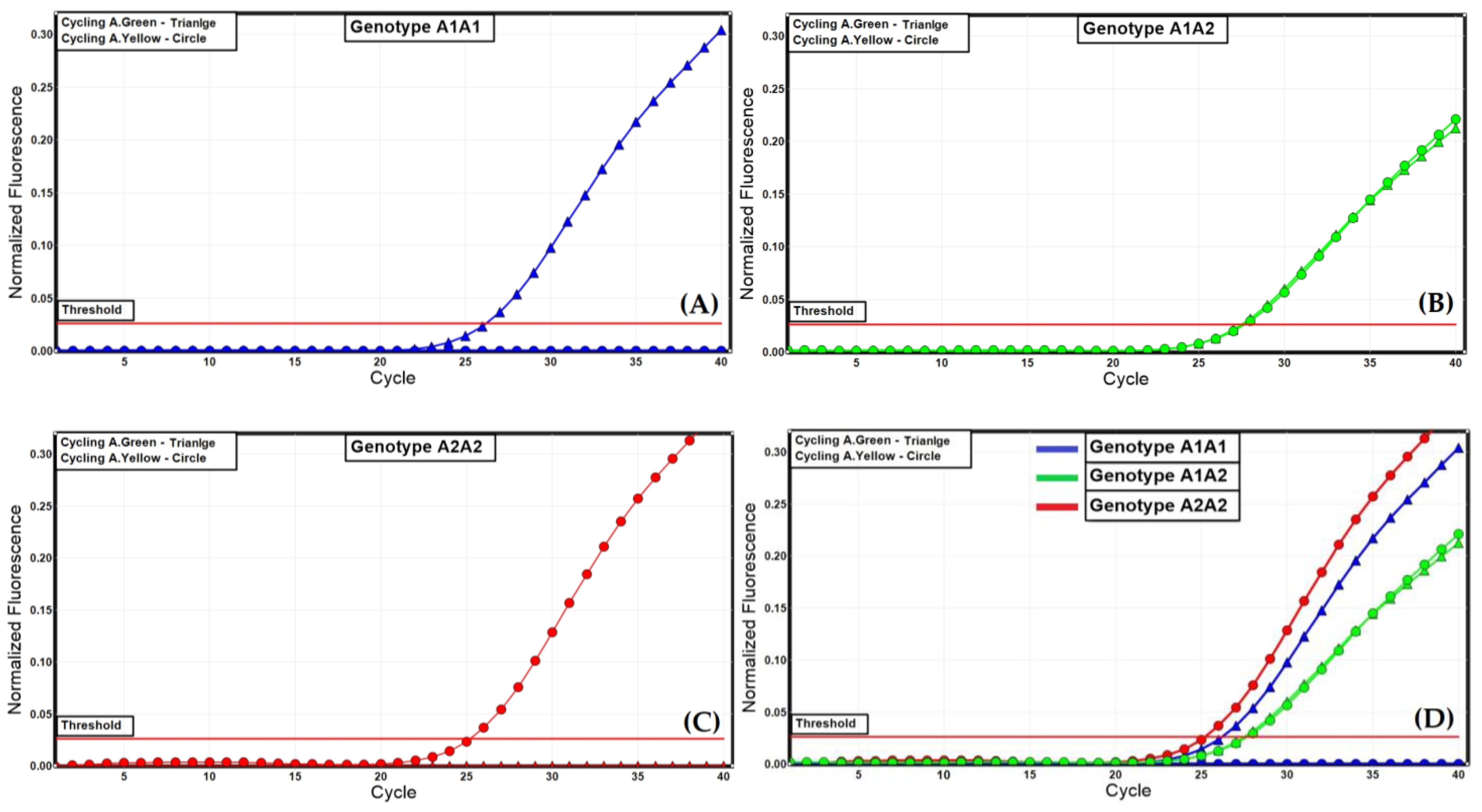
Hedge [
19] reported that the type A1 beta-casein was found in the milk of the breeds of North European origin, such as Friesian, Ayrshire, British Shorthorn and Holstein, while type A2 milk was found in cows of Guernsey, Jersey and the Channel Islands and the Southern French breeds. However, our findings suggest that breeding in high-producing herds alters the genetic structure of the population. In the analyzed Holstein cow population, we observed an increase in the frequency of the A2A2 genotype (57.64%), while still maintaining a sufficient number of A1A2 heterozygotes (37.08%) in the population. The homozygous genotype A1A1 was detected in a few isolated cases with a frequency of 5.28%. Although no national program for breeding Holstein cattle for the
CSN2 gene was implemented in Slovakia, the demand for A2 milk has caused some breeders to select their herds for the A2 variant of CSN2. In our case, one of the farms (Herd 5) implemented a selection in order to increase the proportion of A2A2 cows in the herd. In this farm, the A2A2 genotype was represented by 63.70% and the prevalence of the A2 allele was 80.30%. The farm in question is not related to any A2 organization and delivers milk to retailers as “A2 casein enhanced” milk. Although the selection for the
CSN2 gene was not carried out in the remaining farms, the proportion of the A1A1 genotype was also low in these farms with a frequency of occurrence ranging from 1.59% to 14.96%. The frequency of the A2 allele was higher compared to the A1 allele and ranged from 62.20% to 75% (
Supplementary Table S1). Therefore, the increased frequency of the A2A2 genotype and the A2 allele in the Slovak Holstein cow population may be due to both a deliberate selection for the A2 variant of CSN2, as in the case of Herd 5, but also unintentionally due to the use of bulls with the A2A2 genotype. In Herd 3, which represented the red-and-white variant of the Holstein breed, we found a statistical difference in the genotype frequencies. This difference may have been due to the low number of individuals, as this is a minority population. The high proportion of heterozygotes in this herd could also indicate the use of bulls with the A2A2 genotype in breeding. As reported in this study, Oleński et al. [
26] in the Holstein cattle population, Ganguly et al. [
27] in a population of Holstein crossbreeds and Čítek et al. [
28] in the Simmental population of cattle detected the predominance of the A2A2 genotype, a lower frequency of the A1A2 genotype and the smallest frequency of the A1A1 genotype. The frequencies of the A2 allele in our population were similar to those reported by Beja-Pereira et al. [
29] in Pinzgau cattle. The dominance of the
CSN2 A2 allele, detected by Caroli et al. [
30], was also found in a population of Carora cattle and by Manga et al. [
31] in a population of Czech Spotted and Czech Holstein cattle. In contrast, Hanusová et al. [
32] found a higher frequency of the A1 allele in the Holstein cattle population. Many studies suggested that the A1 beta-casein variant was a health risk factor and was associated with diseases such as type I diabetes mellitus [
13,
18], coronary disease [
12] and sudden infant death syndrome (SIDS) [
14]. According to Boyce et al. [
33], BCM-7, which arises from the A1 variant of beta-casein, in addition to intolerance, may induce a specific immune response leading to allergic reactions. Several authors [
34,
35,
36] identified that the A2 variant can negatively affect the technological properties of milk. They state that the presence of an additional proline in A2 can have a significant effect on the hydrophobicity of the protein, leading to less ordered structures that ultimately affect the casein micelle size, emulsifying and foaming properties and the formation of rennet and acidic curd. Similarly, Chessa et al. [
37] reported that the selection of the A2 variant of beta-casein can lead to a reduction in the frequencies of the A1 and B alleles, which were included in the most favorable genotypes and haplotypes for cheese making and was, therefore, an alert for the loss of useful biodiversity. Therefore, the occurrence of the low frequency of the A1A1 genotype in our population may also have a negative impact on cheese production in the future. The assessment of the genetic structure of the Holstein cattle population was essential for viability status monitoring and was necessary for the determination of suitable conservation units for genetic identification purposes. The loss of the genetic variability caused by the limited population size of the captive population was an important concern. The heterozygosity is widely used because it is proportional to the amount of the genetic variability at a locus and is suitable for theoretically considering the effect of a limited population size on the genetic variation [
38,
39]. Wright´s fixation indices (F
IS), or the F-statistic, are the parameters most widely used to describe the population structure [
24]. In our case, the number of the heterozygous genotypes corresponded to the theoretically expected values. The average F
IS value was −0.0218 and indicated an excess of heterozygotes in Holstein cows compared to the HWE expectations. Despite the selection in Herd 5, there was no reduction in the heterozygotes (F
IS = −0.0493) (
Supplementary Table S2). This suggested that although the selection led to an increase in the proportion of the A2A2 genotype and the A2 allele, a sufficient number of heterozygotes was still retained. In the population of the red-and-white variant of the Holstein cows, which is a minority in Slovakia, a high proportion of heterozygotes (F
IS = −0.5065) was observed, which indicated that genetic variability was maintained. These results confirmed that the selection of herds was random and represented the population of Slovak Holstein cattle.
The milk production data were based on the test day records. The test day records are considered as a more accurate expression of the cattle milk production as a whole lactation data expression. In the Slovak Republic, the test day records are used for the estimation of the breeding values for all the milk traits and the somatic cell counts. There was no statistically significant difference between the groups of CSN2 genotypes for daily milk kilograms and daily fat kilograms. However, this may not be an objective view of the effect of the genotypes on the test day milk yield records because it was a simple descriptive statistic that did not take into account the influence of other effects.
The protein kilograms, fat and protein content were confirmed by the statistically significant differences (p ≤ 0.05) between the least squares means of the analyzed yield according to the genotypes.
The statistical significance was positive in favor of the A2 allele for the protein in kilograms and partially for the protein content percentage. The highest least squares means for the kilograms of protein was found for the genotypes A2A2 (1.07) and A1A2 (1.06). The highest least squares means for the content of protein (%) was found only for the genotype A2A2 (3.32). Only the highest least squares means for the fat percentage was found for the genotype A1A1 (3.75%) compared to the genotype A2A2 (3.68%). This was considered a slightly negative trend.
Morris et al. [
40] showed a positive dominance of the A2 allele for milk yield in the Holstein cattle population. Similar results are available for milk and protein in kilograms [
41,
42] which agree with our results. However, the casein genes were not segregated independently, which was contrary to our approach. Ivankovič et al. [
43] found similar results in conventional and local cattle breeds in Croatia. Also, negative trends only for fat content were found. Our results were in agreement with Gai et al. [
35] who confirmed that the A2 allele was associated with a higher protein yield and the allele A1 was mostly associated with higher fat content. De Vitte et al. [
44] concluded that the amino acid content, fatty acid content and color of the milk could be influenced by the
CSN2 genotypes A1A1, A1A2 and A2A2. They reported that the selective breeding of the genotypes with preferred qualities may improve milk and dairy products, while the genotype A2A2 showed important fatty acids content in milk fats.