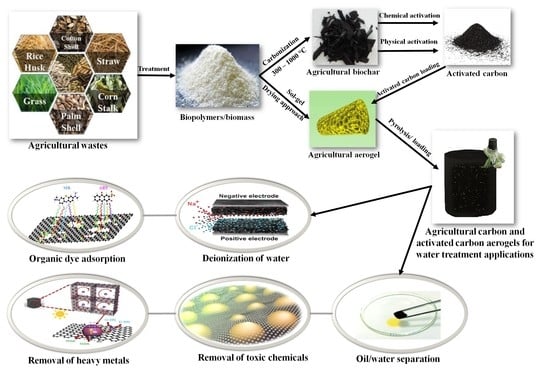
Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications
Abstract
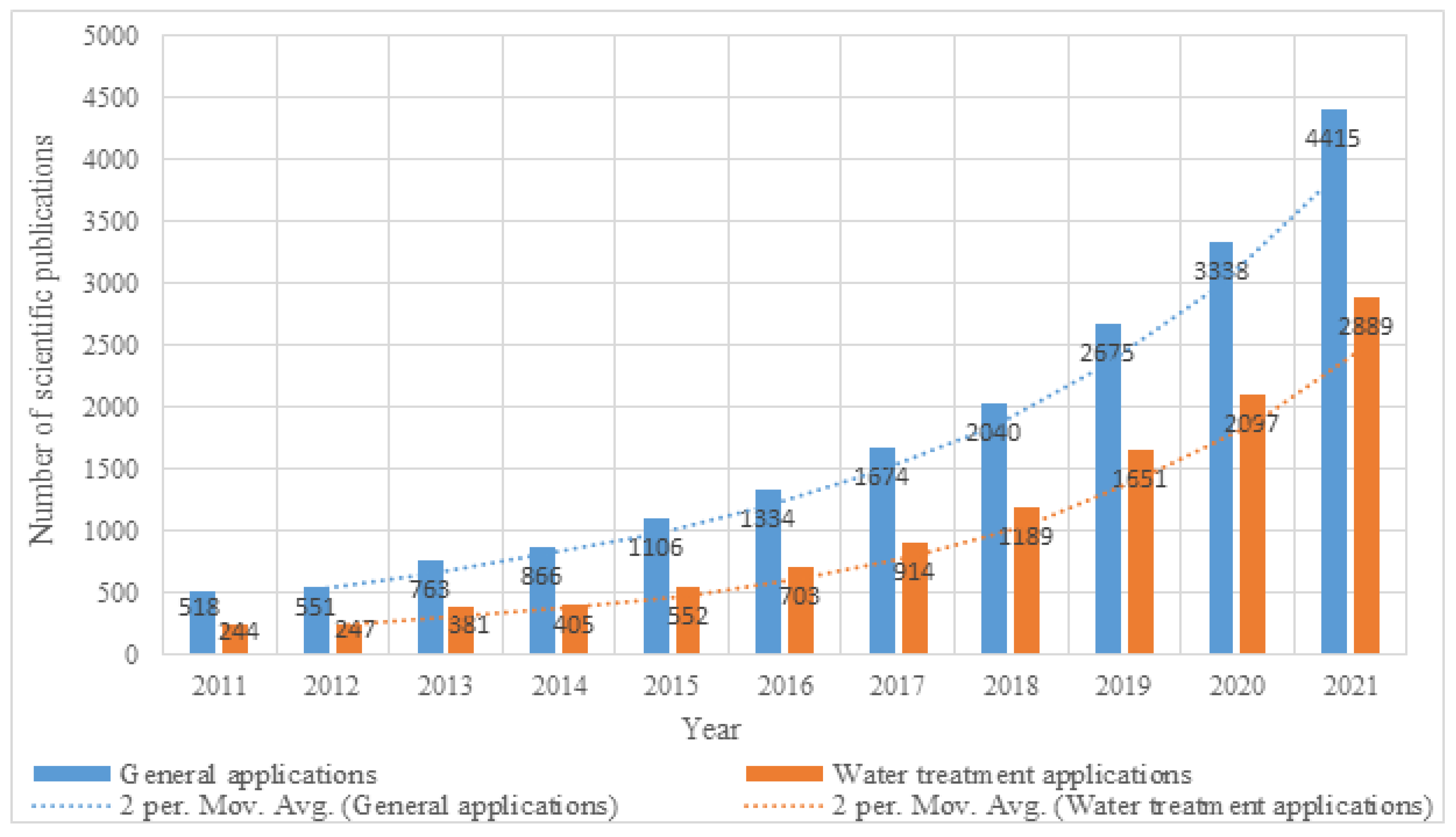
1. Introduction
2. Development of Agricultural-Based Nano-Structured Aerogels
2.1. Classification and Properties of Nano-Structured Aerogels
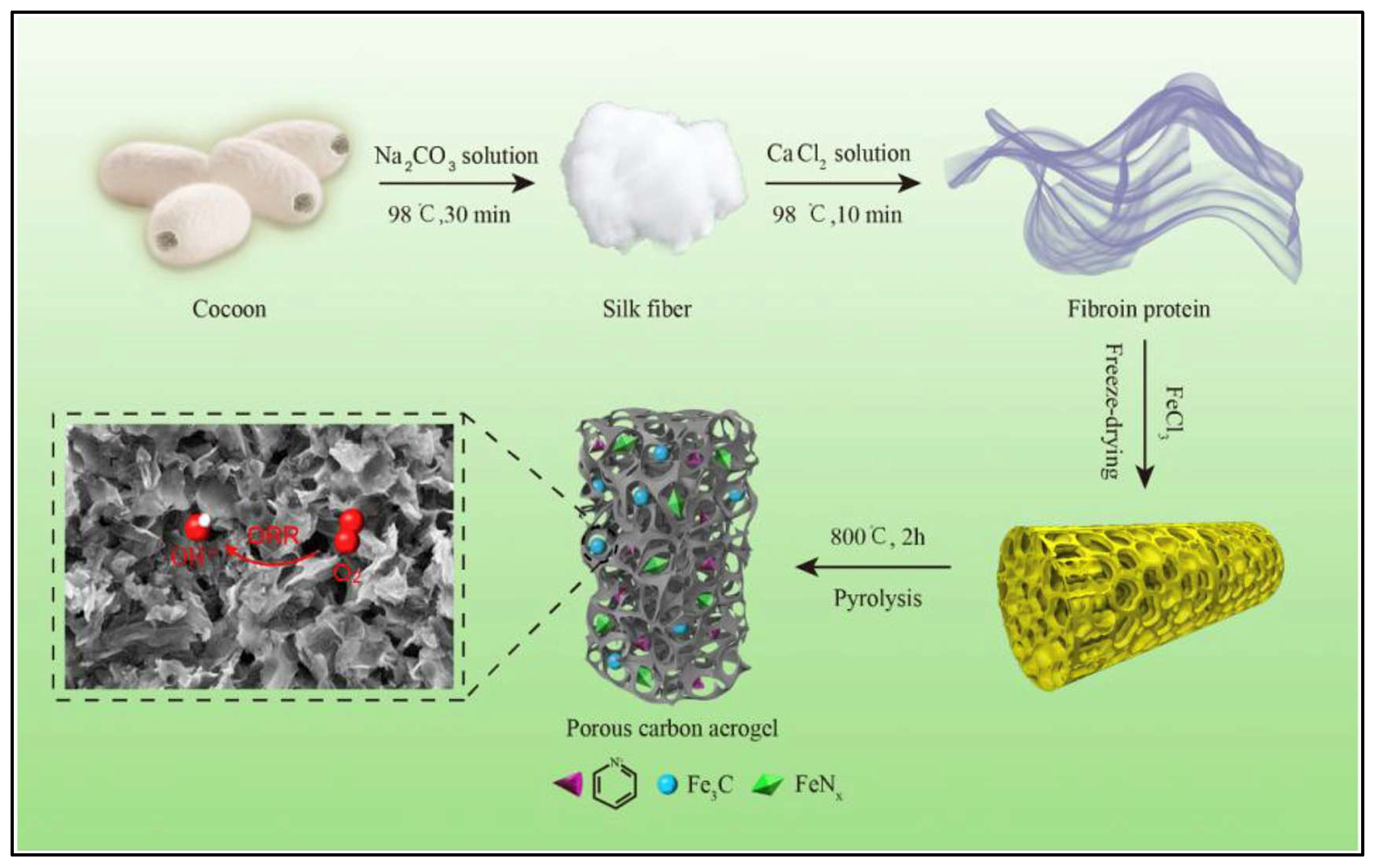
2.2. Fabrication of Agricultral Carbon Nano-Structured Aerogels
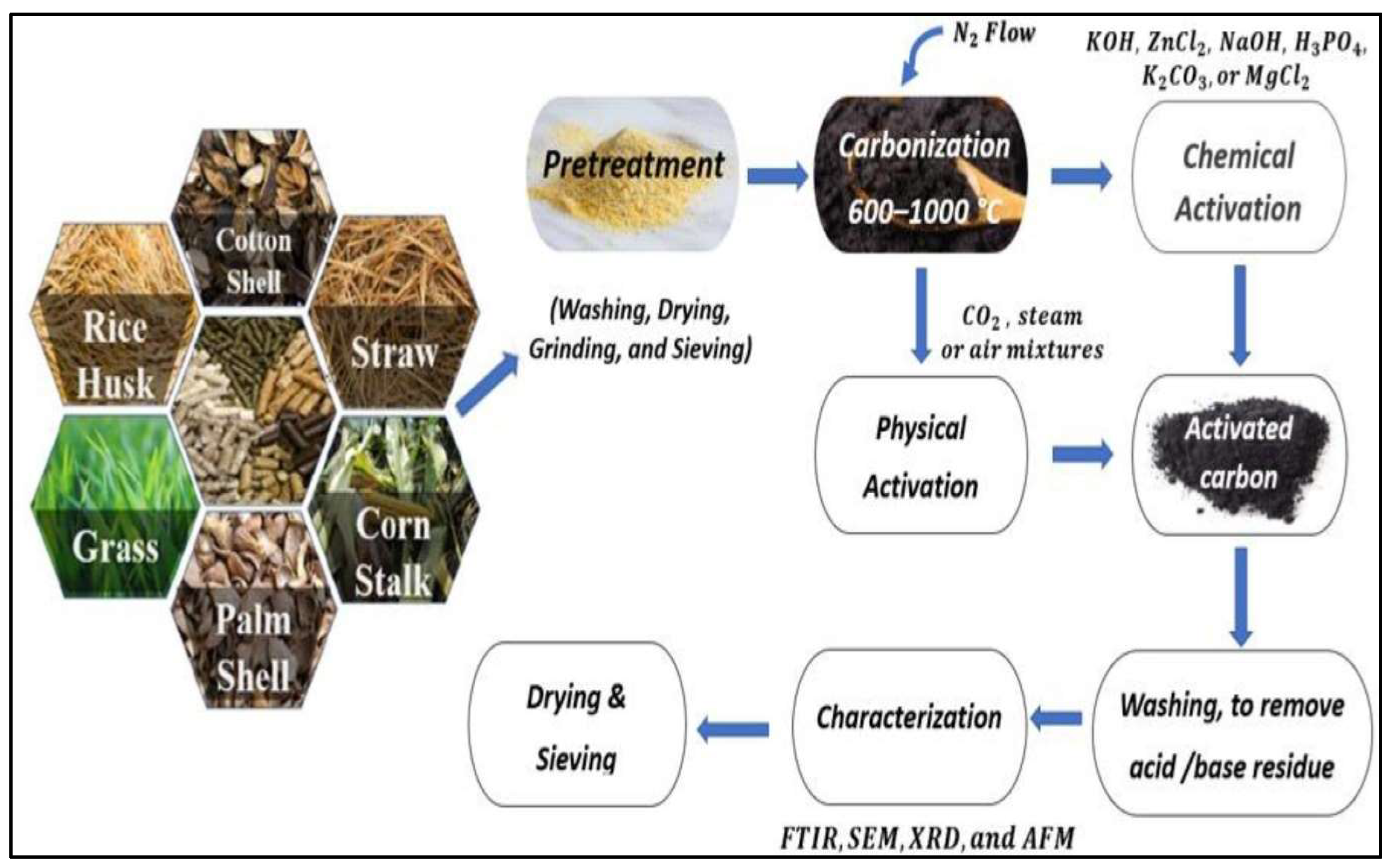
3. Agricultural Activated Carbon and Nano-Structured Aerogels
3.1. Activated Carbon Aerogel
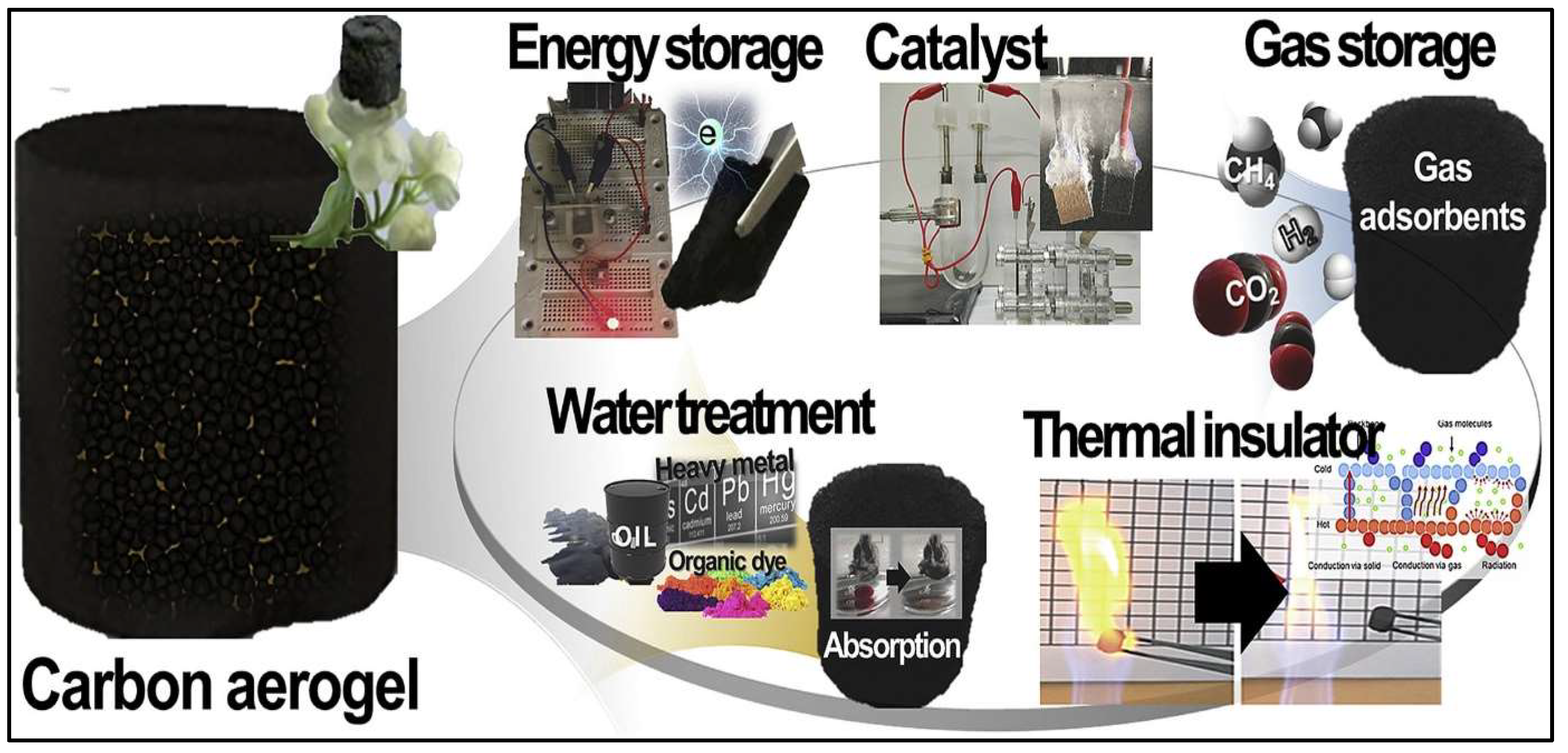
3.2. Applications of Activated Carbon Aerogels
4. Agricultural Activated Carbon Nano-Structured Aerogels for Wastewater Treatment
4.1. Organic Dye Adsorption
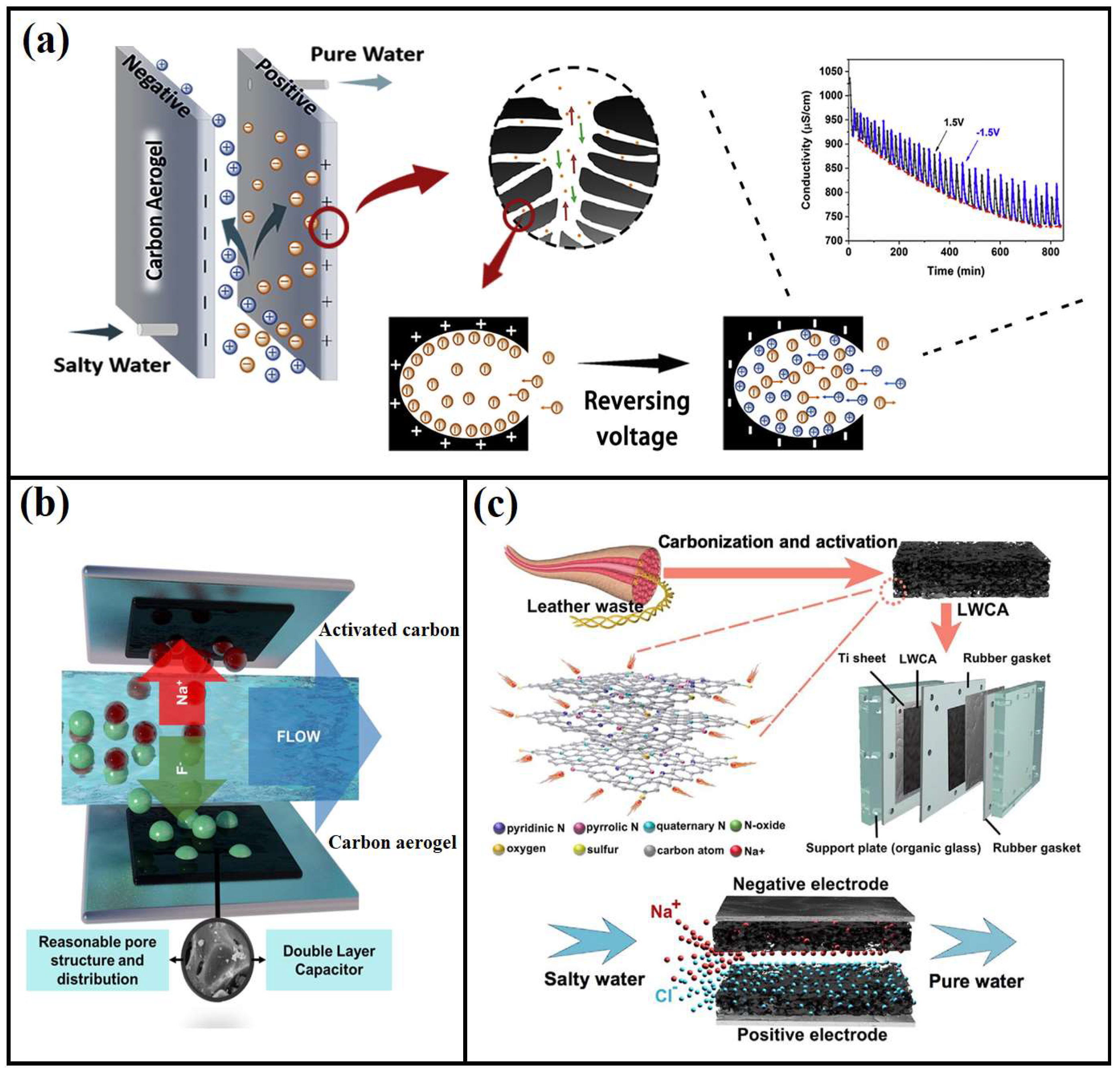
4.2. Deionization of Water
4.3. Removal of Heavy Metals
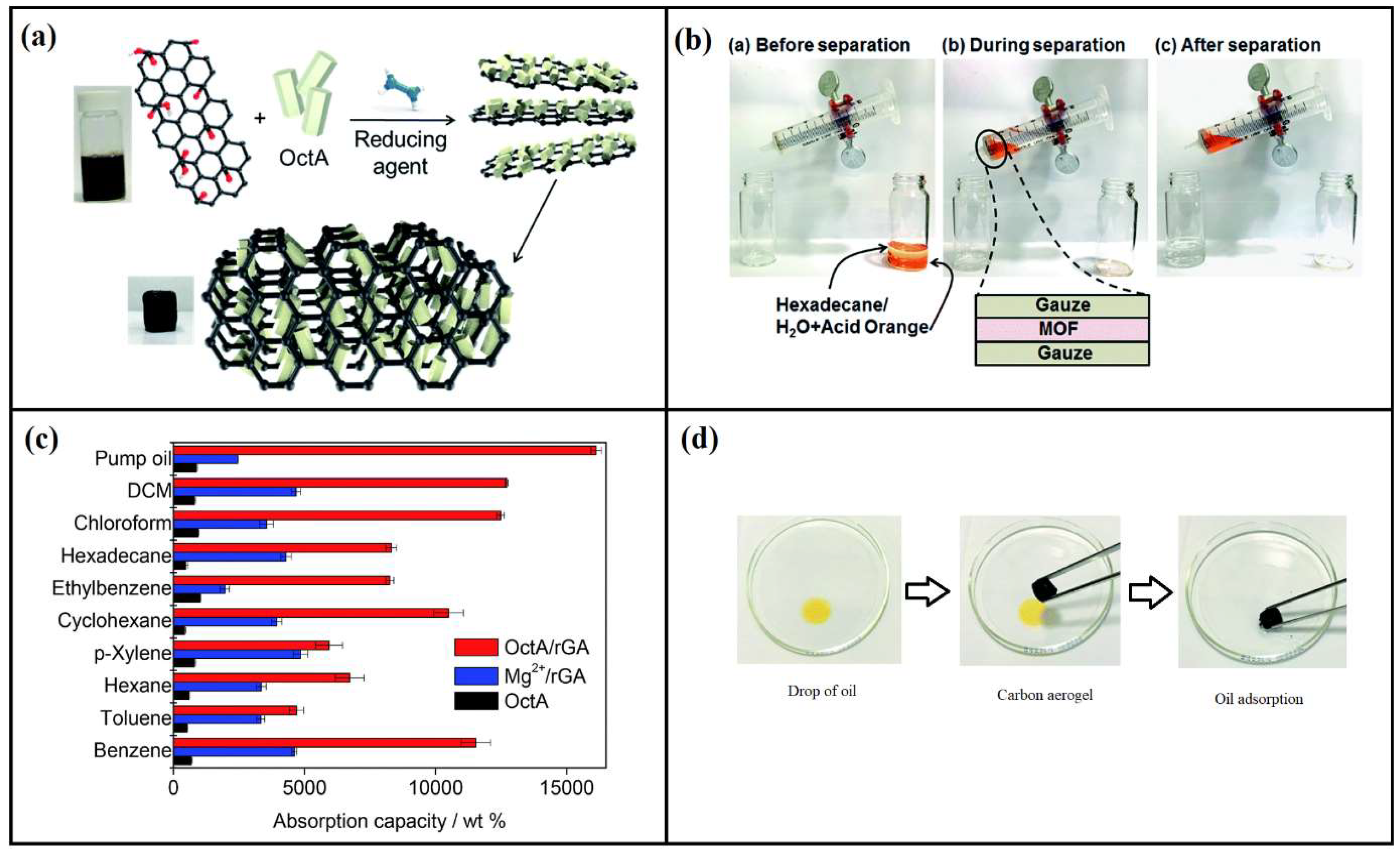
4.4. Oil/Water Separation
4.5. Removal of Toxic Chemicals
4.6. Other Applications
5. Challenges and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alwin, S.; Sahaya Shajan, X. Aerogels: Promising nanostructured materials for energy conversion and storage applications. Mater. Renew. Sustain. Energy 2020, 9, 7. [Google Scholar] [CrossRef]
- Iskandar, M.; Yahya, E.B.; Abdul Khalil, H.P.S.; Rahman, A.; Ismail, M. Recent Progress in Modification Strategies of Nanocellulose-Based Aerogels for Oil Absorption Application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Ren, S.; Li, X.; Fan, J.; Liang, J. Preparation and characterization of organic-inorganic hybrid ZrOC/PF aerogel used as high-temperature insulator. Ceram. Int. 2020, 46, 6326–6332. [Google Scholar] [CrossRef]
- Talebi, Z.; Soltani, P.; Habibi, N.; Latifi, F. Silica aerogel/polyester blankets for efficient sound absorption in buildings. Constr. Build. Mater. 2019, 220, 76–89. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; el Halal, S.L.M.; Bertoldi, F.C.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int. J. Biol. Macromol. 2019, 124, 175–184. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Z.; Wei, M.; Yu, S.; Sun, R.; Wong, C.-P. Synthesis and characterization of 3D CoMoO 4/rGO aerogel for supercapacitor electrodes. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018; pp. 1297–1300. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO42− nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B Environ. 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W. High-performance energy storage and conversion materials derived from a single metal–organic framework/graphene aerogel composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- An, F.; Li, X.; Min, P.; Li, H.; Dai, Z.; Yu, Z.-Z. Highly anisotropic graphene/boron nitride hybrid aerogels with long-range ordered architecture and moderate density for highly thermally conductive composites. Carbon 2018, 126, 119–127. [Google Scholar] [CrossRef]
- Dolai, S.; Bhunia, S.K.; Jelinek, R. Carbon-dot-aerogel sensor for aromatic volatile organic compounds. Sens. Actuators B Chem. 2017, 241, 607–613. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, S.; Zhou, X.; Gu, H.; Ma, C.; Wang, C.; Ding, T.; Shao, Q.; Liu, H.; Guo, Z. Super light 3D hierarchical nanocellulose aerogel foam with superior oil adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Abdul Khalil, H.P.S.; Ahmad, M.I.; Rizal, S.; Muhammad, S. Cleaner approach of preparing antibacterial bioaerogel scaffolds using oil palm waste nanocellulose. Ind. Crops Prod. 2023, 191, 115897. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Albadn, Y.M.; Suriani, A.; Kamaruzzaman, S.; Mohamed, A.; Allaq, A.A.; Yahya, E.B. Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application. Agriculture 2022, 12, 1737. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.K. Characterization of activated carbon paper electrodes prepared by rice husk-isolated cellulose fibers for supercapacitor applications. Molecules 2020, 25, 3951. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; Du, Q. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Peydayesh, M.; Vogt, J.; Chen, X.; Zhou, J.; Donat, F.; Bagnani, M.; Müller, C.R.; Mezzenga, R. Amyloid-based carbon aerogels for water purification. Chem. Eng. J. 2022, 449, 137703. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Thomas, A.; Liao, Y. Ultra-high surface area nitrogen-doped carbon aerogels derived from a schiff-base porous organic polymer aerogel for CO2 storage and supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon Aerogels for Environmental Clean-Up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yu, H.; Li, J.; Liu, S. Wood-Derived Carbon Materials and Light-Emitting Materials. Adv. Mater. 2021, 33, 2000596. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.; Alfatah, T.; Suriani, A.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Wang, H.-L.; Hsu, C.-Y.; Wu, K.C.; Lin, Y.-F.; Tsai, D.-H. Functional nanostructured materials: Aerosol, aerogel, and de novo synthesis to emerging energy and environmental applications. Adv. Powder Technol. 2020, 31, 104–120. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, X.; Deng, Y.; Wang, L.; Hong, H.; Ju, Z. SiO2/N-doped graphene aerogel composite anode for lithium-ion batteries. J. Mater. Sci. 2020, 55, 13023–13035. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, X.; Liu, L.; Yao, J. Highly compressible and durable superhydrophobic cellulose aerogels for oil/water emulsion separation with high flux. J. Mater. Sci. 2021, 56, 2763–2776. [Google Scholar] [CrossRef]
- Öner, E.; Öztürk, A.; Yurtcan, A.B. Utilization of the graphene aerogel as PEM fuel cell catalyst support: Effect of polypyrrole (PPy) and polydimethylsiloxane (PDMS) addition. Int. J. Hydrogen Energy 2020, 45, 34818–34836. [Google Scholar]
- Salimian, S.; Zadhoush, A.; Naeimirad, M.; Kotek, R.; Ramakrishna, S. A review on aerogel: 3D nanoporous structured fillers in polymer-based nanocomposites. Polym. Compos. 2018, 39, 3383–3408. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Wang, C.-T. Photocatalytic activity of nanoparticle gold/iron oxide aerogels for azo dye degradation. J. Non-Cryst. Solids 2007, 353, 1126–1133. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; Xu, K.; Zhang, Y.; Lu, Y.; Nie, Z.; Du, Y.; Zhu, J.; Huang, W. Metallic sandwiched-aerogel hybrids enabling flexible and stretchable intelligent sensor. Nano Lett. 2020, 20, 3449–3458. [Google Scholar] [CrossRef]
- Ahmed, E.; Rothenberger, A. Enhancement in CO2 adsorption capacity and selectivity in the chalcogenide aerogel CuSb2S4 by post-synthetic modification with LiCl. Microporous Mesoporous Mater. 2016, 220, 247–252. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Wang, J.; Liu, Z.; Zhang, K.; Ji, X.; You, Y.; Zhang, X. Reaction-spun transparent silica aerogel fibers. ACS Nano 2020, 14, 11919–11928. [Google Scholar] [CrossRef]
- Gu, W.; Sheng, J.; Huang, Q.; Wang, G.; Chen, J.; Ji, G. Environmentally friendly and multifunctional shaddock peel-based carbon aerogel for thermal-insulation and microwave absorption. Nano-Micro Lett. 2021, 13, 102. [Google Scholar]
- Yang, L.; Li, N.; Guo, C.; He, J.; Wang, S.; Qiao, L.; Li, F.; Yu, L.; Wang, M.; Xu, X. Marine biomass-derived composite aerogels for efficient and durable solar-driven interfacial evaporation and desalination. Chem. Eng. J. 2021, 417, 128051. [Google Scholar] [CrossRef]
- Chen, P.; Bai, D.; Tang, H.; Liu, H.; Wang, J.; Gao, G.; Li, L. Polylactide aerogel with excellent comprehensive performances imparted by stereocomplex crystallization for efficient oil-water separation. Polymer 2022, 255, 125128. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Tu, T.H.; Linh, V.N.P.; Thy, L.T.M.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Preparation of magnetic iron oxide/graphene aerogel nanocomposites for removal of bisphenol A from water. Synth. Met. 2019, 255, 116106. [Google Scholar]
- Batista, M.; Gonçalves, V.S.; Gaspar, F.; Nogueira, I.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Zuo, K.; Wu, J.; Chen, S.; Ji, X.; Wu, W. Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose 2019, 26, 9661–9671. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Tan, Y.; Zhang, S.; Li, J. 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu (II) ions from water. Chem. Eng. J. 2019, 378, 122157. [Google Scholar]
- Follmann, H.D.; Oliveira, O.N.; Lazarin-Bidóia, D.; Nakamura, C.V.; Huang, X.; Asefa, T.; Silva, R. Multifunctional hybrid aerogels: Hyperbranched polymer-trapped mesoporous silica nanoparticles for sustained and prolonged drug release. Nanoscale 2018, 10, 1704–1715. [Google Scholar] [CrossRef]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Juhász, I.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gui, S.-H.; Wu, J.-H.; Xu, D.-D.; Sun, Y.; Dong, X.-Y.; Dai, Y.-Y.; Li, Y.-F. Nanocellulose-Graphene Oxide Hybrid Aerogel to Water Purification. Appl. Environ. Biotechnol. 2019, 4, 11–17. [Google Scholar] [CrossRef]
- Gonçalves, V.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of aging on silica aerogel properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Chandradass, J.; Kang, S.; Bae, D.-S. Synthesis of silica aerogel blanket by ambient drying method using water glass based precursor and glass wool modified by alumina sol. J. Non-Cryst. Solids 2008, 354, 4115–4119. [Google Scholar] [CrossRef]
- Lee, K.-J.; Choe, Y.-J.; Kim, Y.H.; Lee, J.K.; Hwang, H.-J. Fabrication of silica aerogel composite blankets from an aqueous silica aerogel slurry. Ceram. Int. 2018, 44, 2204–2208. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Kow, K.-W.; Yusoff, R.; Aziz, A.A.; Abdullah, E. From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non-Cryst. Solids 2014, 386, 76–84. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.-X.; Song, K.; Wang, Z.-Y. Cost-effective synthesis of silica aerogels from fly ash via ambient pressure drying. J. Non-Cryst. Solids 2010, 356, 2241–2246. [Google Scholar] [CrossRef]
- Gao, G.-M.; Liu, D.-R.; Zou, H.-F.; Zou, L.-C.; Gan, S.-C. Preparation of silica aerogel from oil shale ash by fluidized bed drying. Powder Technol. 2010, 197, 283–287. [Google Scholar] [CrossRef]
- He, P.; Gao, X.-D.; Li, X.-M.; Jiang, Z.-W.; Yang, Z.-H.; Wang, C.-L.; Gu, Z.-Y. Highly transparent silica aerogel thick films with hierarchical porosity from water glass via ambient pressure drying. Mater. Chem. Phys. 2014, 147, 65–74. [Google Scholar] [CrossRef]
- Liu, S.-W.; Wei, Q.; Cui, S.-P.; Nie, Z.-R.; Du, M.-H.; Li, Q.-Y. Hydrophobic silica aerogel derived from wheat husk ash by ambient pressure drying. J. Sol-Gel Sci. Technol. 2016, 78, 60–67. [Google Scholar] [CrossRef]
- Nazriati, N.; Setyawan, H.; Affandi, S.; Yuwana, M.; Winardi, S. Using bagasse ash as a silica source when preparing silica aerogels via ambient pressure drying. J. Non-Cryst. Solids 2014, 400, 6–11. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Hyun, S.; Kim, T.; Kim, G.; Park, H.-H. Synthesis of low-k porous silica films via freeze drying. J. Mater. Sci. Lett. 2000, 19, 1863–1866. [Google Scholar] [CrossRef]
- Nocentini, K.; Achard, P.; Biwole, P.; Stipetic, M. Hygro-thermal properties of silica aerogel blankets dried using microwave heating for building thermal insulation. Energy Build. 2018, 158, 14–22. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Kim, H.S.; Jo, S.M.; Kim, S.Y.; Yang, B.; Cho, J.; Lee, S.; Cha, J.E. Thermally insulating, fire-retardant, smokeless and flexible polyvinylidene fluoride nanofibers filled with silica aerogels. Chem. Eng. J. 2018, 351, 473–481. [Google Scholar] [CrossRef]
- Abbas, N.; Khalid, H.R.; Ban, G.; Kim, H.T.; Lee, H.-K. Silica aerogel derived from rice husk: An aggregate replacer for lightweight and thermally insulating cement-based composites. Constr. Build. Mater. 2019, 195, 312–322. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Jummaat, F.; Adnan, A.; Olaiya, N.; Rizal, S.; Abdullah, C.; Pasquini, D.; Thomas, S. Biopolymers based Aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2022, 131, 101014. [Google Scholar]
- Li, C.; Sun, F.; Lin, Y. Refining cocoon to prepare (N, S, and Fe) ternary-doped porous carbon aerogel as efficient catalyst for the oxygen reduction reaction in alkaline medium. J. Power Sources 2018, 384, 48–57. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Qi, W.; Chen, S.; Tan, Q.; Wei, Z.; Gong, L.; Chen, J.; Zhou, W. The comprehensive evaluation model and optimization selection of activated carbon in the O3-BAC treatment process. J. Water Process Eng. 2021, 40, 101931. [Google Scholar] [CrossRef]
- Tan, X.-F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Song, M.; Jin, B.; Xiao, R.; Yang, L.; Wu, Y.; Zhong, Z.; Huang, Y. The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenergy 2013, 48, 250–256. [Google Scholar] [CrossRef]
- Huang, F.-C.; Lee, C.-K.; Han, Y.-L.; Chao, W.-C.; Chao, H.-P. Preparation of activated carbon using micro-nano carbon spheres through chemical activation. J. Taiwan Inst. Chem. Eng. 2014, 45, 2805–2812. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20. [Google Scholar]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Othman, F.; Yusof, N.; Matsuura, T.; Lau, W.; Jaafar, J.; Ismail, A.; Salleh, W.; Aziz, F. Preparation of nanocomposite activated carbon nanofiber/manganese oxide and its adsorptive performance toward leads (II) from aqueous solution. J. Water Process Eng. 2020, 37, 101430. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Yang, I.; Kwon, D.; Kim, M.-S.; Jung, J.C. A comparative study of activated carbon aerogel and commercial activated carbons as electrode materials for organic electric double-layer capacitors. Carbon 2018, 132, 503–511. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.-H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611. [Google Scholar] [CrossRef]
- Yahya, E.; Abdulsamad, M.A. In-vitro Antibacterial Activity of Carbopol-Essential Oils hydrogels. J. Appl. Sci. Process Eng. 2020, 7, 564–571. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Bouhfid, R. Insight into the bionanocomposite applications on wastewater decontamination. J. Water Process Eng. 2021, 43, 102198. [Google Scholar] [CrossRef]
- Mittal, J. Permissible synthetic food dyes in India. Resonance 2020, 25, 567–577. [Google Scholar] [CrossRef]
- Mota, I.G.C.; Neves, R.A.M.D.; Nascimento, S.S.D.C.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Artificial dyes: Health risks and the need for revision of international regulations. Food Rev. Int. 2021, 1–16. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. 2020, 132, 2082–2086. [Google Scholar] [CrossRef]
- Li, K.; Zhou, M.; Liang, L.; Jiang, L.; Wang, W. Ultrahigh-surface-area activated carbon aerogels derived from glucose for high-performance organic pollutants adsorption. J. Colloid Interface Sci. 2019, 546, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, P.; Min, L.; Tang, J.; Sun, H. Synthesis of cellulose carbon aerogel via combined technology of wet ball-milling and TEMPO-mediated oxidation and its supersorption performance to ionic dyes. Bioresour. Technol. 2020, 315, 123815. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef]
- Ahmed, M.; Wiese, D.N. Short-term trends in Africa’s freshwater resources: Rates and drivers. Sci. Total Environ. 2019, 695, 133843. [Google Scholar] [CrossRef]
- Pradinaud, C.; Northey, S.; Amor, B.; Bare, J.; Benini, L.; Berger, M.; Boulay, A.-M.; Junqua, G.; Lathuillière, M.J.; Margni, M. Defining freshwater as a natural resource: A framework linking water use to the area of protection natural resources. Int. J. Life Cycle Assess. 2019, 24, 960–974. [Google Scholar]
- Elisadiki, J.; Kibona, T.E.; Machunda, R.L.; Saleem, M.W.; Kim, W.-S.; Jande, Y.A. Biomass-based carbon electrode materials for capacitive deionization: A review. Biomass Convers. Biorefinery 2020, 10, 1327–1356. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, H.; Wu, X.; Shen, J. A positive-negative alternate adsorption effect for capacitive deionization in nano-porous carbon aerogel electrodes to enhance desalination capacity. Desalination 2019, 458, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Gu, X.; Wu, N.; Zhang, R.; Shen, Y.; Zheng, B.; Wu, J.; Zhang, W.; Li, S. One-step turning leather wastes into heteroatom doped carbon aerogel for performance enhanced capacitive deionization. Microporous Mesoporous Mater. 2020, 303, 110303. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, Z.; Yang, P.; Wang, J.; Shi, M.; Yu, F.; Ma, J. Industrially-prepared carbon aerogel for excellent fluoride removal by membrane capacitive deionization from brackish groundwaters. Sep. Purif. Technol. 2022, 297, 121510. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Li, J.; Wang, X. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Shen, G.; Xu, Y.; Liu, B. Preparation and adsorption properties of magnetic mesoporous Fe3C/carbon aerogel for arsenic removal from water. Desalination Water Treat. 2016, 57, 24467–24475. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Yang, Z.; Qin, Q.; Zhang, Z.; Wang, X.; Shen, J. Preparation of carbon aerogel electrode for electrosorption of copper ions in aqueous solution. Materials 2019, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, K.-Q.; Zhang, F.; Tang, Z.-R.; Xu, Y.-J. 3D carbon quantum dots/graphene aerogel as a metal-free catalyst for enhanced photosensitization efficiency. Appl. Catal. B Environ. 2018, 233, 11–18. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Waterhouse, G.I.; Sun, J.; Shi, W.; Ai, S. Efficient removal of cadmium ions from water by adsorption on a magnetic carbon aerogel. Environ. Sci. Pollut. Res. 2021, 28, 5149–5157. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Pileidis, F.; Makila, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpaa, M.; Titirici, M.-M. Versatile cellulose-based carbon aerogel for the removal of both cationic and anionic metal contaminants from water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V. Cadmium (II) uptake from aqueous solution by adsorption onto carbon aerogel using a response surface methodological approach. Ind. Eng. Chem. Res. 2006, 45, 6531–6537. [Google Scholar] [CrossRef]
- Song, Z.; Chen, X.; Gong, X.; Gao, X.; Dai, Q.; Nguyen, T.T.; Guo, M. Luminescent carbon quantum dots/nanofibrillated cellulose composite aerogel for monitoring adsorption of heavy metal ions in water. Opt. Mater. 2020, 100, 109642. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Goel, J.; Rajagopal, C. Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J. Hazard. Mater. 2008, 153, 502–507. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. Int. Ed. 2012, 51, 11371–11375. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Chen, J.; Hng, H.H.; Ma, J.; Chen, X. A leavening strategy to prepare reduced graphene oxide foams. Adv. Mater. 2012, 24, 4144–4150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Zhang, Y.-N.; Wang, X.; Chen, J.-F.; Yu, S.-H. Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep. 2014, 4, 4079. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Huang, X.; Wu, X.; Cao, X.; Tan, C.; Yin, Z.; Lu, X.; Sun, L.; Zhang, H. Carbon microbelt aerogel prepared by waste paper: An efficient and recyclable sorbent for oils and organic solvents. Small 2014, 10, 3544–3550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H. Ultra-hydrophobic and mesoporous silica aerogel membranes for efficient separation of surfactant-stabilized water-in-oil emulsion separation. Sep. Purif. Technol. 2019, 212, 597–604. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, L.; Liu, D. Ultralight graphene/carbon nanotubes aerogels with compressibility and oil absorption properties. Materials 2018, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.-C. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Eom, S.; Kang, D.W.; Kang, M.; Choe, J.H.; Kim, H.; Kim, D.W.; Hong, C.S. Fine-tuning of wettability in a single metal–organic framework via postcoordination modification and its reduced graphene oxide aerogel for oil–water separation. Chem. Sci. 2019, 10, 2663–2669. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Gao, Y.; Xu, C.; Lian, Y. Determination of six organophosphorus pesticides in water samples by three-dimensional graphene aerogel-based solid-phase extraction combined with gas chromatography/mass spectrometry. RSC Adv. 2018, 8, 10277–10283. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, B.; Cheng, J.; Xiao, D.; Xie, Z.; Zhao, J. 3D, eco-friendly metal-organic frameworks@ carbon nanotube aerogels composite materials for removal of pesticides in water. J. Hazard. Mater. 2021, 401, 123718. [Google Scholar] [CrossRef]
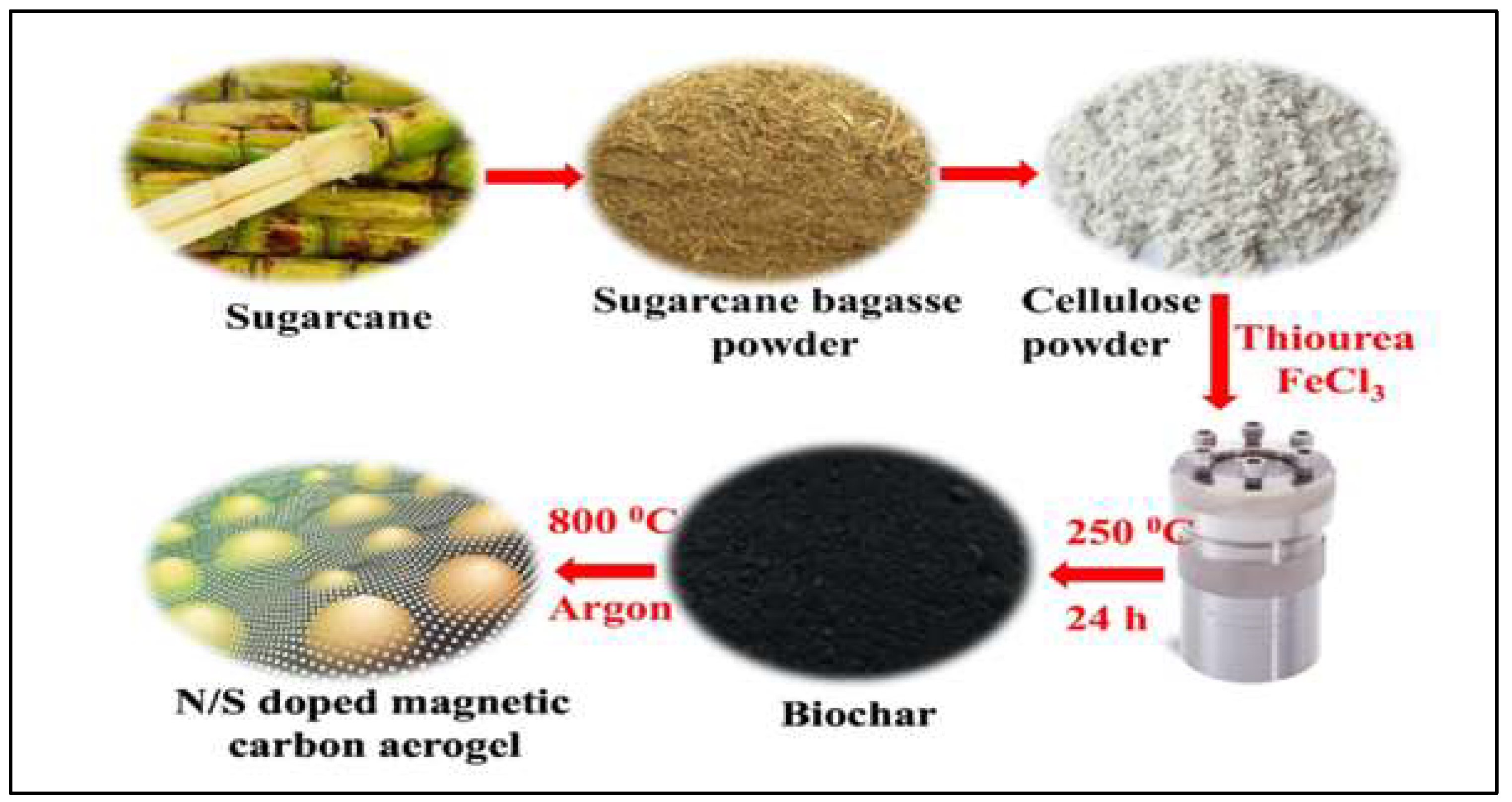
- Ahamad, T.; Naushad, M.; Alhabarah, A.N.; Alshehri, S.M. N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Show, S.; Chakraborty, P.; Karmakar, B.; Halder, G. Sorptive and microbial riddance of micro-pollutant ibuprofen from contaminated water: A state of the art review. Sci. Total Environ. 2021, 786, 147327. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Gunathilake, C.; Abidi, N.; Jaroniec, M. Activated carbon derived from chitin aerogels: Preparation and CO2 adsorption. Cellulose 2018, 25, 1911–1920. [Google Scholar] [CrossRef]
- Aylaz, G.L.N.; Okan, M.; Duman, M.; Aydin, H.M. Study on cost-efficient carbon aerogel to remove antibiotics from water resources. ACS Omega 2020, 5, 16635–16644. [Google Scholar] [CrossRef]
- Mukherjee, S.; Halder, G. A review on the sorptive elimination of fluoride from contaminated wastewater. J. Environ. Chem. Eng. 2018, 6, 1257–1270. [Google Scholar] [CrossRef]
- Ling, Y.; Man, X.; Zhang, W.; Wang, D.; Wu, J.; Liu, Q.; Gu, M.; Lin, Y.; He, P.; Jia, T. Molybdenum trioxide impregnated carbon aerogel for gaseous elemental mercury removal. Korean J. Chem. Eng. 2020, 37, 641–651. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Zhang, R.; Wang, Q.; Liang, P.; Huang, X.; Logan, B.E.; Fellinger, T.P. High-performance carbon aerogel air cathodes for microbial fuel cells. ChemSusChem 2016, 9, 2788–2795. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of biomass-based carbon aerogels in energy and sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.N.; Minh, D.N.; Van Hung, N.; Minh, P.N.; Khoi, P.H. Carbon nanotube and graphene aerogels—The world’s 3D lightest materials for environment applications: A review. Int. J. Mater. Sci. Appl 2017, 6, 277. [Google Scholar]
- Xu, X.; Wang, R.; Nie, P.; Cheng, Y.; Lu, X.; Shi, L.; Sun, J. Copper nanowire-based aerogel with tunable pore structure and its application as flexible pressure sensor. ACS Appl. Mater. Interfaces 2017, 9, 14273–14280. [Google Scholar] [CrossRef] [PubMed]
- Mensing, J.P.; Lomas, T.; Tuantranont, A. 2D and 3D printing for graphene based supercapacitors and batteries: A review. Sustain. Mater. Technol. 2020, 25, e00190. [Google Scholar] [CrossRef]


| Type of Aerogel | Sub-Type | Example | Ref. |
|---|---|---|---|
| Inorganic based aerogels | (1) Inorganic oxide aerogels | Gold/iron oxide aerogel | [38] |
| (2) Metallic aerogels | Vanadium nitride nanosheets aerogel | [39] | |
| (3) Chalcogenide aerogels | Chalcogenide aerogel CuSb2S4 | [40] | |
| (4) Silica aerogels | Transparent silica aerogel fibers | [41] | |
| Organic based aerogels | (5) Carbon aerogels | Shaddock peel-based carbon aerogel | [42] |
| (1) Biomass aerogels | Marine biomass-derived aerogel | [43] | |
| (2) Synthetic organic aerogels | Polylactide aerogel | [44] | |
| (3) Biopolymeric aerogels | Nanocellulose/Chitosan aerogel | [45] | |
| Composites aerogels | (4) Mixed metal oxide aerogels | Magnetic iron oxide/graphene aerogel | [46] |
| (1) Mixed organic aerogels | Alginate-chitosan aerogel | [47] | |
| (2) Mixed inorganic aerogels | Mesoporous Fe-silica aerogel | [48] | |
| (3) Organic/inorganic aerogels | Nanocellulose/silica aerogel | [49] | |
| (4) Other composite aerogels | Nanocellulose/poly ethylenimine aerogel | [50] |
| Functionality | Conventional Techniques | Rapid Prototyping Techniques |
|---|---|---|
| Time consuming and computer-aid | Consume longer time, without computer aid | Rapid fabrication with computer-aid |
| Easiness and Manpower requirements | Require more manpower | Minimize manpower required |
| Aerogel homogeneity | Difficult to obtain homogeneous structures | Easier to obtain homogeneous structures |
| Accurate controllable properties and aerogel shape | Relatively unspecific | Highly specific |
| Aerogel porosity and pore shape | Random and irregular pores shape | Highly regular and interconnected pores |
| Aerogel toxicity | Depending on the technique (more toxic) | Less toxic in some techniques |
| Production costs | More expensive, consume more materials | Consume minimum materials, generally low cost of production |
| Precursor Material/s | Preparation Technique | Type of Heavy Metal | Adsorption Capacity | Ref. |
|---|---|---|---|---|
| Versatile cellulose-based carbon aerogel | Carbonization and freeze-drying approach | Cr(VI) and Pb(II) | 68 and 240 mg/g | [108] |
| Modified carbon aerogel | Sol–gel approach | Cd(II) | 15.53 mg/g | [109] |
| Luminescent carbon quantum dots/nanocellulose aerogel | Freeze-drying under a vacuum atmosphere | Cr3+ | 104.5 mg/g | [110] |
| Commercial carbon aerogel | sol–gel polymerization of metal alkoxides | Pb(II), Hg(II) and Cd(II) | 34.72, 34.96 and 15.53 respectively | [111] |
| Straw biochar-loaded N-doped carbon aerogel | Hydrothermal carbonization process | Pb(II) and Cd(II) | 205.07 and 105.56 | [104] |
| Amyloid fibrils-based carbon aerogels | Hydrothermal carbonization process | Au(III) | 650.08 mg/g | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Marwan, M.; Albadn, Y.M. Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications. Agriculture 2023, 13, 208. https://doi.org/10.3390/agriculture13010208
Muhammad S, Yahya EB, Abdul Khalil HPS, Marwan M, Albadn YM. Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications. Agriculture. 2023; 13(1):208. https://doi.org/10.3390/agriculture13010208
Chicago/Turabian StyleMuhammad, Syaifullah, Esam Bashir Yahya, H. P. S. Abdul Khalil, M. Marwan, and Yonss M. Albadn. 2023. "Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications" Agriculture 13, no. 1: 208. https://doi.org/10.3390/agriculture13010208
APA StyleMuhammad, S., Yahya, E. B., Abdul Khalil, H. P. S., Marwan, M., & Albadn, Y. M. (2023). Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications. Agriculture, 13(1), 208. https://doi.org/10.3390/agriculture13010208