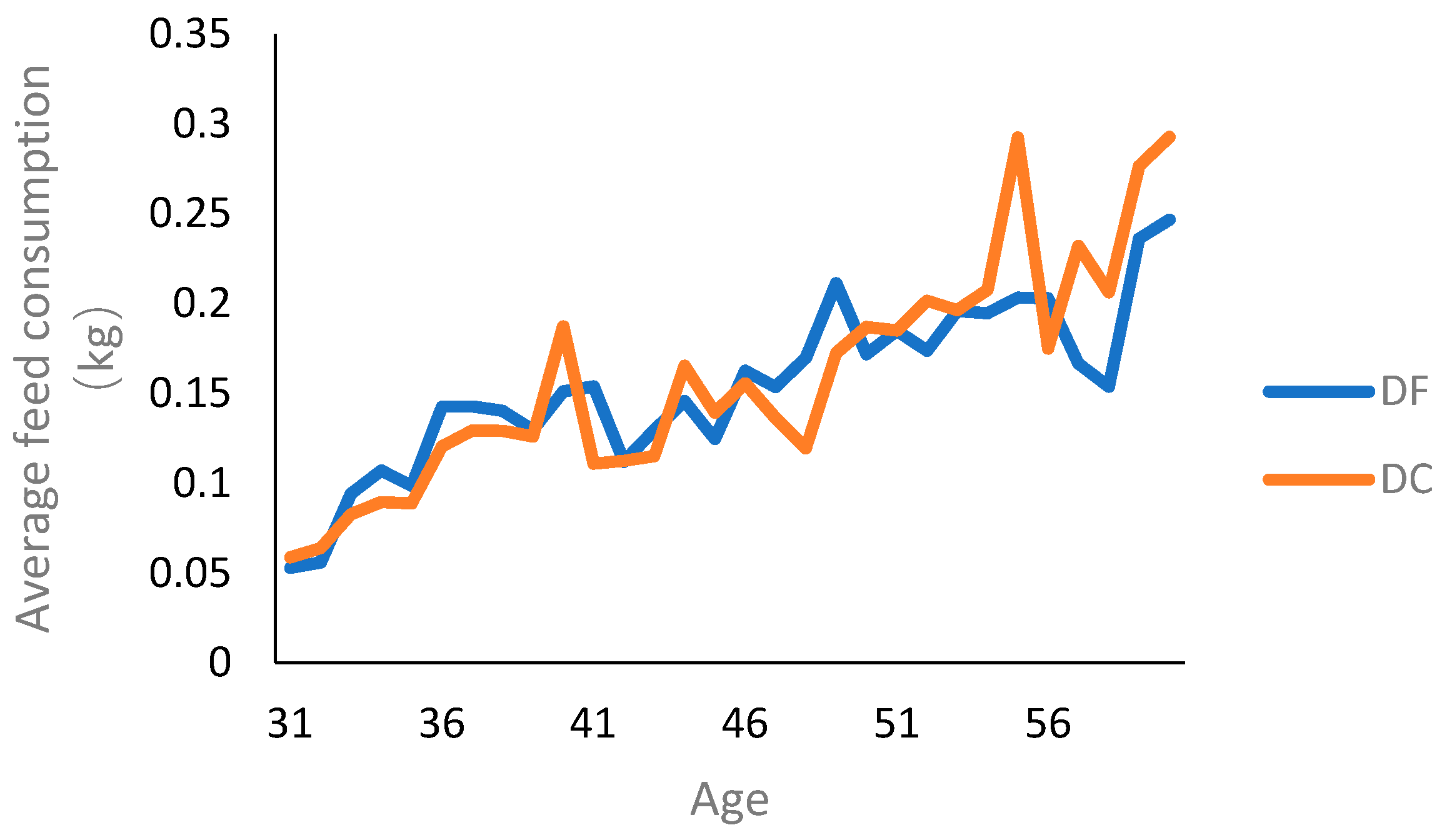Abstract
The combination of planting and breeding, an agricultural production system, makes full use of land, ecological space and time, increasing the utilization rate and yield of both plant and animal production. This experiment aimed to explore the effects of the grape-duck farming system by analyzing behavior, meat quality, and fecal microbiota of ducks and the soil microbiota. The results differed significantly on the expressions of positive behaviors between the breeding group and the combination of planting and breeding group, including actions of foraging, feeding and walking (p < 0.05), while the free-range group showed more stereotyped behavior of no apparent purpose or significance. In terms of meat quality, the yellow value b* of the combination group was significantly higher than that of the free-range group (p < 0.05). The drip loss rate of the planting and breeding group was 1.80%, and the drip loss rate of the combination group was 3.80%, with a significant difference (p < 0.05). The combination of planting and rearing increased the alpha diversity of soil microbiota (p < 0.05), but it had no obvious effect on the fecal microbiota of ducks. PCoA showed that soil microorganisms and fecal microbiota are obviously separated and clustered. In conclusion, the combination production mode of “grape-duck” had a positive effect on duck behavior, meat quality, and soil microorganisms, however, it changed virtually nothing in duck production performance and fecal microbiota.
1. Introduction
In recent years, agricultural recycling and sustainable development have been advocated on a gigantic scale. Actually, humans have been developing critical and longstanding practices of crop production and animal husbandry, integrated farming systems, for eight to ten millennia [1]. The concept of “combination of planting and breeding” is a mode of circular agricultural system for sustainable development, one which combines plant and animal production [2].
The combination of planting and breeding has been reported in diverse modes, and integrated crop-livestock models have been conducted all over the world, e.g., a mulberry-dike-fish-pond, a rice-duck-fish complex ecosystem in China, a crop–livestock systems in North America [3,4], and in Italy they raise geese in vineyards [5]. As it is practiced, the mixed crop-livestock farming system plays a profound effect on the improvement of modern agricultural management.
More than agricultural productions, the combination of planting and breeding modes acts as an ecological system, one which creates a high-efficiency and three-dimensional comprehensive agriculture zone, enhances the utilization rate of land resources and soil quality, improves animal production quality, and even welfare and health [6,7]. At present, the research frontiers on the combination of planting and breeding focus on animal welfare and behaviors [8], meat quality [9], the impact on plant growth and development, soil physical and chemical properties, and the resource utilization of fecal sewage [1]. However, there is no report in the literature on the impact of the combination of planting and breeding on animal growth and development, animal products, and soil microorganisms in feces.
Therefore, this experiment aims to explore the effect of the integration of a grape-duck farming system by testing duck production and slaughter performance, animal behavior, meat quality, and fecal and soil microorganisms. The study will provide insights on farming systems of ecological breeding and the combination of planting and breeding.
2. Materials and Methods
2.1. Experimental Design Descriptions
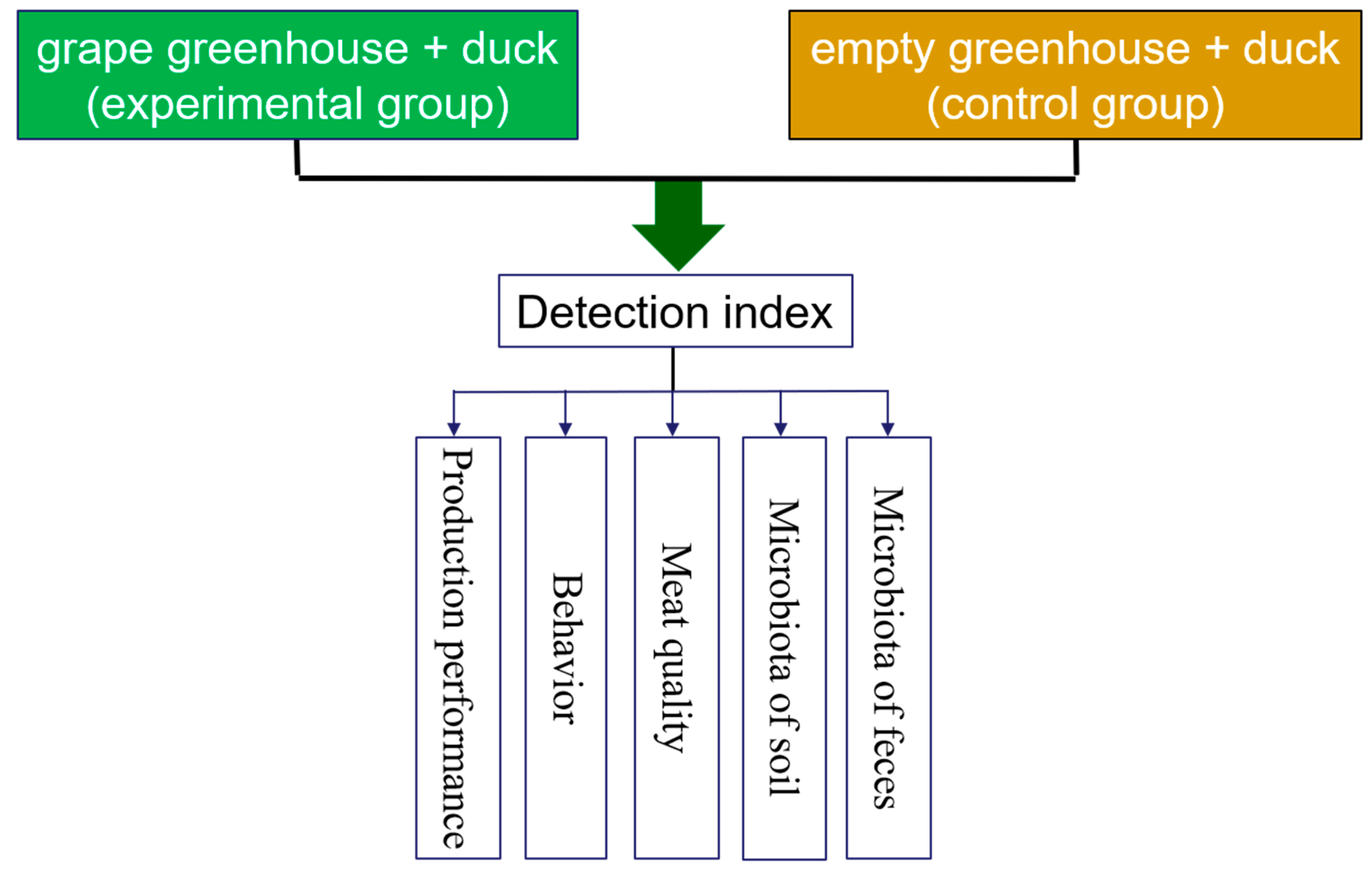
The experimental protocols and animal care were approved by the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection (AW40103202-1-1). The experiment was performed in Guangzong County, Hebei Province, China. The farm in this study is mainly dedicated to greenhouse grape production. At the age of 25 days, one hundred healthy Jinding ducks (a Chinese local breed, provided by Baiyuan family farm, Guangzong County, Hebei Province, China) were randomly divided into two groups. The experimental group was combination of planting and breeding (DC), in which 50 ducks were fed in a greenhouse (25.82 m × 103.28 m), and grapes (Vitis labrusca × vinifera ‘Shine Muscat’, 9.95 m2/plant) were planted, and weeds were grown in the soil. The control group was a badling of free ranged ducks in a greenhouse (DF), consisting of 50 in a greenhouse (25.82 m × 103.28 m) with spare grapes and weeds. The diet (a commercial starter and a grower formula) was kept consistent in DC and DF ducks. DC ducks had access to a commercial diet in addition to the natural pasture, while DF ducks were fed only with a commercial diet. The grower diet was fed to 30 days till the end of the experiment. The temperature in the greenhouse was 28.0–34.4 °C, and the humidity (RH) was 36.2–56.8%, which was recorded by the smart agriculture IoT system (China Unicom). The environmental conditions were comfortable in order to meet the demands of ducks’ health and welfare. During the trials, body weight and the number of living ducks were recorded weekly, and total feed consumption was recorded daily. The duck maintenance behaviors were recorded at the age of 45 to 47 days, and soil and fecal samples were collected at 25 and 61 days of age for subsequent microbial analysis, respectively. At the age of 61 days, five female ducks in each group were humanely slaughtered. The slaughter weight was recorded, and the dressed weight, half-eviscerated weight, eviscerated weight, and net muscle weight of both large and small legs were recorded during slaughter process [10] (Figure 1).

Figure 1.
Experimental design blueprint (the experimental group is the integration system of grape-duck, the control group is the conventional farming system of free range).
2.2. Behavioral Observations
The maintenance behaviors of ducks were recorded at the age of 45 to 47 days from 7:00 to 10:00 a.m. and 2:00 to 5:00 p.m. (birds were in a state of excitement and activity during these periods [11]). The behaviors were recorded through two videos (DS-7808N-E2/8P, JVC, Yokohama, Japan) in different views. The recorded behaviors were classified and counted according to the duck behavior ethogram (Table 1) [12].

Table 1.
Behavior ethogram of ducks.
2.3. Meat Quality of Ducks
The peroneus longus muscle was dissected from duck thigh for analysis [13]. Briefly, the process is as follows:
Thirty minutes after slaughter, the color parameters (L*, a*, b*) of whole left leg meat were measured by a colorimeter (CS-200, Color spectrum technologies, Hangzhou, China). Connective tissue, blood stasis and visible fat were avoided at the time of measurement. Each sample was measured three times and recorded separately.
Ultimate pH (pHu) was measured at 24 h with a portable pH meter (FE20, METTLER TOLEDO) after slaughter. Each sample was measured three times, and the final pH value used was the mean of the three measurements.
The drip loss was estimated by suspending 10 g muscle, the fat and connective tissue of which were removed, in a polyethylene bag, keeping the samples vertically downward but not reaching the bag. Refrigerating at 4 °C for 24 h, weighed after drying the surface with filter paper. The drip loss was calculated according to the formula: (10-weight after suspending)/10 × 100%.
To determine cooking loss, samples were pre-weighed and subsequently placed in sealed polyethylene bags. The bags were then transferred into continuously heated boiling water until the samples reached an internal temperature of 72 °C, as measured by a portable thermometer (Hengko, Shenzhen, China). The bags were then removed from the boiling water and cooled to 25 °C, as determined by the portable thermometer, at which point surface water was gently wiped from the sample using a paper towel. Cooking loss values were calculated as the difference between the initial and final weights of the samples, and the results are expressed as a percentage of the initial weight.
Shear force values were measured with a tenderness tester (RH-N50, Run Hu Instrument Co., Guangzhou, China) by cutting meat samples (1 cm × 1 cm × 6 cm) obtained from cooked samples perpendicular to the fiber direction. Each strip was cut on the tenderness tester 3 times, and the average value of the cut values was calculated.
2.4. Microbial Analysis of Soil and Fecal Samplings
Soil and fecal samples were collected at 25 and 61 days of age, respectively. In each plot, 40 cm of soil was taken from each sample plot (five-point sampling method) [14]. Soil coverings (plants, litter, and visible soil animals) were removed and a 6 cm diameter drill was used to collect the sample. Three samples were taken from the same plot and mixed as one soil test. At the age of 25 days, five ducks were randomly selected for feces analysis. The procedure involved dipping the feces sample in the mucous membrane of the duck cloaca with a sterile cotton swab before immediately putting them into a 1.5 mL freezing tube for further analysis. At the age of 61 days, five rectal fecal samples in each group were collected after ducks had been slaughtered. All samples were frozen in dry ice, and then stored in a −80 °C refrigerator for subsequent microbial analysis. The name of each categorical group was defined as BSMC: soil microorganisms in the counterpart group before the experiment; BSME: soil microorganisms in the experimental group before the experiment; CSM: soil microorganisms in the counterpart group after the experiment; ESM: soil microorganisms in the experimental group after the experiment; BFM: fecal microorganisms before the experiment; CFM: fecal microorganisms in the counterpart group after the experiment; EFM: fecal microorganisms in the experimental group after the experiment.
For 16S rDNA sequencing, total bacterial DNA was isolated from samples using the Power Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s recommendations. DNA quality and quantity were assessed by the ratios of 260/280 and 1% agarose gel electrophoresis before being used to construct libraries. The V3-V4 region of the bacterial 16S rRNA gene was amplified with the common primers [15] (forward primer, 5′-ACTCCTACGGGAGGCAGCA-3′; reverse primer, 5′-GGACTACHVGGGTWTCTAAT-3′) combined with adapter sequences and barcode sequences. High-throughput sequencing analysis of bacterial rRNA genes was performed on the purified, pooled sample using the Illumina NovaSeq 6000 platform (2 × 250 paired ends).
Raw reads were filtered by sequencing used Trimmomatic v0.33 [16]. The primer sequences were recognized and removed using cutadapt 1.9.1 software to yield high-quality reads which excluded primer sequences [17]. Using FLASH v1.2.7 [18], high-quality reads for each sample were spliced by overlaps, which acquired clean reads. UCHIME v4.2 software [19] was used to identify and remove chimera sequences and produce final valid data (effective reads). Reads were clustered at 97% similarity level using USEARCH [20], OTUs (Operational Taxonomic Units) were filtered using 0.005% of all sequence counts sequenced as a threshold [11]. The feature sequences were taxonomically annotated using a naive Bayes classifier using Silva [21] and Unite [22] as reference databases to obtain species taxonomic information corresponding to each feature. The OTU representative sequences were used to count the community composition of each sample at taxonomic level (phylum, class, order, family, genus, species) QIIME2 [23]. was used to evaluate the sample alpha diversity and beta diversity analysis to compare the similarity of different samples in species diversity [24], and Alpha diversity reflected species richness and diversity between different groups. Alpha diversity includes Chao1, ACE, Shannon, Simpson, Goods_coverage and PD_whole_tree. Chao1 and ACE indices measured species richness. Shannon and Simpson indexes are used to measure species diversity. The greater the Shannon index and Simpson index, the higher the species diversity of the sample. In addition, Goods_coverage is an indicator of the sequencing depth, representing the higher the probability of species being measured in the sample, and the lower the probability of not being measured. PCoA (Principal Co-ordinates Analysis) was used to sort through a series of eigenvalues and eigenvectors, selecting the main first few eigenvalues, adopting the idea of dimension reduction, and finding the most important coordinates in the distance matrix, so as to distinguish the differences between individuals or groups.
3. Results
3.1. Production Performance
3.1.1. Survival Rate
The survival rate of ducks was 100% and 96% in DC and DF groups, respectively.
3.1.2. Feed Consumption
There was no significant difference in average feed consumption between DF (0.154 ± 0.0021) and DC (0.158 ± 0.0039) (Figure 2).

Figure 2.
Feed consumption of ducks between DC and DF groups. DC: ducks kept within the combination of planting and breeding; DF: ducks kept in free range.
3.1.3. Body Weight
The body weight changes of each group were shown in Table 2. At the beginning of the experiment, there was no significant difference between the two groups, and then significant differences appeared in later measurements (p < 0.01).

Table 2.
Effects of conventional system of free range and grape-duck system on duck body weight (unit: g).
3.1.4. Slaughter Performance
There was no significant difference between DF and DC ducks concerning slaughter weight, dressed weight, half-eviscerated weight, eviscerated weight, leg muscle weight, dressed percentage, and other slaughter performance (Table 3).

Table 3.
Determination of slaughter performance of ducks.
3.2. Behaviors
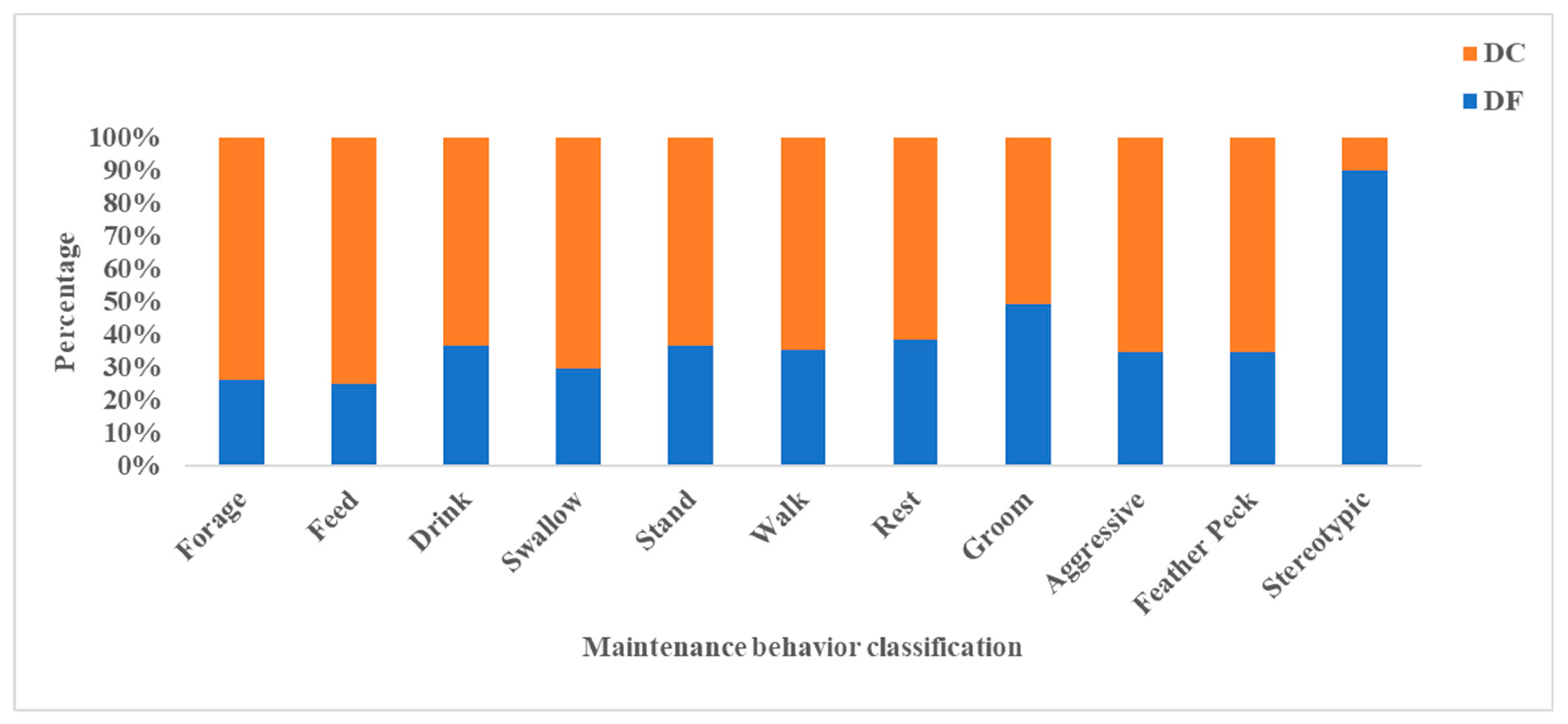
According to the data analysis, the frequency of foraging, feeding, swallowing, drinking, standing, rest, and walking in the DC group were significantly higher than that in DF group (p < 0.05). The incidence of stereotyped behavior in DF group was significantly higher than that in DC group (p < 0.05). In addition, no significant difference on behaviors of grooming, aggressive, and feather pecking was expressed between DF and DC ducks (Figure 3).

Figure 3.
Maintenance behavior. DC: ducks kept within the combination of planting and breeding; DF: ducks kept in free range (n = 50).
3.3. Meat Quality
Amongst data, b* in DC group was significantly higher than DF (p < 0.05), and drip loss rate in DF group was significantly lower than DC group (p < 0.05). There were no significant differences in the other meat quality indexes which are illustrated in Table 4.

Table 4.
Meat quality traits in each group.
3.4. Effects on Microbiota of Soil and Duck Feces
3.4.1. Soil Microbial Composition
Raw Data Processing and Quality Control
A total of 1,034,468 effective sequences were obtained from four groups of samples, including 49,507 high-quality reads in BSMC group, 53,460 in BSME group, 56,473 in CSM group, and 47,455 in ESM group; the average values of Q20 and Q30 were 98% and 94%, respectively.
OTU Analysis
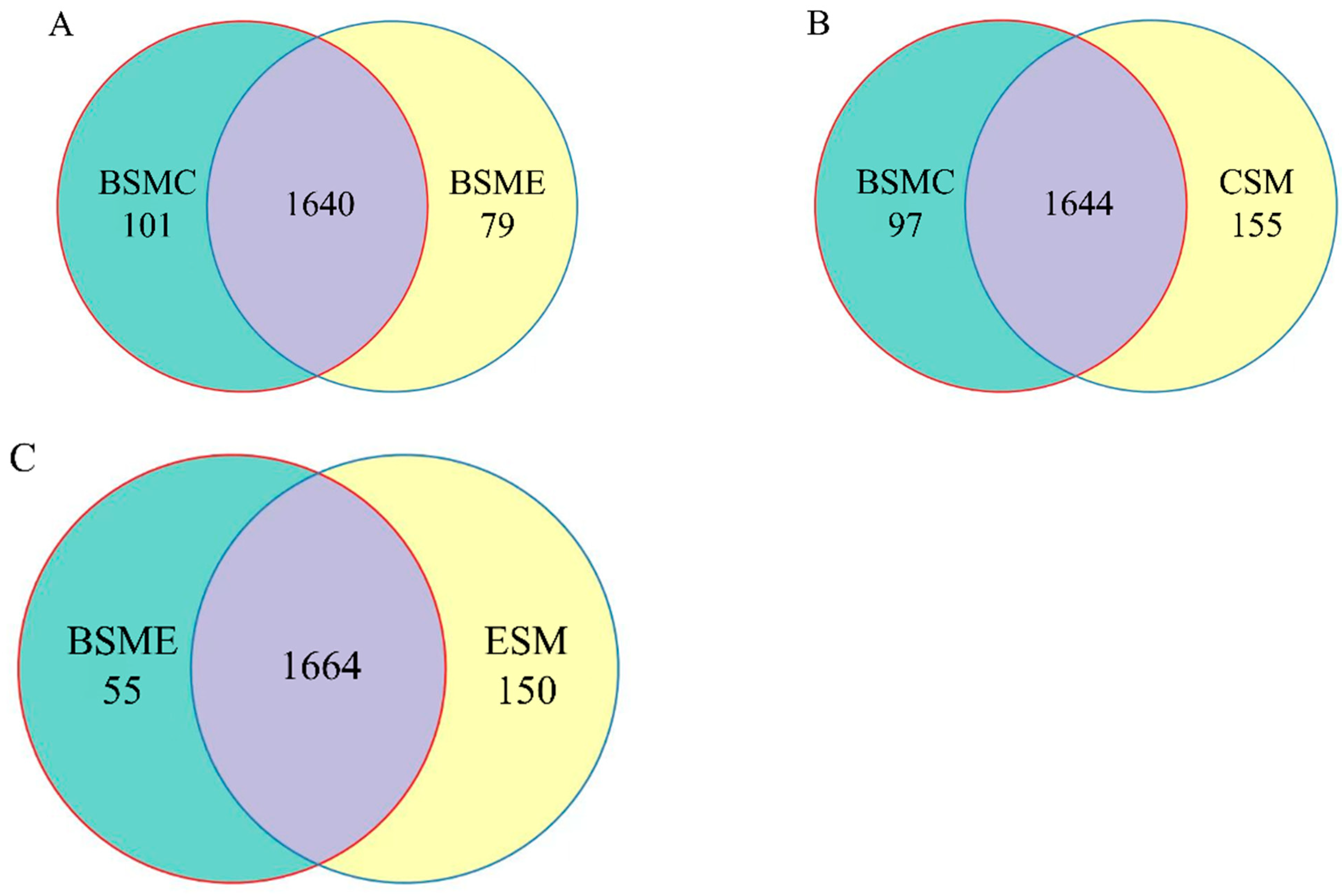
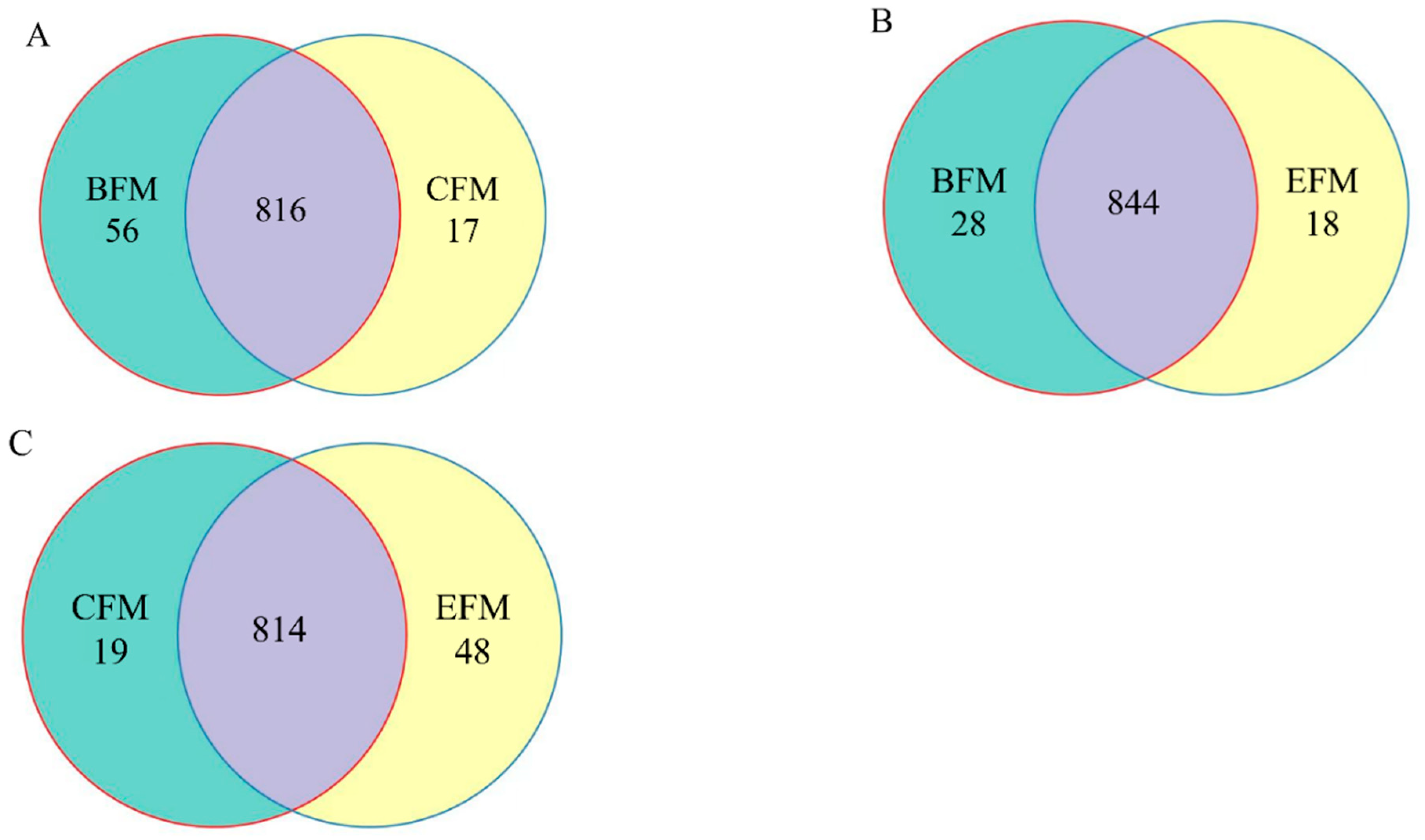
The similarity and overlap of operating units (OTUs) composition among different groups were studied through a Venn diagram of classified OTUs (Figure 4). The total number of OTUs in BSME, BSME, BSMC, and CSM groups were 1741, 1719, 1799 and 1814, respectively.
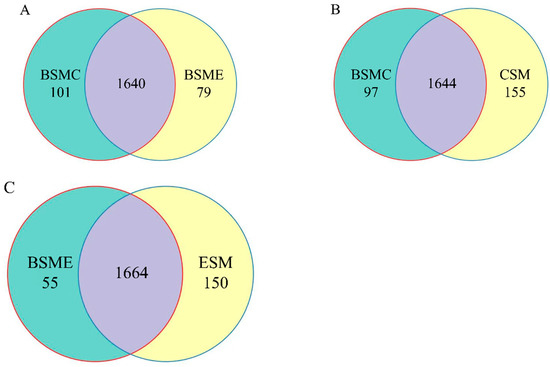
Figure 4.
Venn diagram of OTUs among groups. (A) OTUs Venn diagram of greenhouse soil microorganisms between BSMC and BSME groups in DC and DF groups; (B) BSMC and CSM groups; (C) BSME and ESM groups. BSMC: soil microorganisms in the counterpart group before the experiment; BSME: soil microorganisms in the experimental group before the experiment; CSM: soil microorganisms in the counterpart group after the experiment; ESM: soil microorganisms in the experimental group after the experiment.
Alpha Diversity
The statistics of the alpha diversity index of each sample was shown in Table 5. Alpha diversity reflects the species richness and diversity of a single sample. There are several indicators: Chao1, ACE, Shannon, Simpson, Goods_coverage and PD_whole_tree. The CSM group and the ESM group, concerning the index of ACE and Chao1, were higher than that of the BSMC and BSME groups. However, Shannon, Simpson, Goods_coverage and PD_whole_tree showed no difference among the groups.

Table 5.
Alpha diversity of soil samples.
Microbial Relative Abundance Analysis in Soils
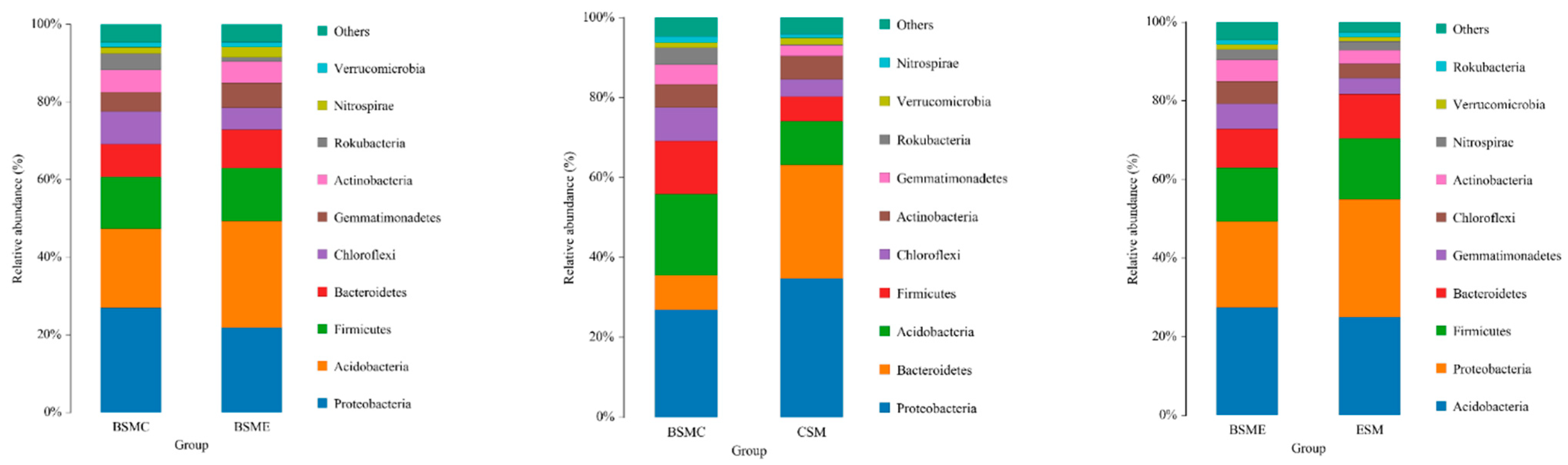
Figure 5 illustrates the comparison of microbial relative abundance of three different comparison strategy groups at the phylum level. At the phylum level, the dominant microflora were Acidobacteria, Proteus, Firmicutes, Bacteroidea, Blastomonas, Curvularia, and Actinomycetes, which accounted for more than 90% of the soil microbial population. Through the comparison of BSMC and BSME groups, it can be seen that the relative abundance of Acidobacteria, Firmicutes, Bacteroidea, and Bacillus increased, while the relative abundance of Proteus, Campylobacter, and Actinomycetes decreased in the grape planting group; the comparison of BSMC and CSM groups showed that compared with ordinary soil, the relative abundance of Proteus and Bacteroidetes in duck group increased, while the relative abundance of Acidobacteria, Firmicutes, Curvularia, actinomycetes, and Blastomonas decreased; through the comparison of BSME and ESM groups, it can be seen that, compared with ordinary grape greenhouse soil, the relative abundance of Proteus, Firmicutes and Bacteroidetes in duck grape greenhouse soil increased, while the relative abundance of Acidobacteria, Blastomonas, Campylobacter, and Actinomycetes decreased compared with ordinary soil (Figure 5).
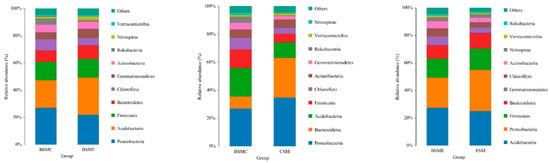
Figure 5.
Histogram of relative abundance of horizontal species of the phylum. BSMC: soil microorganisms in the counterpart group before the experiment; BSME: soil microorganisms in the experimental group before the experiment; CSM: soil microorganisms in the counterpart group after the experiment; ESM: soil microorganisms in the experimental group after the experiment.
3.5. Duck Feces Microbial Composition
3.5.1. Raw Data Quality Control and Processing
A total of 1,190,235 effective sequences were obtained from the three groups of samples, including 58,156 high-quality sequences in the BFM group, 65,890 in the CFM group, and 55,844 in the EFM group. The average values of Q20 and Q30 are 98% and 94%, respectively. The sample sequence number and quality of this test are sufficient and meet the requirements of subsequent analysis.
3.5.2. OTU Analysis
The similarity and overlap of OTUs composition among different groups were studied through the Venn diagram of the OTUs (Figure 6). The total number of OTUs in BFM, CFM, and EFM groups were 872, 833, and 862, respectively.
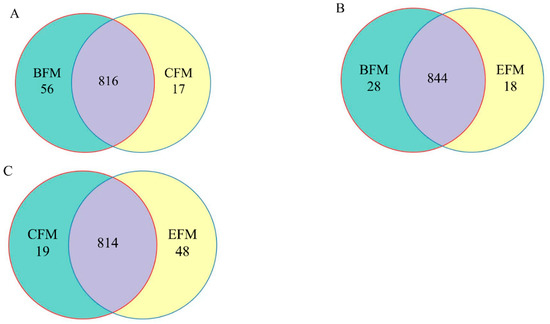
Figure 6.
Venn diagram based on OTU feature. (A) OTUs Venn diagram of fecal microbiota between BFM and CFM groups; (B) BFM and EFM groups; (C) CFM and EFM groups. BFM: fecal microbiota before the experiment; CFM: fecal microbiota in the counterpart group after the experiment; EFM: fecal microbiota in the experimental group after the experiment.
3.5.3. Alpha Diversity Analysis Indices
The statistics of alpha diversity index values of each sample are shown in Table 6. Feature, Chao1, ACE, Shannon, Simpson, Goods_coverage and PD_whole_tree revealed no difference among BFM, CFM, and EFM groups.

Table 6.
Alpha diversity of fecal samples among groups.
3.5.4. Relative Abundance of Microbial Composition in Feces
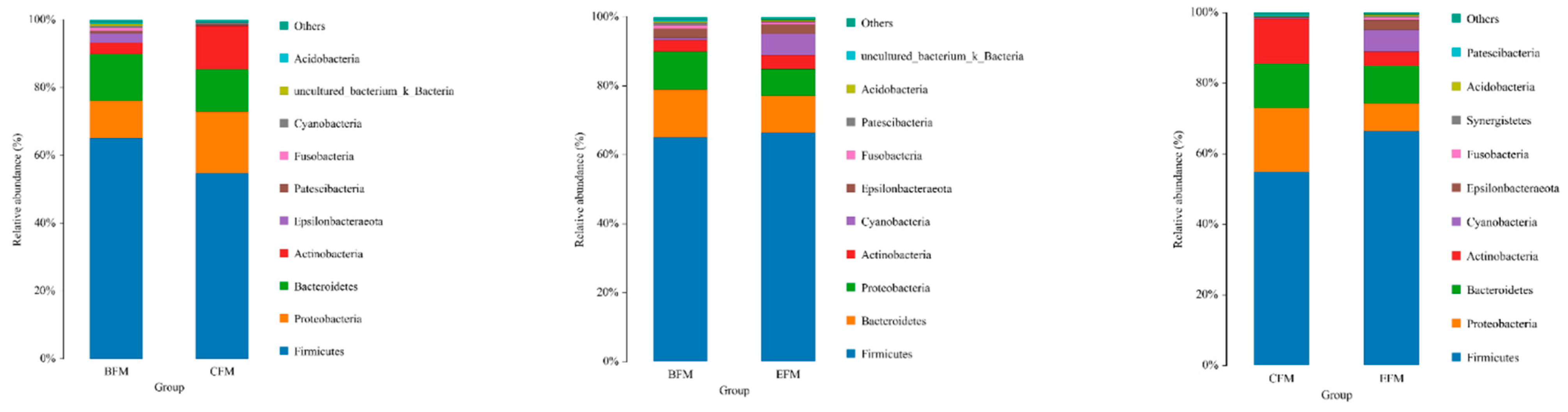
The species of duck fecal microorganisms in each group are mainly concentrated in Firmicutes, Bacteroides, Proteobacteria, Actinobacteria, and Cyanobacteria. These microorganisms account for more than 95% of the duck intestinal microbial population, including Firmicutes, and Proteobacteria and Bacteroidetes are the three most important phyla in each group. Through the comparison of CFM and EFM groups, it can be seen that the abundance of Firmicutes and Cyanobacteria in duck fecal microorganisms in the test group increased and the abundance of Proteus and Actinomycetes decreased compared with the control group (Figure 7).
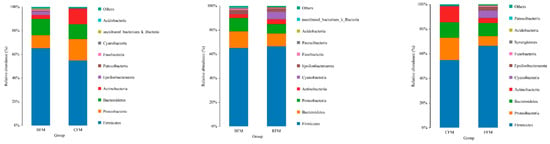
Figure 7.
Microbial relative abundance at the phylum level. BFM: fecal microbiota before the experiment; CFM: fecal microbiota in the counterpart group after the experiment; EFM: fecal microbiota in the experimental group after the experiment.
3.6. Beta Diversity Analysis
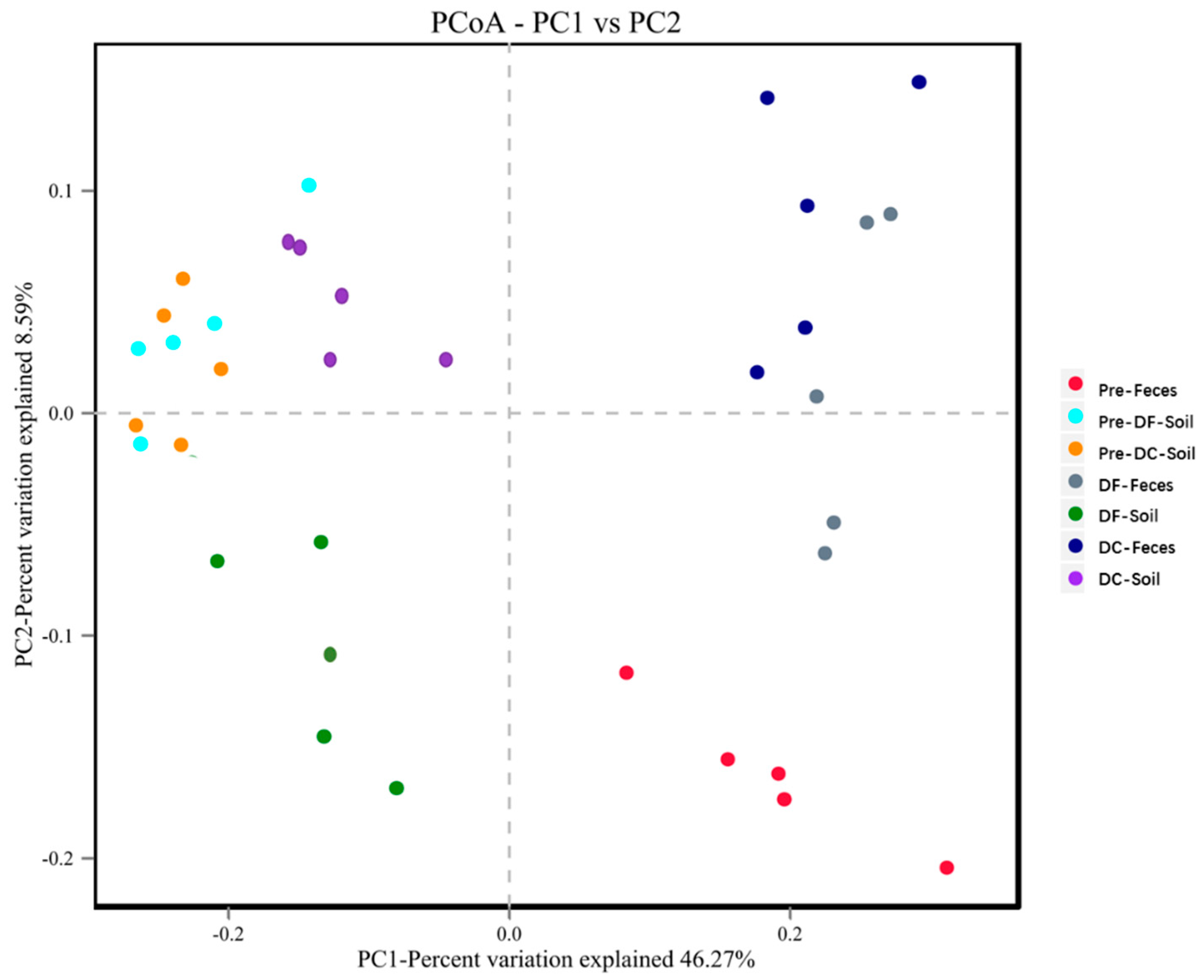
The results of PCoA analysis between groups are as follows (Figure 8). Principal component analysis based on the OTUs level of microbial communities showed that different groups of soil and feces could significantly separate communities before and after the experiment. Before the experiment, the soil microbial community structure of the DC group and the DF group were similar, but after the experiment, the DC group and the DF group were significantly separated. For fecal microorganisms, the microbial communities were significantly separated before and after the experiment, but the differences between the DC group and the DF group were not significant. Soil microorganisms and fecal microbiota are obviously separated and clustered.
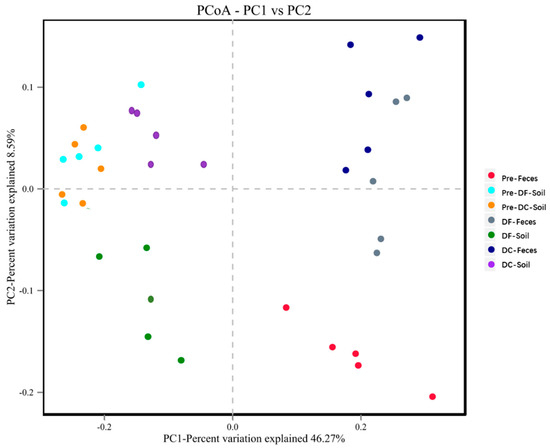
Figure 8.
PCoA analysis of soil microbes and fecal microbiota. Points represent each sample respectively; different colors represent different groups; the abscissa represents the first principal component, and the percentage represents the contribution value of the first principal component to the sample difference; the ordinate represents the second principal component, and the percentage represents the contribution of the second principal component to the sample difference. Pre−Feces: fecal microorganisms before the experiment; Pre−DF-Soil: soil microorganisms in the counterpart group before the experiment; Pre−DC−Soil: soil microorganisms in the experimental group before the experiment; DF−Feces: fecal microorganisms in the counterpart group after the experiment; DF−Soil: soil microorganisms in the counterpart group after the experiment; DC−Feces: fecal microorganisms in the counterpart group after the experiment; DC−Soil: soil microorganisms in the experimental group after the experiment.
4. Discussion
4.1. Effects of Duck Behaviors
In our study, the combination of planting and breeding provides enriched environments for ducks. Our results showed that the frequency of foraging, feeding, swallowing, standing, walking, and resting behaviors in the combined planting and breeding group were significantly higher than those in the conventional system. In the combined planting and breeding group, due to supplementary extra feeding, ducks needed to explore for food, which may have increased the possibility of activity and resting behaviors. Previous study has found that environmental enrichments affect behavior expressions in laying hens [25,26]. In an enriched environment, individuals show more positive behaviors, such as foraging and preening, and reduced expression of negative behaviors, such as stereotyped behaviors and feather pecking [27,28,29]. Our results showed the interaction effects between birds and the environment concerning behavior. Drinking is generally considered as the response of livestock and poultry to stress [30]. The combination planting and breeding group needed to continuously explore the enriched environment, resulting in increased foraging causing some positive stimulation itself. The stereotypical behavior in the conventional system is more expressed, which may be due to the single raising environment of the empty greenhouse where the individual is located, the lack of freshness, the extra feed supply, and the monotonous life. The stereotypical behavior is similarly in accord with the finding that poor environment in the early stage may lead to abnormal behavior of brooding hens [31]. The behaviors in the combined planting and breeding group imply that ducks in enriched environments can exhibit expected positive behaviors.
4.2. Effects on Production and Slaughter Performance of Ducks
Our results showed that the growth curves of ducks between the two modes are similar, which is in accordance with previous research results [32]. There are significant differences in body weight and feed consumption between the conventional free range and the integration of grape-duck system. However, there are differences in the reports of feeding methods affecting poultry production performance. The reason for the above test results may be that the feed of the conventional system is free to feed during the test, while the combined planting and breeding group feeds on the weeds in the grape greenhouse and is supplemented with feed. The combined group increases the amount of exercise in the process of grazing, thus increasing the individual energy consumption, resulting in the weight of the conventional system being higher than that of the ducks in the combined planting and breeding group. In the grape greenhouse with an enriched environment, ducks spent a lot of time exploring the surrounding environment to obtain various foods. In the process of foraging, the amount of exercise has increased and the physique has been exercised, which may improve disease resistance, so it helps to improve the immunity performance of ducks to the enriched environment. Unfortunately, the survival rate of the conventional system is lower than that of the grape-duck system, which may be attributed to the unstable and poor feeding environment.
In the study of broilers, the slaughter performance is closely related to genetic factors [31]. Because the same batch of Jinding ducks was used in this experiment, there was no genetic significant difference in slaughter performance between the conventional and grape-duck system. The richness of the living environment provided by the grape-duck or conventional system did not greatly affect the slaughter performance of ducks. One of the qualities of poultry meat production performance is reflected in the dressed percentage. If the dressed percentage reaches more than 80%, it shows that the variety is suitable for meat [33]. In our experiment, the dressed percentage of the conventional system was 90.60%, and that of the combination group was 90.30%, which was above the standard. The dressed percentage is similar to the ducks raising dietary Quebracho tannins, which positively improved carcass yields [34]. Above all, the slaughter performance improvement was attributed to the combination mode of planting and breeding.
4.3. Effects on Ducks’ Meat Quality
Meat quality is used to measure the comprehensive performance of meat animals, including observed appearance characteristics, determination of nutritional components by physical or chemical methods, and food safety [35]. The color of meat is the most direct expression of physiological and biochemical changes, alterations which can reflect some characteristics to a certain extent and whether it can be further processed [36]. After slaughter, muscle tissue will undergo anaerobic glycolysis of muscle glycogen, and pH can indicate the degree of this process. Too high or too low pH will induce abnormal meat quality [37]. In our experiment, the 24 h pH value of duck leg muscle in the conventional system and the grape-duck system were in the range of 6.28–6.39. The amount of fluid lost by the muscle protein system only under the action of gravity is defined as drip loss, which can reflect whether the muscle is full of juice and tender [38]. Some studies have shown that the pH value measured by the muscles of free range broilers will decrease, and the drip loss will also decrease compared with ordinary captive broilers [39]. The pH value of the combined planting and breeding group is relatively less than that of the conventional system, and the drip loss is significantly higher than that of the conventional system. Previous research shows that the dripping loss and cooking loss of meat samples are inversely proportional to the water-holding capacity (the WHC was calculated as the fraction of water retained by the meat [1 (expressible juice/total moisture content)]) [40], and our tested results are in line with it. Shear force can be used to quantify the tenderness of cooked meat. There was no significant difference in pH, cooking loss, and shear force between the two groups. Our study showed that the mixed condition model exerts improved effects on meat quality.
4.4. Effects on Microorganisms in Soil and Duck Feces
As known, animals and plants are commonly and persistently inhabited by microorganisms [41]. Previous reviews have summarized the proposition that the larger community of soil microorganisms can increase plant health and crop productivity [42], and even can rapidly improve plant fitness in response to environmental changes [43]. In addition, grazing plays a positive role in the improvement of soil microbial community in the soil’s organic carbon, the important positive role of soil microbial α-diversity in a meadow steppe ecosystem [44]. Similarly, soil microbial community composition and functions were very sensitive to the impact of livestock grazing in the Tibetan alpine grassland [45]. In our study, it was found that the soil microbial structure changed after planting grapes and raising ducks, and the fecal microbial composition structure of ducks also changed under different growth conditions concerning the OTUs. The relative abundance of soil Acidobacteria is the highest in the BSME group, and the relative abundance of soil Proteus is the highest in the BSMC group. Compared with the BSMC group, the abundance of Proteus and Bacteroidea in the CSM group is higher, while the abundance of Firmicutes and Acidobacteria is lower. The abundance of soil Proteobacteria in the ESM group growing grapes and raising ducks is the highest. As known, Acidobacteria has the photosynthetic ability and can degrade plant litter, Firmicutes have strong stress resistance and can produce spores to resist external harmful substances [46]. Besides, Proteus plays an important role in decomposing organic matter and promoting soil carbon, nitrogen, and sulfur cycle [47]. In particular, the CSM group and ESM group’s index of ACE and Chao1 were increased compared with those of the BSMC and BSME groups. The above results show that duck breeding can increase the richness of soil microorganisms compared with the only-grape-planting group. Also, the combination of planting and breeding system can reduce the adverse effects on soil microbial structure and activity. The structure and diversity of the soil microbial community are richer after raising ducks, which is more conducive to the recovery of soil fertility after resisting external threats. The reasons for the change of soil microbial diversity are controversial. The research of Watts et al. [48] shows that exogenous bacteria will be introduced when manure is applied in the field, which affects the dynamic change of soil microbial community. In similar experiments, when livestock manure was applied to the soil with long-term chemical fertilization, it had little impact on the soil microbial community, and the soil pH value is an important factor in determining the microbial community [49].
With the increase of duck age, the abundance of Firmicutes decreased, the abundance of Proteus and Actinomycetes increased, and the abundance of Bacteroidetes and Proteus decreased, the abundance of Firmicutes and Proteus increased. The abundance of fecal microorganisms Bacteroidetes, Proteus, and Actinomycetes in the CFM group of ducks is higher than that in the EFM group of ducks raised in the grape greenhouse, and the abundance of Firmicutes and Cyanobacteria is lower, but the uniformity of fecal microorganisms in the EFM group is higher. The dominant flora with the highest fecal microbial abundance in the three groups is Firmicutes, and the microbial diversity is not significant although there are some differences. The microbial community in the feces can appropriately reflect the level of microbial population in the intestine [50]. In the research results of poultry intestinal microorganisms, the influencing factors of intestinal microbial community are multifaceted. The similarity of intestinal microbial community in Tibetan chicken is related to geographical distance; the closer the distance is, the closer the DGGE fingerprint of Tibetan chicken population is [51]. When 60 to 120 day old ducks were restricted to feed, their intestinal microbial diversity was altered, with an increased abundance of Firmicutes and a decreased abundance of Bacteroidetes [52]. In the previous experiments, ducks were reared using fermentation beds, high feeding density had a negative effect on the beneficial bacteria in the intestinal tract of ducks, and there were large differences in the microbial populations in the fermentation bedding and cecum [53]. Therefore, no significant differences in duck fecal microbial community alterations were observed among the results of our experiment, which may be due to the small variation in environment and feeding. However, since grapes have been planted in both greenhouses, plants will have a certain impact on soil microorganisms. Although the control group is an empty greenhouse, soil memory still affected duck intestinal microorganisms. In this experiment, the content of Proteobacteria in the EFM group was higher, which may be due to the sudden transfer from a single feeding environment to the grape greenhouse, resulting in the change of flora.
5. Conclusions
Comparing to the conventional system, ducks in the grape-accompanied system exhibited similar feed consumption and growth performance, but demonstrated a higher survival rate, increased positive behaviors and improved meat qualities. Feeding ducks in grape greenhouses to a certain degree reduced the negative effect of planting on the soil, and the combination of planting and breeding mode changed the composition of soil microorganisms and increased the diversity of the soil microbial populations, but the effect on duck intestinal microorganisms was not obvious. The grape-duck system is technically and productionally feasible for practice, and within it, ducks can live out their days with better welfare conditions.
Author Contributions
Conceptualization, X.Z.; methodology, D.C.; software, D.C.; validation, Y.Z., C.Y. and X.Z.; formal analysis, C.Y.; investigation, Y.Z., C.Y., D.C. and C.Z.; resources, X.Z.; data curation, Y.Z., C.Y. and D.C.; writing—original draft preparation, Y.Z., C.Y. and D.C.; writing—review and editing, C.Y. and X.Z.; visualization, Y.Z. and D.C.; supervision, X.Z.; project administration, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Joint Project of Hebei Guangzong Professor Workstation (Number: 201705511523).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection (AW40103202-1-1).
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request.
Acknowledgments
We express sincere thanks to Qindong Wang and Yanbin Hu for their support of the experiments.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Thornton, P. The Emergence of Agriculture: Bruce D. Smith. Scientific American Library, Distributed by W. H. Freeman & Co. Ltd, New York and Oxford, 1995. 231 pp. Price: £19.95, US$ 32.95 (Hardback). ISBN 0 7167 5055 4. 1996; Volume 51, pp. 496–497. Available online: https://www.sciencedirect.com/science/article/abs/pii/0308521X96814892?via%3Dihub (accessed on 29 November 2022).
- Bell, L.W.; Moore, A.D. Integrated crop–livestock systems in Australian agriculture: Trends, drivers and implications. Agric. Syst. 2012, 111, 1–12. [Google Scholar] [CrossRef]
- Faust, D.R.; Kumar, S.; Archer, D.W.; Hendrickson, J.R.; Kronberg, S.L.; Liebig, M.A. Integrated Crop-Livestock Systems and Water Quality in the Northern Great Plains: Review of Current Practices and Future Research Needs. J. Environ. Qual. 2018, 47, 1–15. [Google Scholar] [CrossRef]
- Russelle, M.P.; Entz, M.H.; Franzluebbers, A.J. Reconsidering integrated crop-livestock systems in north America. Agron. J. 2007, 99, 325–334. [Google Scholar] [CrossRef]
- Massaccesi, L.; Cartoni Mancinelli, A.; Mattioli, S.; De Feudis, M.; Castellini, C.; Dal Bosco, A.; Marongiu, M.L.; Agnelli, A. Geese Reared in Vineyard: Soil, Grass and Animals Interaction. Animals 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.C.F.; Peterson, C.A.; Nunes, P.A.A.; Martins, A.P.; de Souza Filho, W.; Bertolazi, V.T.; Kunrath, T.R.; de Moraes, A.; Anghinoni, I. Animal production and soil characteristics from integrated crop-livestock systems: Toward sustainable intensification. J. Anim. Sci. 2018, 96, 3513–3525. [Google Scholar] [CrossRef]
- Patrizi, N.; Niccolucci, V.; Castellini, C.; Pulselli, F.M.; Bastianoni, S. Sustainability of agro-livestock integration: Implications and results of Emergy evaluation. Sci. Total Environ. 2018, 622, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Cartoni Mancinelli, A.; Mattioli, S.; Menchetti, L.; Dal Bosco, A.; Chiattelli, D.; Angelucci, E.; Castellini, C. Validation of a behavior observation form for geese reared in agroforestry systems. Sci. Rep. 2022, 12, 15152. [Google Scholar] [CrossRef]
- Mancinelli, A.; Mattioli, S.; Bosco, A.; Piottoli, L.; Ranucci, D.; Branciari, R.; Cotozzolo, E.; Castellini, C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim. Sci. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Chen, K.; Gao, Y.; Wang, Z.; Wang, Y.; Zhang, X. Performance Ferms and Measurement for Poultry; China Agriculture Press: Beijing, China, 2004. [Google Scholar]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Jones, T.A.; Dawkins, M.S. Effect of environment on Pekin duck behaviour and its correlation with body condition on commercial farms in the UK. Br. Poult. Sci. 2010, 51, 319–325. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, B.; Mei, X.; Jiang, S.; Li, W. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poult. Sci. 2019, 98, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.K.; Sharma, J.C.; Dahiya, S.S.; Dhankar, J.S. Collection and preparation of soil samples. Environ. Sci. 2004, 37–45. [Google Scholar]
- Dennis, K.L.; Wang, Y.; Blatner, N.R.; Wang, S.; Saadalla, A.; Trudeau, E.; Roers, A.; Weaver, C.T.; Lee, J.J.; Gilbert, J.A.; et al. Adenomatous Polyps Are Driven by Microbe-Instigated Focal Inflammation and Are Controlled by IL-10-Producing T Cells. Cancer Res. 2013, 73, 5905–5913. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Gloeckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Lawley, B.; Tannock, G.W. Analysis of 16S rRNA Gene Amplicon Sequences Using the QIIME Software Package. In Oral Biology: Molecular Techniques and Applications, 2nd ed.; Seymour, G.J., Cullinan, M.P., Heng, N.C.K., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1537, pp. 153–163. [Google Scholar]
- Rai, S.N.; Qian, C.; Pan, J.; Rai, J.P.; Song, M.; Bagaitkar, J.; Merchant, M.; Cave, M.; Egilmez, N.K.; McClain, C.J. Microbiome data analysis with applications to pre-clinical studies using QIIME2: Statistical considerations. Genes Dis. 2021, 8, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.S. Behavioral deprivation—A central problem in animal-welfare. Appl. Anim. Behav. Sci. 1988, 20, 209–225. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ozaki, T.; Watanabe, T.; Tanida, H.; Yoshimoto, T. Effects of Perches on Behavior and Performance of Caged Hens. Jpn. Poult. Sci. 2008, 30, 183–189. [Google Scholar] [CrossRef]
- Liao, S.C.; Lyu, P.X.; Shen, S.Y.; Hsiao, C.C.; Lien, C.Y.; Wang, S.D.; Lin, T.Y.; Tu, P.A. Effects of Swimming Pool Conditions and Floor Types on White Roman Geese’s Physical Condition Scores and Behaviors in an Indoor Rearing System. Animals 2022, 12, 3273. [Google Scholar] [CrossRef]
- Colton, S.; Fraley, G.S. The effects of environmental enrichment devices on feather picking in commercially housed Pekin ducks. Poult. Sci. 2014, 93, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Savory, C.J.; Gentle, M.J.; Yeomans, M.R. Opioid modulation of feeding and drinking in fowls. Br. Poult. Sci. 1989, 30, 379–392. [Google Scholar] [CrossRef]
- Riber, A.B.; Wichman, A.; Braastad, B.O.; Forkman, B. Effects of broody hens on perch use, ground pecking, feather pecking and cannibalism in domestic fowl (Gallus gallus domesticus). Appl. Anim. Behav. Sci. 2007, 106, 39–51. [Google Scholar] [CrossRef]
- Massuquetto, A.; Panisson, J.C.; Marx, F.O.; Surek, D.; Krabbe, E.L.; Maiorka, A. Effect of pelleting and different feeding programs on growth performance, carcass yield, and nutrient digestibility in broiler chickens. Poult. Sci. 2019, 98, 5497–5503. [Google Scholar] [CrossRef]
- Ju, X.; Shu, J.; Zhang, M.; Liu, Y.; Tu, Y.; Ji, G.; Shan, Y.; Zou, J. Comparison analysis of meat quality and flavor of different breeds and feeding periods of broilers. Chin. J. Anim. Nutr. 2018, 30, 2421–2430. [Google Scholar]
- Castillo, A.; Schiavone, A.; Cappai, M.G.; Nery, J.; Gariglio, M.; Sartore, S.; Franzoni, A.; Marzoni, M. Performance of Slow-Growing Male Muscovy Ducks Exposed to Different Dietary Levels of Quebracho Tannin. Animals 2020, 10, 979. [Google Scholar] [CrossRef]
- Jung, D.Y.; Lee, D.; Lee, H.J.; Kim, H.-J.; Jung, J.H.; Jang, A.; Jo, C. Comparison of chicken breast quality characteristics and metabolites due to different rearing environments and refrigerated storage. Poult. Sci. 2022, 101, 101953. [Google Scholar] [CrossRef]
- Owens, C.M.; Hirschler, E.M.; Mckee, S.R.; Martinez-Dawson, R.; Sams, A.R. The characterization and incidence of pale, soft, exudative turkey meat in a commercial plant 1. Poult. Sci. 2000, 79, 553–558. [Google Scholar] [CrossRef]
- Juqing, W.U.; Chunbao, L.I.; Guanghong, Z.; Xinglian, X.U.; Xiuxiang, J.U.; Yichen, C. Changes of Meat Color and Tenderness of Chilled Beef and Pork during Postmortem Aging. Food Sci. 2008, 29, 136–139. [Google Scholar]
- He, F.; Wang, Z.; Zhang, C.; Ge, C.; Zhang, D. Effect of Drip Loss of Lamb from Different Varieties on the Meat Quality. J. Chin. Inst. Food Sci. Technol. 2018, 18, 239–247. [Google Scholar]
- Castellini, C.; Mugnai, C.; Bosco, A.D.J.J.o.M.F.; Medicine, N. Effect of conventional versus organic method of production on the broiler carcass and meat quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Fletcher, D.L.; Northcutt, J.K.; Russell, S.M. The relationship of broiler breast color to meat quality and shelf-life. Poult. Sci. 1998, 77, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Sachs, J.L. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef]
- Phour, M.; Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Interkingdom signaling in plant-rhizomicrobiome interactions for sustainable agriculture. Microbiol. Res. 2020, 241, 126589. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kong, W.; Ji, M.; Zhao, K.; Chen, H.; Yue, L.; Dong, X. Grazing greatly reduces the temporal stability of soil cellulolytic fungal community in a steppe on the Tibetan Plateau. J. Environ. Sci. 2022, 121, 48–57. [Google Scholar] [CrossRef]
- Dai, J.; Tian, P.; Zhang, Y.; Su, J.Y. Rhizobacteria community structure and diversity of six salt-tolerant plants in Yinbei saline soil. Acta Ecol. Sin. 2019, 39, 2705–2714. [Google Scholar]
- Yang, Y.; Xu, M.; Zou, X.; Chen, J.; Zhang, J.; Zhang, J. Effects of different vegetation types on the characteristics of soil bacterial communities in the hilly area of central Guizhou. J. Ecol. Rural Environ. 2021, 37, 518–525. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A.; Feng, Y.; Prior, S.A. Soil Microbial Community Dynamics as Influenced by Composted Dairy Manure, Soil Properties, and Landscape Position. Soil Sci. 2010, 175, 474–486. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Zheng, J.; Wen, C.; Ji, C.; Zhang, D.; Chen, Y.; Hou, Z.; Yang, N. Efficacy of Fecal Sampling as a Gut Proxy in the Study of Chicken Gut Microbiota. Front. Microbiol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, X.; Yang, C.; Ma, B.; Lei, C.; Xu, C.; Zhang, A.; Yang, X.; Xiong, Q.; Zhang, P.; et al. Cecal microbiota of Tibetan Chickens from five geographic regions were determined by 16S rRNA sequencing. Microbiologyopen 2016, 5, 753–762. [Google Scholar] [CrossRef]
- Li, J.-P.; Wu, Q.-F.; Ma, S.-C.; Wang, J.-M.; Wei, B.; Xi, Y.; Han, C.-C.; Li, L.; He, H.; Liu, H.-H. Effect of feed restriction on the intestinal microbial community structure of growing ducks. Arch. Microbiol. 2022, 204, 85. [Google Scholar] [CrossRef]
- Wang, J.-M.; Gan, X.-M.; Pu, F.-J.; Wang, W.-X.; Ma, M.; Sun, L.-L.; Hu, J.-W.; Hu, B.; Zhang, R.-P.; Bai, L.-L.; et al. Effect of fermentation bed on bacterial growth in the fermentation mattress material and cecum of ducks. Arch. Microbiol. 2021, 203, 1489–1497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).