Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Potassium Silicon Treatments
2.3. Phenological and Pomological Traits
2.4. Agronomical Traits and Vegetative Growth
2.5. Fruit Physicochemical Parameters
2.6. Fruit Composition Analysis
2.7. Phenolic Compounds Extraction and Quantification
2.8. Vitamin B5, Vitamin C Extraction and Quantification
2.9. Decay Incidence, Ethylene Biosynthesis and Chilling Injury (CI) Symptoms
2.10. Statistical Analysis
3. Results
3.1. Phenological Traits
3.2. Tree Vigor, Vegetative Growth, Yield and Nutritional Composition
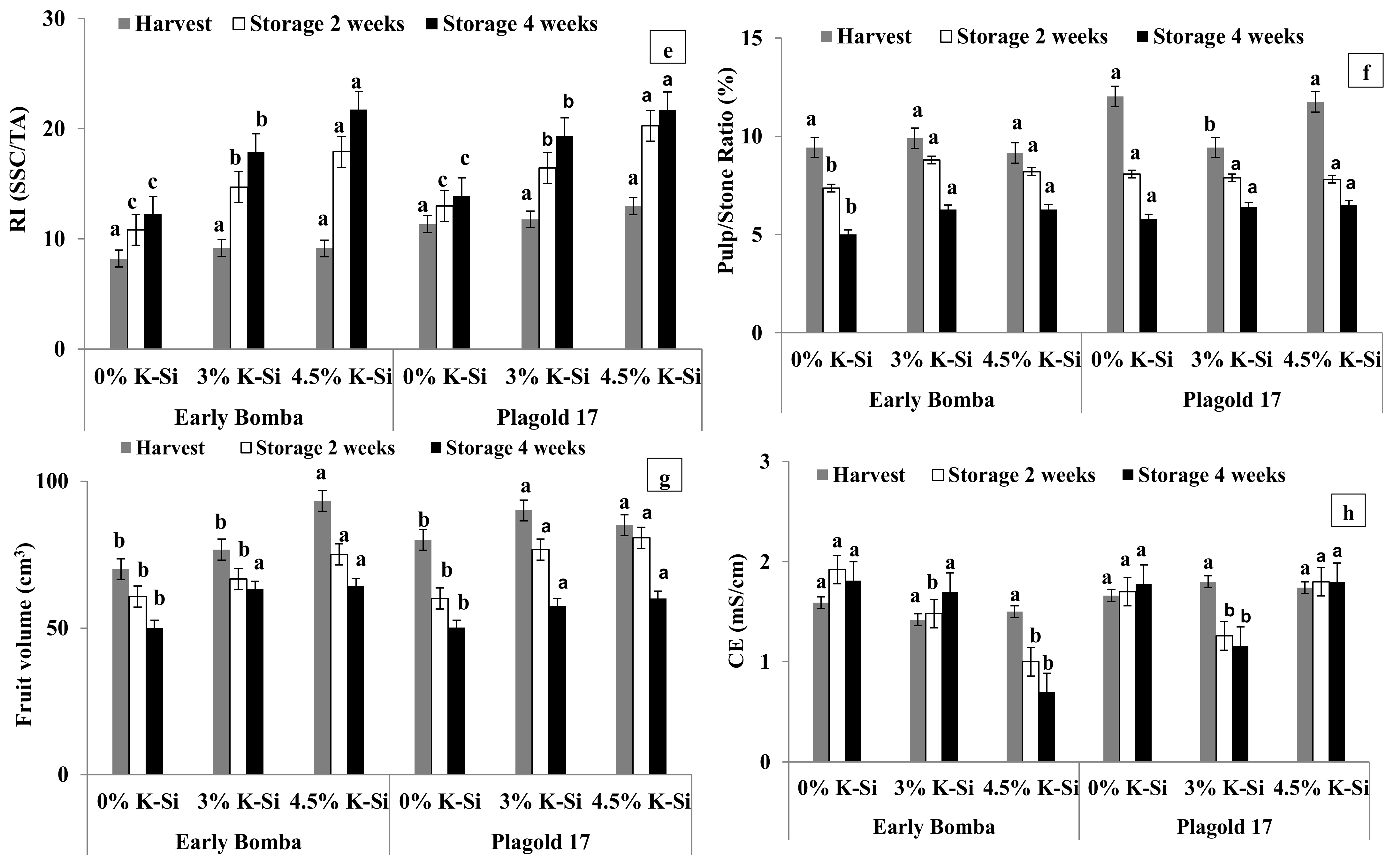
3.3. Physicochemical Parameters
3.4. Phenolic Compounds Content
3.5. Vitamin B5 and Vitamin C Analysis
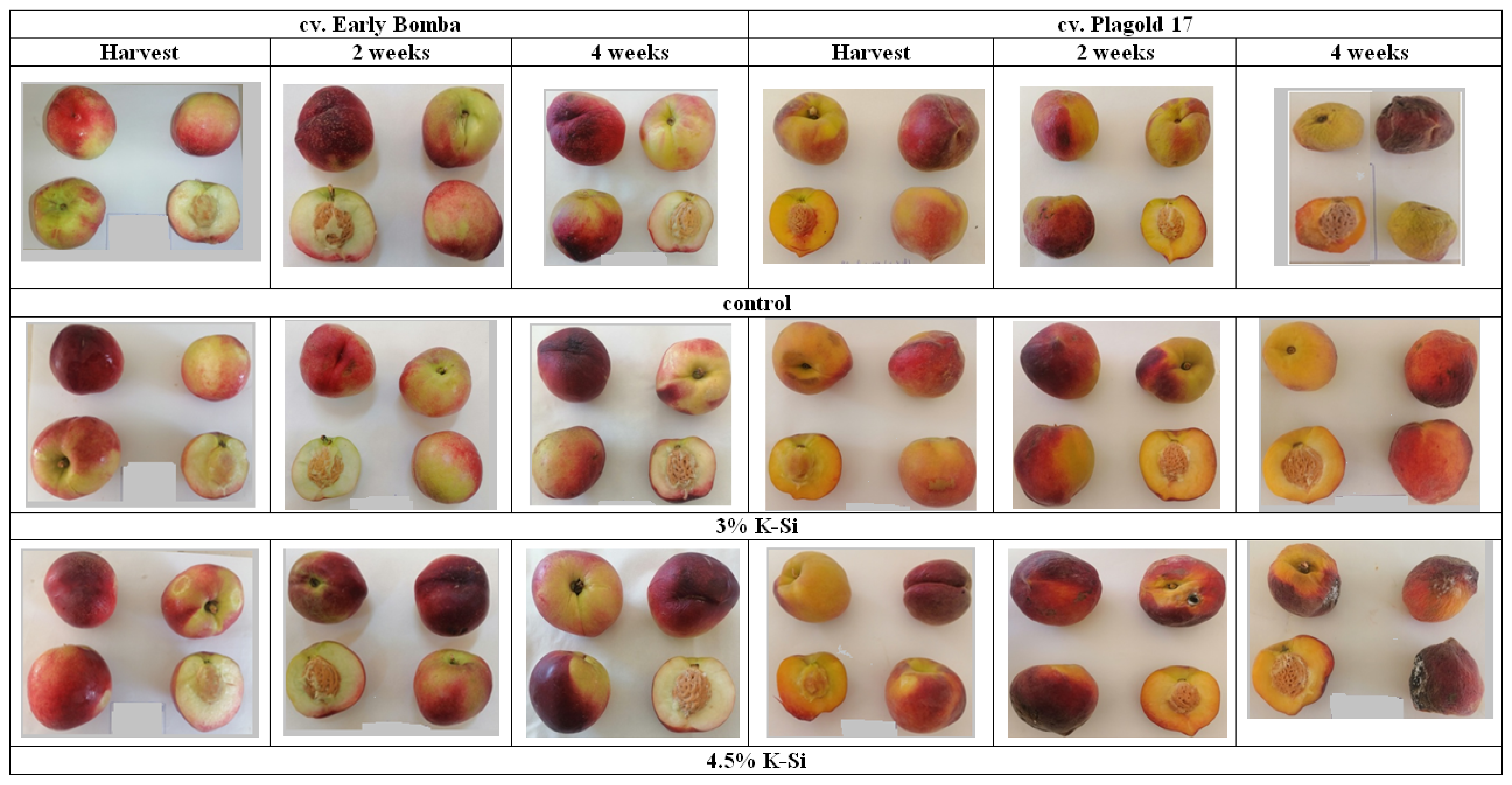
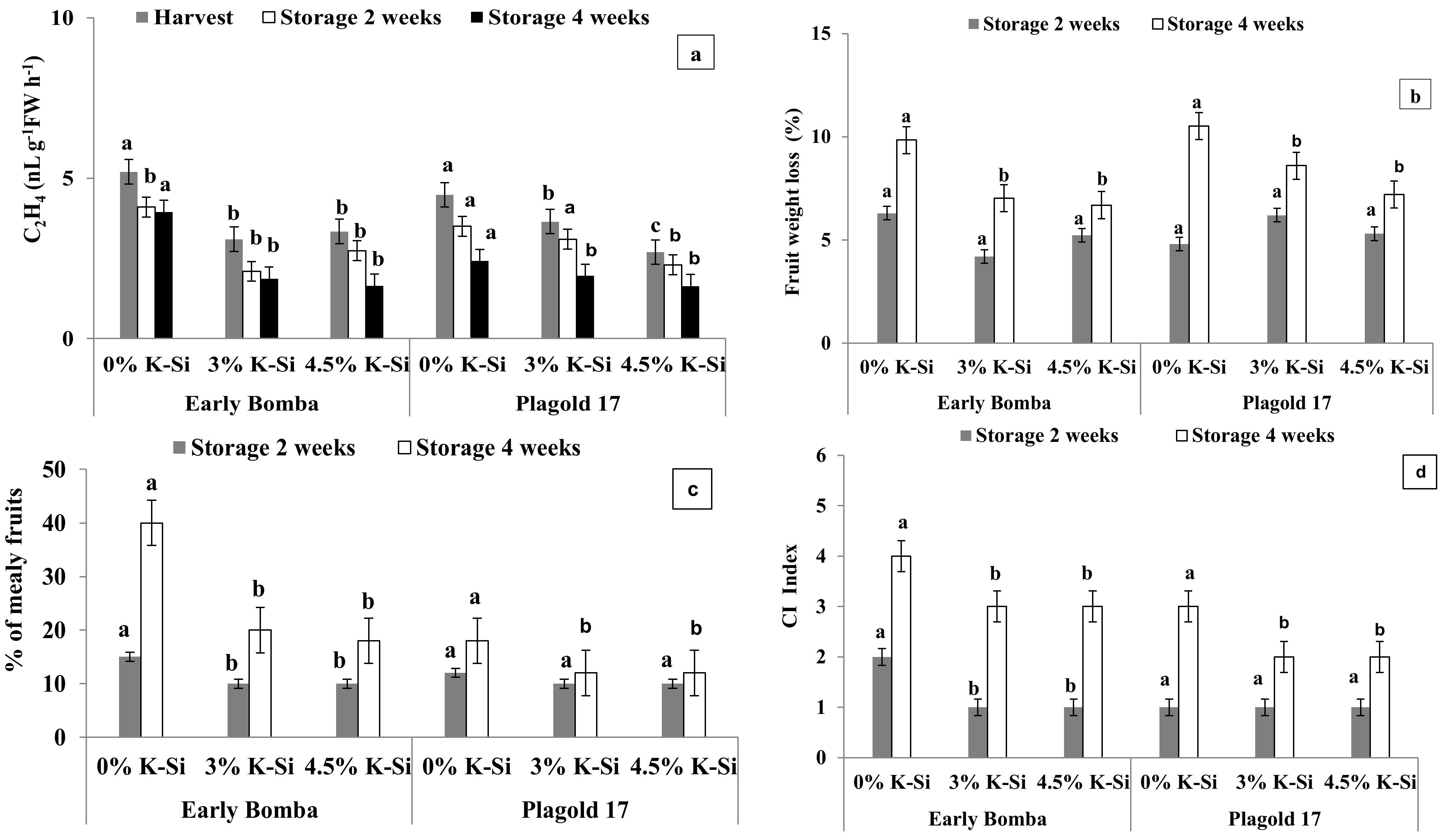
3.6. Ethylene Biosynthesis, Fruit Decay and Chilling Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2022. Available online: http://www.faostat.fao.org (accessed on 1 December 2022).
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Studies on enhancing the keeping quality of peach (Prunus persica Batsch) cv. Elberta by gamma-irradiation. Rad. Physiol. Chem. 2008, 77, 473–481. [Google Scholar] [CrossRef]
- Lalithya, K.A.; Bhagya, H.P.; Choudhary, R. Response of silicon and micro nutrients on fruit character and nutrient content in leaf of sapota. Biolife 2014, 2, 593–598. [Google Scholar]
- Okba, S.K.; Mazrou, Y.; Elmenofy, H.M.; Ezzat, A.; Salama, A.M. New Insights of Potassium Sources Impacts as Foliar Application on ‘Canino’ Apricot Fruit Yield, Fruit Anatomy, Quality and Storability. Plants 2021, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis melo L.) case study. Plant Soil 2010, 335, 117–131. [Google Scholar] [CrossRef]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.; Mohapatra, P.; Nguyen, N.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Ajay; Kundu, S.; Rao, A.S. A Case for Silicon Fertilization to Improve Crop Yields in Tropical Soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatinriboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Mditshwa, A.; Bower, J.P.; Bertling, I.; Mathaba, N. Investigation of the Efficiency of the Total Antioxidants Assays in Silicon-Treated Lemon Fruit (Citrus limon). Acta Hort. 2013, 1007, 93–102. [Google Scholar] [CrossRef]
- Ramina, A.; Tonutti, P.; McGlasson, B. Ripening, nutrition, and postharvest physiology. In The Peach Botany, Production and Uses; CABI: Oxfordshire, UK, 2008; pp. 550–574. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Barreto, C.F.; Ferreira, L.V.; Navroski, R.; Benati1, J.A.; Cantillano, R.F.F.; Vizzotto, M.; Nava, G.; Antunes, L.E.C. Effect of potassium fertilizers associated with cold storage on peach (Prunus persica L.) quality. Austral. J. Crop Sci. 2021, 15, 319–324. [Google Scholar] [CrossRef]
- Abidi, W.; Cantín, C.; Jiménez, S.; Giménez, R.; Moreno, M.A.; Gogorcena, Y. Influence of antioxidant compounds, Total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica L. Batsch) progeny. J. Sci. Food Agric. 2015, 95, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidi, W.; Jiménez, S.; Moreno, M.Á.; Gogorcena, Y. Evaluation of antioxidant compounds and total sugar content in a nectarine [Prunus persica (L.) Batsch] progeny. Int. J. Mol. Sci. 2011, 12, 6919–6935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254, 637. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Akram, N.A.; Alqurainy, F.; Foolad, M.R. Chapter five—Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agronomy 2011, 111, 249–296. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.L., Jr.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Effect of pectin-based coating on the kinetics of quality change associated with stored avocados. J. Food Process. Preserv. 2008, 32, 621–643. [Google Scholar] [CrossRef]
- Cantín, C.M.; Crisosto, C.H.; Ogundiwin, E.A.; Gradziel, T.; Torrents, J.; Moreno, M.A.; Gogorcena, Y. Chilling injury susceptibility in an intra-specific peach [Prunus persica (L.)Batsch] progeny. Postharvest Biol. Technol. 2010, 58, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Swierczynski, S.; Zydlik, Z.; Kleiber, T. The Influence of Foliar Nutrition of Apple Trees with Silicon on Growth and Yield as Well as Mineral Content in Leaves and Fruits. Agronomy 2022, 12, 1680. [Google Scholar] [CrossRef]
- Saleem, Q.T.S.; Joody, A.T. Effect of Silicon, Calcium and Boron on Apple Leaf Minerals Content. Iraqi J. Agric. Sci. 2019, 50, 296–301. [Google Scholar]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Hussien, M.A.; Kassem, M.S. Influence of spraying kaolin, silicon and calcium on productivity and quality of Sultani Fig. Egipt. J. Hort. 2021, 48, 9–18. [Google Scholar] [CrossRef]
- Abd El-Rhman, I.E.; Diab, S.M.; Sahar, A.F. Effect of reflective particles spraying on productivity and quality of ‘Anna’ apple Malus domestica. Middle East J. Agric. Res. 2020, 9, 871–879. [Google Scholar]
- El Kholy, M.F.; Mahmoud, A.A.; Mehaisen, S.M.A. Impact of Potassium Silicate Sprays on Fruiting, Fruit Quality and Fruit Storability of Loquat trees. Middle East J. Agric. Res. 2018, 07, 139–153. [Google Scholar]
- Abdrabboh, G.A. Effect of some preharvest treatments on quality of Canino apricot fruits under cold storage conditions. J. Hortic. Sci. Ornam. Plants 2012, 4, 227–234. [Google Scholar]
- Kaur, A.P.; Singh, H.; Jawandha, S.K. Effect of pre-harvest application on nutrients and growth regulator on fruit quality of sub-tropical peach. Asian J. Hort. 2012, 7, 565–568. [Google Scholar]
- Gill, P.S.; Ganaie, M.Y.; Dhillon, W.S.; Singh, N.P. Effect of foliar sprays of potassium on fruit size and quality of ‘Patharnakh’ pear. Indian J. Hort. 2012, 69, 512–516. [Google Scholar]
- Tarabih, M.E.; El-Eryan, E.E.; El-Metwally, M.A. Physiological and pathological impacts of potassium silicate on storability of Anna apple. Am. J. Plant Physiol. 2014, 9, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.X.; Cai, D.L.; Liu, Z.M. New progress in silicon-improvement of quality of crops. In Proceedings of the 5th International Conference on Silicon in Agriculture, Beijing, China, 13–18 September 2011; p. 77. [Google Scholar]
- Rudell, D.R.; Fellman, J.K.; Mattheis, J.P. Preharvest application of methyl jasmonate to ‘Fuji’ apples enhances red coloration and affects fruit size, splitting, and bitter pit incidence. HortScience 2005, 40, 1760–1762. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.T.; Sajid, M.; Khan, N.U.; Rab, A.; Amin, N.U.; Arif, M.; Haleema, B.; Saeed, S. Peach antioxidant and phenolic activities influenced by the application of 1-Methylcyclopropene (1-MCP) at post-harvest. Acta Sci. Pol. Hortorum Cultus 2019, 18, 71–85. [Google Scholar] [CrossRef]
- Bal, E. Combined Treatment of Modified Atmosphere Packaging and Salicylic Acid Improves Postharvest Quality of Nectarine (Prunus persica L.) Fruit. J. Agric. Sci. Technol. 2016, 18, 1345–1354. [Google Scholar]
- Su, X.W.; Wei, S.C.; Jiang, Y.M.; Huang, Y.Y. Effects of silicon on quality of apple fruit and Mn content in plants on acid soils. Shandong Agric. Sci. 2011, 6, 59–61. [Google Scholar]
- Tesfay, S.Z.; Bertling, I.; Bower, J.P. Effects of postharvest potassium silicate application on phenolics and other antioxidant systems aligned to avocado fruit quality. Postharvest Biol. Technol. 2011, 60, 92–99. [Google Scholar] [CrossRef]
- Mditshwa, A.; Bower, J.P.; Bertling, I.; Mathaba, N.; Tesfay, S.Z. The potential of postharvest silicon dips to regulate phenolics in citrus peel as a method to mitigate chilling injury in lemons. Afr. J. Biotechnol. 2013, 12, 1482–1489. [Google Scholar]



| Traits | Plagold 17 | Early Bomba |
|---|---|---|
| Fruit type | peach | nectarine |
| Fruit shape | round | round |
| Peel color | red | red |
| Flesh color | yellow | white |
| Flesh texture | non-melting | melting |
| Stone adherence | clingstone | freestone |
| Flower type | non-showy | showy |
| Petiol gland shape | reniform | reniform |
| Cultivars | cv Early Bomba | cv Plagold 17 | ||
|---|---|---|---|---|
| Seasons | 2021 | 2022 | 2021 | 2022 |
| Traits | ||||
| Initial blooming | 01/02 | 06/02 | 10/02 | 18/02 |
| Full blooming | 05/02 | 10/02 | 15/02 | 24/02 |
| End blooming | 20/02 | 23/02 | 01/03 | 10/03 |
| Harvest | 15/05 | 25/05 | 20/05 | 05/06 |
| Fruit development | 84 | 91 | 80 | 87 |
| Peach and Nectarine Cultivars | ||||||
|---|---|---|---|---|---|---|
| Early Bomba | Plagold 17 | |||||
| Traits | T0 | T1 | T2 | T0 | T1 | T2 |
| Tree vigor | ||||||
| Height (m) | 2.7 ± 0.1 a | 2.4 ± 0.2 b | 2.5 ± 0.3 b | 2.7 ± 0.1 a | 2.3 ± 0.1 b | 2.6 ± 0.2 a |
| Canopy (m) | 2.4 ± 0.2 a | 2.5 ± 0.4 a | 2.3 ± 0.3 a | 1.9 ± 0.4 b | 2.2 ± 0.2 a | 2.2 ± 0.2 a |
| TCSA (cm2) | 56.7 ± 2.5 b | 59.0 ± 5.3 a | 61.7 ± 4.9 a | 52.3 ± 2.9 a | 49.3 ± 1.5 a | 58.7 ± 9.8 a |
| Yield | ||||||
| Yield (ton/ha) | 25.3 ± 0.5 b | 28.5 ± 0.2 a | 30.0 ± 0.1 a | 20.0 ± 0.5 a | 20.5 ± 0.3 a | 22.0 ± 0.4 a |
| C.Y (ton/ha) | 48.3 ± 0.5 b | 50.0 ± 0.2 a | 55.6 ± 0.3 a | 38.1 ± 0.5 a | 40.3 ± 0.2 a | 42.5 ± 0.7 a |
| Y.E (kg/cm2) | 0.81 ± 0.1 a | 0.88 ± 0.2 a | 0.88 ± 0.2 a | 0.70 ± 0.2 a | 0.76 ± 0.1 a | 0.68 ± 0.2 a |
| Vegetative growth | ||||||
| Shoot lenght (cm) | 53.3 ± 5 a | 55.0 ± 5 a | 61.6 ± 7 b | 32.7 ± 4 a | 29.3 ± 1 b | 33.3 ± 3 a |
| Internode number | 14.3 ± 2.0 a | 16.7 ± 1.5 a | 15.3 ± 2.5 a | 20 ± 1 a | 19.7 ± 1.5 a | 15 ± 1 b |
| Shoot D (cm) | 0.4 ± 0.1 b | 0.50 ± 0.1 a | 0.60 ± 0.1 b | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.5 ± 0.2 a |
| Internode L (cm) | 1.70 ± 0.1 a | 1.8 ± 0.2 a | 1.5 ± 0.3 b | 2.1 ± 0.4 a | 2.0 ± 0.1 a | 1.5 ± 0.3 b |
| Leaf Area (cm2) | 22.5 ± 0.2 b | 24.7 ± 0.1 b | 30.4 ± 0.1 a | 27.5 ± 0.2 b | 30.8 ± 0.1 a | 34.5 ± 0.2 a |
| Fruit composition | ||||||
| Water content (%) | 83.50 ± 3 b | 87.20 ± 3 a | 85.50 ± 3 a | 82.50 ± 2 b | 85.80 ± 2 a | 87.80 ± 2 a |
| Carbohydrates (%) | 6.50 ± 1 b | 7.20 ± 1 a | 8.30 ± 1 a | 7.20 ± 1 b | 8.20 ± 1 a | 10.20 ± 1 a |
| Ash (%) | 0.39 ± 0.1 a | 0.42 ± 0.1 a | 0.45 ± 0.1 a | 0.45 ± 0.1 a | 0.50 ± 0.1 a | 0.52 ± 0.1 a |
| Protein (%) | 0.50 ± 0.1 a | 0.55 ± 0.1 a | 0.58 ± 0.1 a | 0.51 ± 0.2 a | 0.53 ± 0.2 a | 0.56 ± 0.2 a |
| Fiber (%) | 1.65 ± 0.2 a | 1.72 ± 0.2 a | 1.85 ± 0.2 a | 1.60 ± 0.2 a | 1.70 ± 0.1 a | 1.80 ± 0.2 a |
| Storage Period | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| T0 (0% K-Si) | |||||
| Early Bomba | |||||
| Harvest | 46.32 a | 35.12 a | 16.93 b | 38.98 a | 25.64 b |
| 2 weeks storage | 46.18 a | 31.83 a | 21.13 a | 38.20 a | 33.42 a |
| 4 weeks storage | 43.52 a | 33.90 a | 19.98 a | 39.34 a | 30.54 a |
| Plagold 17 | |||||
| Harvest | 28.05 b | 24.52 a | 16.18 a | 29.37 a | 33.42 a |
| 2 weeks storage | 38.15 a | 23.35 a | 14.88 a | 27.68 a | 32.61 a |
| 4 weeks storage | 36.35 a | 24.98 a | 12.43 b | 27.90 a | 26.65 b |
| T1 (3% K-Si) | |||||
| Early Bomba | |||||
| Harvest | 50.90 a | 27.70 a | 23.30 a | 36.19 a | 40.03 a |
| 2 weeks storage | 44.32 b | 27.13a | 19.23 a | 33.25 a | 35.37 a |
| 4 weeks storage | 46.90 b | 27.50a | 14.20 b | 27.81 b | 38.53 a |
| Plagold 17 | |||||
| Harvest | 34.30 b | 25.40 a | 10.70 b | 27.56 b | 22.78 b |
| 2 weeks storage | 42.25 a | 22.92 a | 23.40 a | 32.75 a | 45.56 a |
| 4 weeks storage | 31.40 b | 25.20 a | 12.10 b | 27.95 b | 25.64 b |
| T2 (4.5% K-Si) | |||||
| Early Bomba | |||||
| Harvest | 47.90 a | 41.50 a | 24.60 a | 48.24 a | 30.54 a |
| 2 weeks storage | 42.58 a | 31.92 b | 17.73 b | 36.51 b | 29.24 a |
| 4 weeks storage | 41.20 a | 33.30 b | 14.00 b | 36.12 b | 22.78 b |
| Plagold 17 | |||||
| Harvest | 35.30 b | 25.40 a | 16.40 b | 30.23 b | 33.02 b |
| 2 weeks storage | 41.48 a | 25.68 a | 21.47 a | 33.47 b | 40.03 a |
| 4 weeks storage | 40.40 a | 24.50 a | 25.50 a | 42.90 a | 36.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidi, W.; Akrimi, R.; Hajlaoui, H.; Rejeb, H.; Gogorcena, Y. Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars. Agriculture 2023, 13, 195. https://doi.org/10.3390/agriculture13010195
Abidi W, Akrimi R, Hajlaoui H, Rejeb H, Gogorcena Y. Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars. Agriculture. 2023; 13(1):195. https://doi.org/10.3390/agriculture13010195
Chicago/Turabian StyleAbidi, Walid, Rawaa Akrimi, Hichem Hajlaoui, Hichem Rejeb, and Yolanda Gogorcena. 2023. "Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars" Agriculture 13, no. 1: 195. https://doi.org/10.3390/agriculture13010195
APA StyleAbidi, W., Akrimi, R., Hajlaoui, H., Rejeb, H., & Gogorcena, Y. (2023). Foliar Fertilization of Potassium Silicon Improved Postharvest Fruit Quality of Peach and Nectarine [Prunus persica (L.) Batsch] Cultivars. Agriculture, 13(1), 195. https://doi.org/10.3390/agriculture13010195








