Abstract
Skin separation (puffiness) is a critical physiological disorder that significantly reduces the date palm (Phoenix dactylifera L.) fruit’s visual appearance and quality. The objectives of this study were to analyze fruit anatomy in skin-separated and normal date palm (var. Mejhoul) fruit and to assess the microclimatic conditions (temperature and relative humidity) during the fruit developmental stages and their association with skin separation. Fruit anatomy analysis was carried at different growth stages (Kimri-Khalal, Khalal, Rutab and Tamr) for two growing seasons. In addition, microclimatic conditions (specifically, temperature, and relative humidity) as well as soil moisture content were assessed across the study period 2020 and 2021. The anatomical analysis of the date palm fruit revealed that the exocarp or skin (cuticle, epidermis, hypodermis) thickness were quite similar across the developmental stages and over the study period (2020, 2021). Conversely, significantly more sclereid cells were found in skin-separated fruit (compared to normal fruit) at the Tamr stage. At that stage (Tamr), the percentage increase in sclereid cell number in skin-separated fruit ranged from 34–183%, sclereid clusters number 23–92%, cluster area 37–206%, and cluster perimeter 25–64%, as compared to normal fruit. In addition, sclereid cells at skin-separated areas of the fruit were clustered together, forming a chain of aggregates underneath the fruit skin, and were closer (42–50%, than normal) to the cuticle, leading to the partial separation of the exocarp from the fruit mesocarp. Although the weather data were similar across the growing seasons (temperatures, 33–34 °C; relative humidity, 45–46%), skin separation occurred in 14–15% of total fruit in 2020 and 30–34% in 2021. Therefore, we believe that skin separation disorder is not as heavily weather dependent as it seems to have been thought. In conclusion, changes in microclimate conditions were not the conclusive component for inducing the skin separation physiological disorder in date palm fruit. Further studies are required to identify the main factors that stimulate the development of sclereid clusters aggregates and the potential of agricultural practices to reduce skin separation.
1. Introduction
Date palm (Phoenix dactylifera L.) is considered one of the most important fruit crops in many regions of the world, especially those with hot and dry climates. Date palms thrive in hot, arid climates, with the majority of their production taking place in the Southern Mediterranean regions [1]. In fact, date palm trees are adapted to a wide range of soil conditions including mineral nutrients, salinity, and water stress [2]. Worldwide, the total area grown is estimated to be ca 1.3 million ha, and total production is about 13 million tons [3]. In 2017, the total number of date trees cultivated globally was about 100 million, out of which ~90% were grown in the Middle East and North Africa regions [4]. In Jordan, the total cultivated area is about 3244 ha, and total production is estimated to be 30,000 tons [3].
Dates are an important component of healthy diets in many nations and are recognized as the main food crop in those regions [2]. In fact, the date palm has played a key role in food security in the Middle East and North African regions by providing nutritional food for humans for the last 5000 years [4]. Date carbohydrate (total sugars) content ranges from 44 to 88%, fiber 6.4 to 11.5%, protein 2.3 to 5.6%, fat 0.2 to 0.5%, oil 0.2–0.5%, and there are 15 types of salts and minerals [1]. Furthermore, the seed (accounting for 5.6–14.2% of the date weight) contains 7.7 to 9.7% oil, 14 types of fatty acids [1], and 15% of fiber (mainly water-insoluble mannan fibers). Dates are a rich source of phenolic antioxidants (1–2%), especially condensed tannin and fiber (6.5 to 11.5%), as reported by Ghnimi et al. [4]. Thus, date fruit and seeds are used in some food products as a rich source of fiber and antioxidants [4]. The high demand for date palm fruit worldwide due to its potential source of antioxidants and sugars makes it an ideal crop to help with the development of low-income countries, where poverty is dominant [5].
The fruit development of date palm as reported by Al-Hajjaj et al. [6] can be classified into five stages, namely, Hababouk (1–5 weeks from pollination, WAP), Kimri (6–16 WAP), Khalal (17–20 WAP), Rutub (21–24 WAP), and Tamr (25–27 WAP). The fruit at the Tamr stage have low moisture content (less than 25%), which is appropriate for long-term storage [2,7]. Date sorting is normally done according to shape, size, maturity, weight, condition of the flesh (ranges from soft to dry), and skin quality [5,8]. The absence of visual defects, deformity, skin puffiness, sunburn, insect damage, uniformity of color and size, decay, fermentation, and mechanical damage are some of the criteria that affect the quality grades of the date fruit [8]. However, fruit quality at the time of harvest and during storage is influenced by pre-harvest growth conditions during the growing season [9]. These effects may alter the fruit skin and cause certain subsurface cellular structural alterations, which may have an impact on the quality and shelf life of the fruit after harvest [9].
The majority of producing countries have large losses during harvest and postharvest processing and marketing as a result of physical and physiological disorders, especially those associated with skin appearance [2,7]. In most horticultural crops, including date palm, the visual appearance of the fruit skin is essential for determining its market value [10]. Physiological disorders that are associated with skin potentially lower the visual quality of the fruit and result in possible economic loss to the growers [10]. Skin separation of ripe date fruit (especially “Mejhoul”) lowers the cultivar market value [11]. The price range for fruit with 30–50% skin separation (puffiness) is about 80% lower than that with an intact, normal skin (no puffiness) [12]. Skin separation (puffiness) normally occurs when the date skin is dry, hard and brittle [13]. This physiological disorder develops during ripening and leads to the separation of the skin from the flesh [13]. A few research studies have been carried out to understand and reduce skin separation [11,14]. However, the main causes of skin separation in the date palm fruit is not yet fully understood. Kader and Hussein [13] suggested that high relative humidity and temperature at early stages of ripening might lead to skin separation in dates. Al-Hajjaj and Ayad [11] found that boron foliar application at the rate of 1600 mg L−1 significantly increased date yield (67.7 vs. 53.4 kg tree−1) and fruit size (50 vs. 45 mm) but did not reduce skin separation. The deficit irrigation technique at the fruit ripening stage stimulated the ripening of the date palm fruit (while maintain yield) but had limited effect (not significant improvement) on skin separation [6]. Considering the high economic value of the date palm fruit and the potential losses associated with skin separation, as well as the uncertainty of identifying the main causes of this physiological disorder, the study of date palm fruit anatomy and microclimate is critical to progress the research efforts associated with skin separation. The objectives of this study were to evaluate the anatomy component in skin-separated and normal date fruit and to assess the microclimatic conditions (temperature and relative humidity) during the fruit developmental stages and its association with skin separation.
2. Materials and Methods
2.1. Site Selection
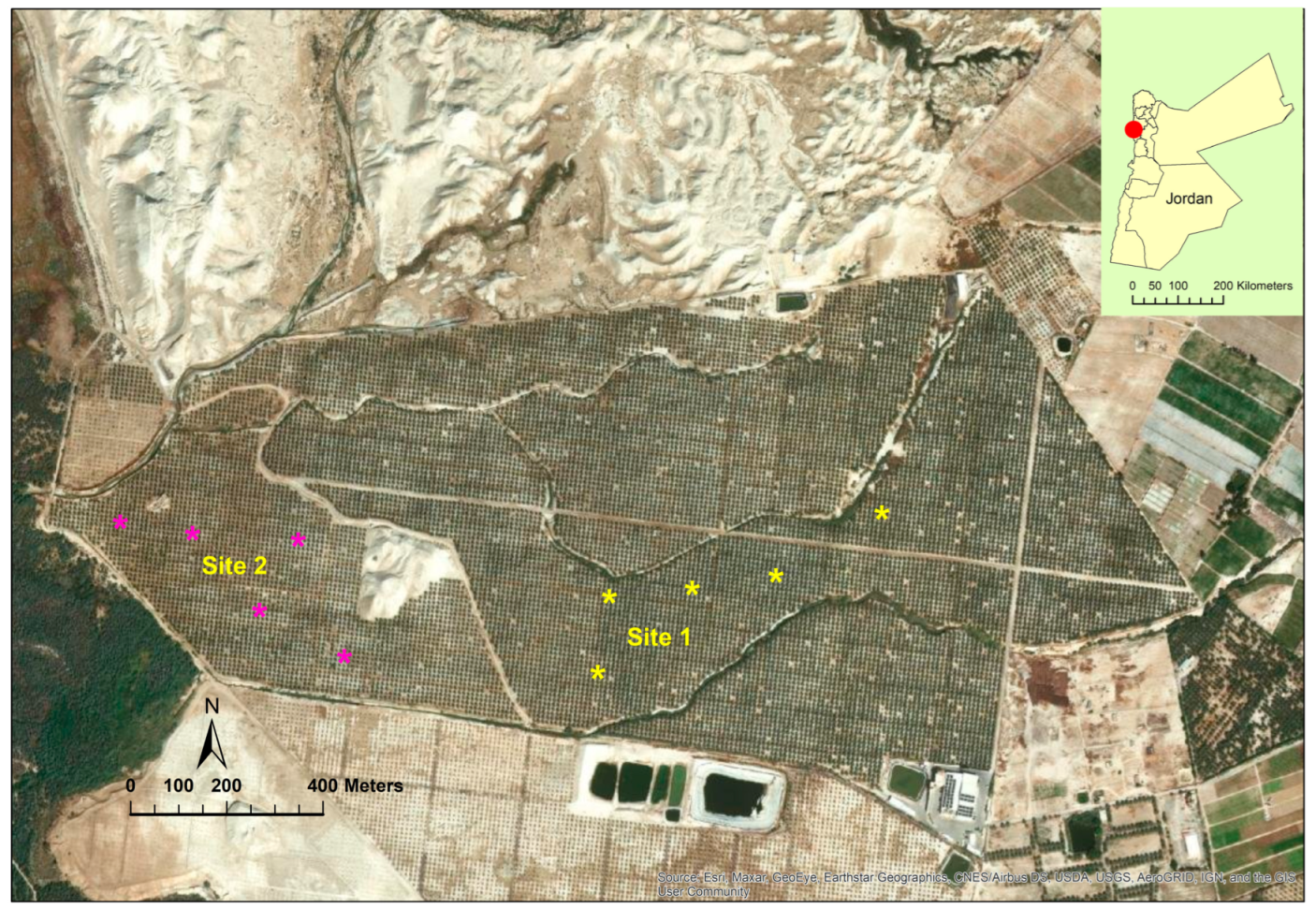
This study was conducted at The Medjool Village farm, Jordan Valley, Jordan (long. 31° 58’41.14″ N, lat. 35° 33’2534″ W, elevation 325 m below sea level) from March 2021 to October 2022 (Figure 1). The farm was awarded GLOBAL G.A.P. certification for applying international standards for crop production. The farm’s total area is about 128 ha, and the total number of date palm trees (Phoenix dactylifera L.) var. “Mejhoul” (previously known as “Medjool”) is about 17,700. Although several date palm cultivars (>10) are grown in Jordan including “Barhi” and “Khalas”, “Mejhoul” account for 62% of the total grown area [11]. The study was carried out for two growing seasons in two selected sites at the farm (Figure 1). The soil chemical analysis for both sites is presented in Table 1. Trees from both sites were about 8 years old and spaced 9 m within and between rows. Trees from both sites were subjected to similar agricultural practices including irrigation, fertilizers, and bunch thinning. Due to COVID-19, workers removed only the lower one third of the bunch by cutting back the tips of the stands at pollination period. During the growing seasons (2020, 2021), the mean temperatures ranged from 33–34 °C and relative humidity between 35 and 46%. The total irrigation per tree per year was 45 m3 using a drip irrigation system.
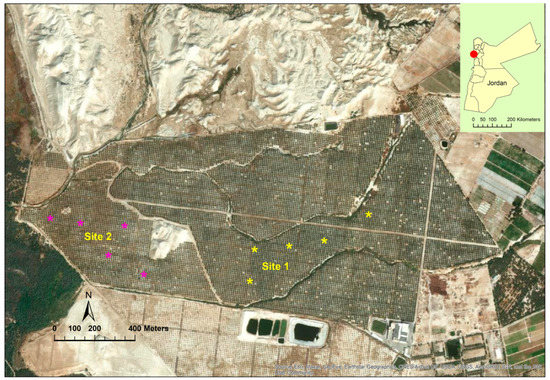
Figure 1.
Study sites at The Medjool Village, Jordan Valley, Jordan. Asterisk (*) represents the tree location (site 1 and 2) from which the fruit were sampled for anatomical observations.

Table 1.
Soil analysis of experimental sites at the Medjool Village, Jordan Valley, Jordan.
2.2. Fruit Anatomy and Skin Separation
For the anatomical observations during the 2020 and 2021 growing seasons, 40 fruit of each maturity stage [unripe (kimri and khlal) and ripe (Rutab and Tamr)] were collected (5 date palm trees per site). A 0.5 cm long section of fruit skin (with pulp) was taken. For each mature and ripe stages, the fruit sections were immediately soaked in Formalin–Acetic Acid fixative solution (FAA) following the procedure of Huang et al. [15]. 5 mm-thick transverse sections were mounting on slides, dried, and stained in hematoxylin and eosin stain. Fruit anatomy sections (cross and longitudinal) were analyzed using a light microscope (B-Scope Trino E-plan, Euromax Microscopes Holland, Arnhem, The Netherlands) supplied with a 12 pixel camera (CMEX 12, Euromax Microscopes Holland, Arnhem, The Netherlands). Fruit anatomy variables were measured using image analysis software (Image Focus Plus, Euromax Microscopes Holland, Arnhem, The Netherlands). Skin separation was determined for both growing seasons and across sites (1 and 2) using fruit internal quality system (TrueSort™, Ellips, Eindhoven, The Netherlands). The sorting system can accurately detect internal defects in dates and is able to see completely through the exocarp.
2.3. Weather Data and Soil Moisture Content
Air temperature and relative humidity were measured continuously in three randomly selected locations at both sites using external temperature/relative humidity data logger (MX2302A, HOBO, Bourne, MA, USA). Sensors were installed on the top of the trees. In addition, soil moisture content was measured daily at 11:00 a.m. using time-domain reflectometry (TDR 100, Spectrum Technologies, Inc. Aurora, IL, USA).
2.4. Data Analysis
Skin separation data were analyzed using SAS statistical software (version 9.2; SAS Institute, Cary, NC, USA). The analysis of variance (ANOVA) and the least significant difference test (p < 0.05) in SAS were used to identify differences between treatments.
3. Results
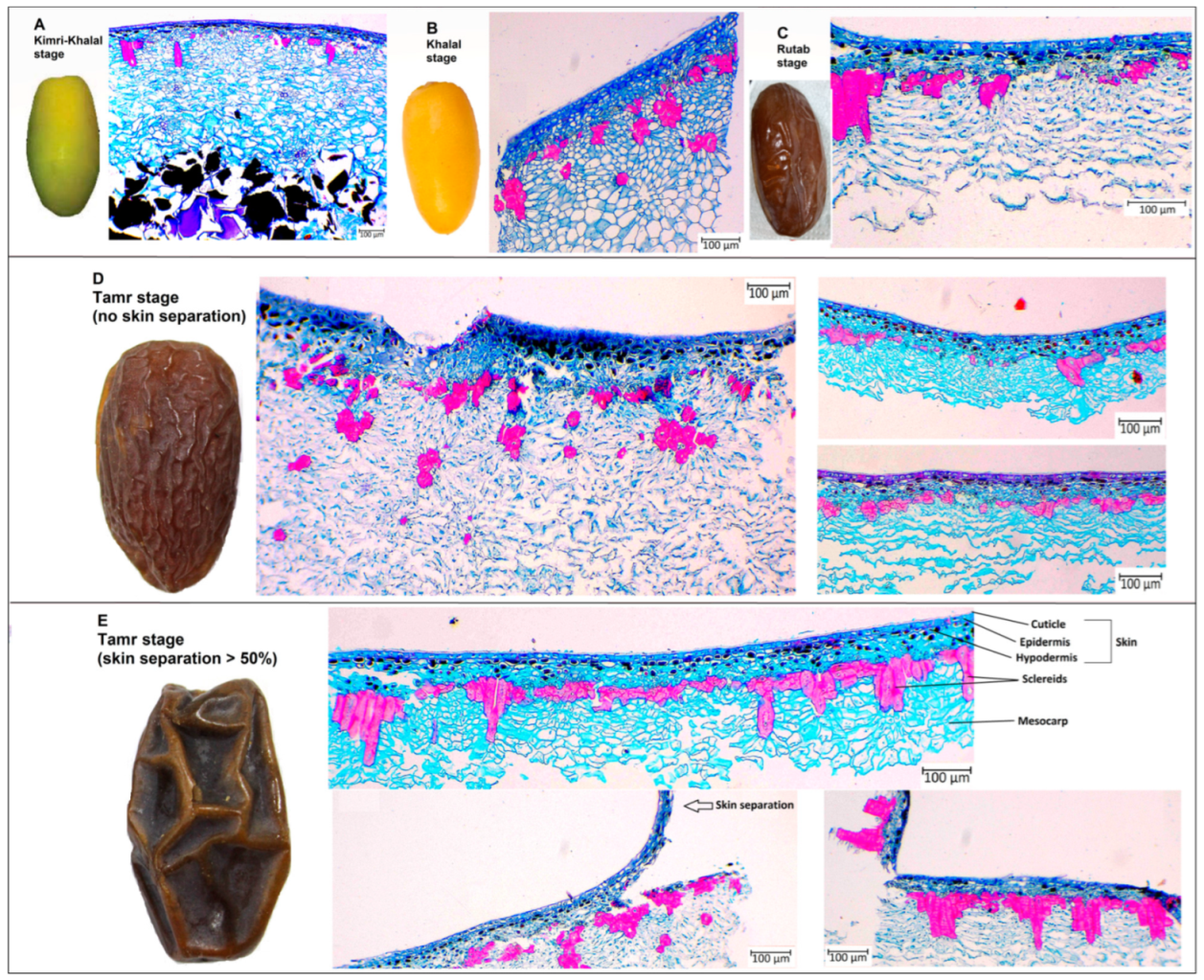
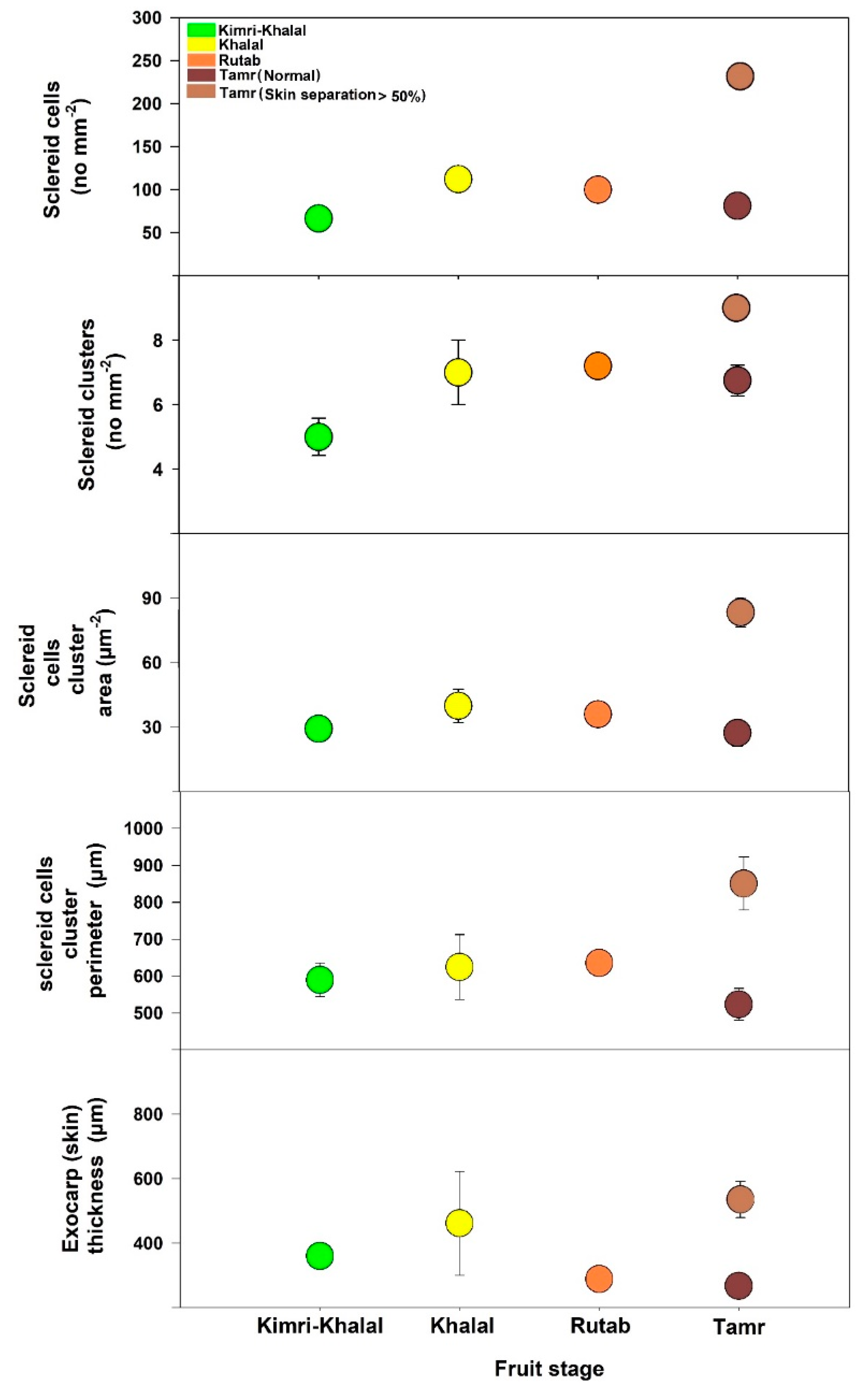
The anatomy analysis of date palm fruits was carried out at the Kimri-Khalal, Khalal, Rutab, and Tamr stages in 2020 (Figure 2 and Figure 3). The sclereid cell numbers and clusters at the early fruit developmental stage (Kimri-Khalal) were lower than the next succeeding stages. However, the sclereid cluster area and perimeter were similar across the four stages, except at Tamr in fruit with more than 50% skin separation (Figure 2). During the Kimri-Rutab fruit developmental stages, the skin (cuticle + epidermis + hypodermis) thickness ranged from 350–400 µm.
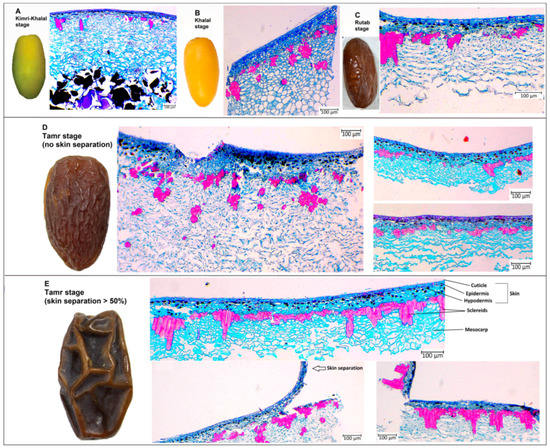
Figure 2.
Date palm fruit anatomy at (A) Kimri-Khalal, (B) Khalal, (C) Rutab, and (D) Tamr with no skin separation and (E) with skin separation >30%.
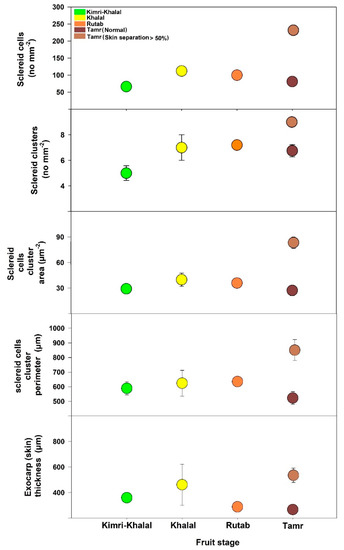
Figure 3.
Sclereid total cell number, cluster number, area, and perimeter and exocarp (skin) thickness of date palm fruit ‘Mejhoul’ at four developmental stages.
Table 2 shows the anatomical differences in date palm fruit ‘Mejhoul’ at the Tamr stage in normal fruit and those in which skin separation exceeded 50%. In both years (2020 and 2021), the sclereid cell number in normal fruit was significantly lower than in skin-separated ones. In addition, the sclereid cluster number, area, and perimeter in normal fruit was extremely lower than those that had the skin separation physiological disorder.

Table 2.
Anatomical changes in date palm fruit ‘Mejhoul’ at the Tamr stage, September 2020 and 2021.
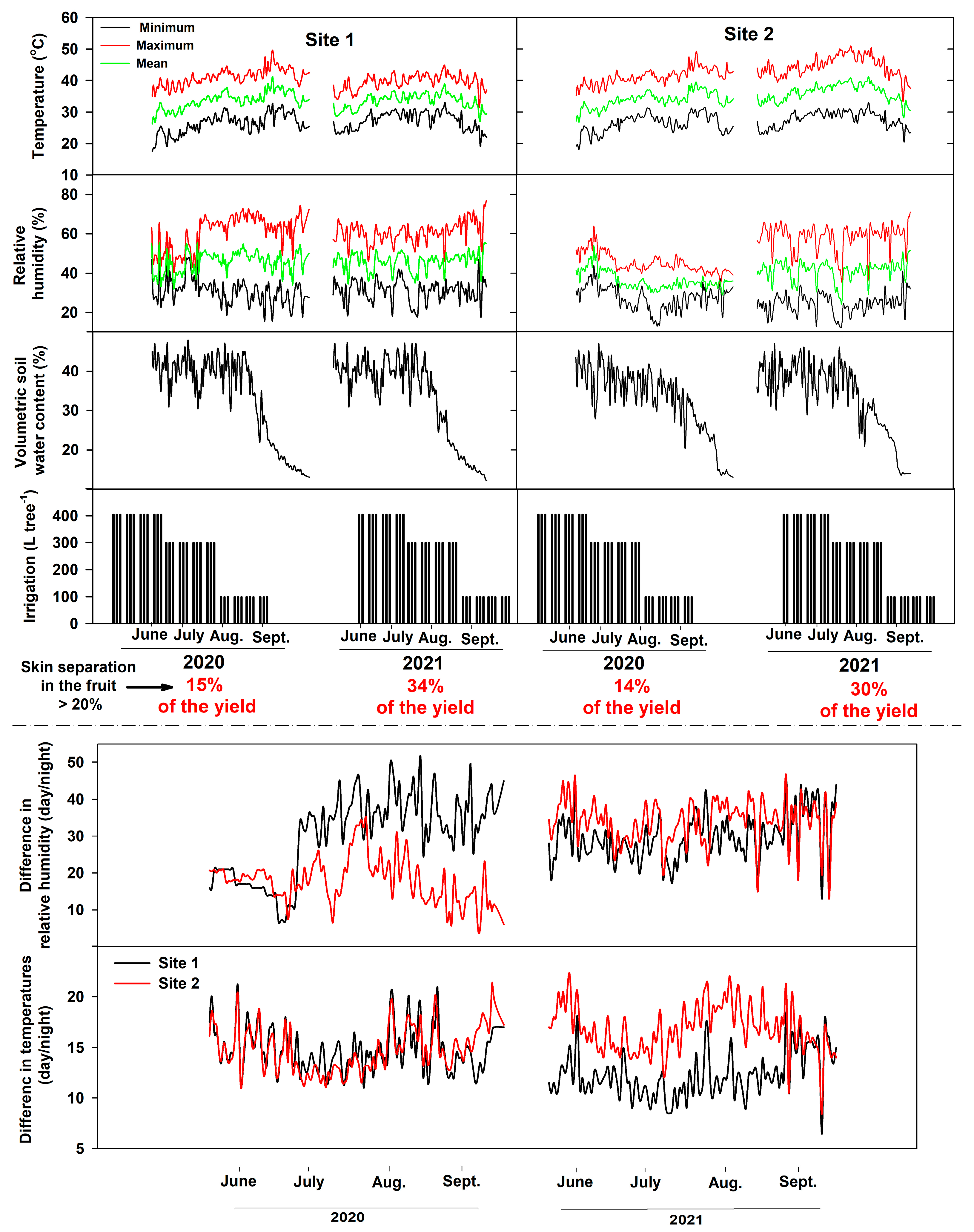
Weather data (temperature and relative humidity), volumetric soil water content, and irrigation during the fruit ripening stages (June–October, 2020 and 2021) are shown in Figure 4. For site 1, mean air temperatures (2020, 2021) were between 33.7 and 33 °C, and mean relative humidity was between 45.8 and 46%. Site 2 mean temperatures (2020, 2021) were between 33.9 and 36 °C, whereas mean relative humidity was between 36.3 and 41%. The difference in temperatures between day and night at site 1 during the 2020 growing season ranged from 12–21°C (mean 14.7 °C) and from 11–21°C (mean 14.8 °C) at site 2. Differences in relative humidity (day/night) for 2020 ranged from 7 to 51% (mean 30%) at site 1 and from 5 to 35% (mean 18%) at site 2. In 2021, temperature differences at site 1 ranged between 6.5–18.5°C (mean 12 °C) at site 1 and 8.5–22 °C (mean 16.8 °C) at site 2. In addition, the difference in relative humidity between day and night in 2021 at site 1 was between 13–46% (mean 29.6%) and 13.2–46.5% (mean 34%) at site 2.
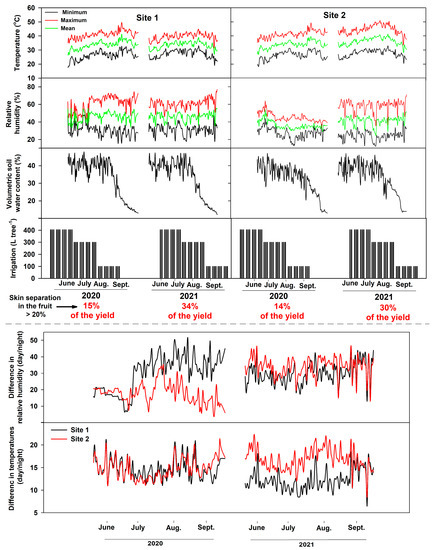
Figure 4.
Weather macroclimate, irrigation per tree, and volumetric soil water content during the study period in both sites at the Medjool Village, Jordan Valley, Jordan.
4. Discussion
4.1. Date Palm Industry and Skin Separation
Date fruits are commonly available in the global market, predominantly at the mature Tamr stage [4]. Although the date fruit is used in several food products worldwide, large amounts of dates ends up as waste [16]; this is approximately 20–30% of the total production in Jordan [12]. Low-quality date fruit (loss) has more fibers and a harder texture than the high-grade ones [16]. In horticultural crops, the cuticle, epidermal, and hypodermal cells represent the structural backbone of the skin during fruit development and ripening [17]. Interestingly, the visual appearance of a fruit is associated with mechanical characteristics of its skin as well as the skin integrity [17]. Overall, studies dealing with skin quality are essential for date palm industries due to fact that the expected value of loss caused by the skin physiological disorders (specifically, skin separation) is extremely high and could cost millions of dollars each year. In this study, the skin separation (>30% per fruit) ranged from 14.5% (2020) to 34% (2021) across the studied sites. The skin separation of ripe fruit is a common physiological disorder which significantly reduces the quality of the fruit and, consequently, its commercial value [18,19]. Generally, skin separation does not reduce the nutritional value or taste of the fruit, but these symptoms do affect the visual appearance of the fruit and consequently compromise its price value at the point of sale [20]. Therefore, these disorders, including microcracking and separation, are of considerable commercial importance [20]. Lustig et al. [19] concluded that when skin separation in the fruit exceeds 10%, it becomes disqualified for export. Considering the high percentage of skin separation (this study: 14.5–32%, Jordan national statistics: 20–40%) of fruit yield, and due to its negative effect on date marketing value (Jordan: ~$30 million loss), understanding the main causes of this physiological disorder is of great interest to date palm growers.
4.2. Fruit Anatomy
In this study, the anatomical analysis of the date palm fruit revealed that the exocarp or skin (cuticle, epidermis, hypodermis) and mesocarp thickness were quite similar across the study period (2020, 2021), except for sclereid cells (Figure 2 and Figure 3). During the ripening stages (Kimri-Khalal, Khalal, Rutab and Tamr), the sclereid cell numbers and clusters gradually increased and became prominent, especially in skin-separated fruit at the Tamr stage. In fact, the anatomical analysis of the date revealed that the percentage increase in sclereid cell number in skin-separated fruit at the Tamr stage ranged from 34–183%, sclereid clusters number 23–92%, cluster area 37–206%, and cluster perimeter 25–64% when compared to normal fruit (no skin separation), as shown in Table 2. In addition, the sclereid cells in skin-separated fruit were clustered together, forming a chain of aggregates underneath the fruit skin, leading to the partial separation of exocarp from the flesh of the fruit (mesocarp), as shown in Figure 2E. In pears (Pyrus communis, P. bretschneideri and P. pyrifolia), stone (or sclereid) cells appeared in cluster structures 60 days after pollination [21]. In addition, these stone cells were clustered and moved closer to the epidermis in P. pyrifolia pear than in P. communis and P. bretschneideri [21]. In our study, the distance between cuticle and sclereid clusters in skin-separated fruit (compared to normal skin date) was 42–50% shorter (Table 2). Overall, in this study, the skin separation of date fruits at the Tamr stage occurred when the stone cells clustered, aggregated, and moved closer to the exocarp.
Stone (sclereid) cells form sclerenchyma tissue that is developed by the deposition of lignin onto the primary cell wall of parenchyma cells, leading to an increase in the cell wall thickness [22,23]. High numbers of stone cells stimulate the development of rough skin fruit and, thus, reduce fruit quality. Normally, stone cells’ numbers at early fruit developmental stages are low but gradually increase at later stages, especially in rough-skin fruit [23]. In loquat fruit, the content of stone cells can reach 1.6% (w/w) and size 1000 × 500 μm [22]. Stone cells content in sand pears (Pyrus pyrifolia) was between 2.8 and 29.0%, lignin was between 8.8–55.3% and cellulose 11.5–30.6% [24]. Due to the high density of lignified stone cells that were significantly correlated with the firmness and the lignin content of the bulk flesh, Huang et al. [15] concluded that the increase in lignified cell number is a key factor that reduces the quality of the postharvest loquat (Eriobotrya japonica) fruit. Therefore, in order to identify the microclimatic stress factors that stimulate the development of rough-skinned fruit, the analysis and assessment of physiological and molecular mechanisms of stone cell formation is essential [23]. The main components of stone cells in horticultural crops are lignin cellulose and hemicellulose, [23,25]. During cell development stages, lignin and cellulose gradually fill the lignified cells, leading to significant morphological changes in fruit [15]. In the date palm fruit, George et al. [25] postulated that parenchyma and sclereid cells contain high levels of lignin and phytolith (inorganic particles of silicon dioxide). The development of sclereids (stone cells) is closely related to the synthesis, transfer, and deposition of lignin [26]. These sclereids are sclerenchyma cells that are developed by the deposition of lignin on the primary walls of parenchyma cells [26]. During the deposition process, lignin molecules and microfibrils are alternatively arranged until they fill up the whole cell cavity, ending in the development of stone cells [26]. In the Japanese pear fruit (Pyrus serotina), the young sclereids formed secondary cell walls by the accumulation of lignin [27]. This lignification process induced vesicular structures inside the cytoplasm, hence the destruction of the cytoplasm [27].
Understanding the main structural organization of the networks formed by lignin and silica (phytolith) in the fruit of the date palm is essential to assess their functional roles in the mechanical strength and hardness as well as adaptation to biotic and abiotic stresses [25]. The silicified plant tissue is named phytoliths [28], hence both lignin and silica (phytolith) may contribute to the rigidity of date fruit [25]. Silica is an amorphous (non-crystalline) material that does not establish specific bonds (antigen contact) with biostructures, therefore, the rate of silicic acid polymerization and structure may be controlled via silicic acid oligomers [28]. George et al. [25] found a significant heterogeneity in date fruit silica phytoliths and the lignified structures. In their study, lignin presented independently from both silica in the secondary cell walls of sclereid cells and the spheroid phytoliths located around the sclereid cells. Spheroid phytoliths were abundant mainly around the sclereid cells within the skin of the date fruit [25]. Bauer et al. [28] stated that the bio-silicification in the plant involves the uptake of silicic acid from the soil solution, after which it is condensed into solid silica (polymerized silica is one of the solidest materials in the plant tissue), mainly in the epidermis. Bio-silicification positively correlates with transpiration rates of the tissue (polymerization of the silica as water evaporates) [28]. Bio-silicification in wheat was found in the epidermis cell wall and in sclerenchyma cells near the vascular bundles, but not in the stomata, proposing that an active transport oriented the soluble silica away from the water evaporation stream [29]. Silicon induces changes in the mechanical properties of cell walls in plants. Silicon promotes the extensibility of cell walls (cell elongation but not cell division) in the growing zone in the apical and subapical zones by decreasing the mechanical hardening of these regions (elastic moduli and viscosity coefficients) [30,31].
4.3. Weather Data
Microclimate conditions (temperature and relative humidity) during the pre-harvest period affect fruit quality at the time of harvest and during post-harvest stages, specifically at long-term storage [9]. These conditions may result in changes in the composition and cellular structures of the skin and sub-surface cellular layer, leading to significant impact on fruit postharvest quality and storability [9]. Inconsistent or low relative humidity at the ripening stage could induce some physiological disorders, including skin separation [2]. Most of these physiological disorders are often increased during fruit ripening and postharvest operations, especially under high temperatures or relative humidity, leading to potential reduction in fruit quality and severe economic losses [32]. For example, high relative humidity reduces the mechanical resistance of the cuticle and induces the development of skin microcracks [32]. Transpiration is a physical process that occurs in ripening fruit via the stomata and cuticle. Fruit transpiration rate correlated positively with temperature and negatively with relative humidity [33]. Transpiration affects the water relations and balance of the fruit skin. For example, in banana, the cumulative transpiration increased linearly with the development of fruit stages [33]. However, unbalanced water relation across the fruit layers may lead to fruit skin splitting and skin browning. In this study, mean air temperatures for both growing seasons (2020, 2021) were between 33 and 34 °C, and mean relative humidity were between 45 and 46%. In addition, management practices (irrigation, fertilization, pruning, etc.) were similar across the growing seasons (2020, 2021) and in both sites (1 and 2). However, skin separation was found in 14 to 15% of the total fruit in 2020, and 30 to 34% in 2021 growing season. Although the mean temperature and relative humidity as well as management practices were similar across the seasons, the skin separation amount of total yield was potentially higher in 2021 than 2020. These results indicate that the weather conditions, specifically temperatures and relative humidity, are not the only factors that stimulate the skin separation physiological disorder in date palm fruits. Overall, further studies are required to identify the main factors that stimulate the development of sclereid cells and the potential of agricultural practices.
5. Conclusions
In conclusion, in both growing seasons and across the study sites, the number, clusters, and aggregate of stone (sclereid) cells increased during the fruit ripening stages (khimri to Tamr), specifically, in skin-separated fruit at the Tamr stage. In addition, these sclereid cells were clustered and moved closer to the exocarp (skin). However, although the microclimate conditions (mean temperatures and relative humidity) were similar across the growing seasons (2020, 2021), the skin separation percentage in fruit yield in 2020 ranged from 14 to 15% and in 2021 from 30 to 34%; this demonstrates that the weather conditions are not the only components that induce the development of skin separation in the date palm fruit. A future study is intended to explore the possible interactive effects of management practices and the micro-environment with the abrupt increase of sclereid clusters and aggregates in skin-separated fruit as well as the chemical and mechanical assessment of fruit skin.
Author Contributions
Conceptualization, N.A. and Y.O.; methodology, N.A., N.E.-A. and Y.O.; software, M.A.-A. and J.A.; validation, N.A., Y.O. and N.E.-A.; formal analysis, N.A. and Y.O.; investigation, N.A. and Y.O.; data curation, N.A., M.A.-A. and Y.O.; writing—original draft preparation, N.A. and Y.O.; writing—review and editing, N.E.-A., J.S. and J.A.; supervision, Y.O.; project administration, N.A.; funding acquisition, N.A. and Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at the University of Jordan, grant number 2366.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Medjool Village farm, Jordan Valley, for supplying date palm fruits and conducting our study at their farm. Appreciation is also extended to Engs. Yazan Nabulsi, Mohammad Al-Edwan, Ehab Al-Balawneh, Ibrahim Al-Bakeet and Tala A’saf for their assistance in the field and laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Shahib, W.; Marshall, R. The fruit of the date palm: Its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.; Yahia, E.; Kader, A. Biology and Postharvest Physiology of Date Fruit. In Dates: Postharvest Science, Processing Technology and Health Benefits, 1st ed.; Siddiq, M., Aleid, S., Kader, A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2021. Available online: https://www.fao.org/faostat/en/#home (accessed on 27 November 2022).
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- El-Juhany, L. Degradation of date palm trees and date production in Arab countries: Causes and potential rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar]
- Al-Hajjaj, H.; Ayad, J.; Othman, Y.; Abu-Rayyan, A. Foliar potassium application improves fruits yield and quality of ‘medjool’ date palm. Fresenius Environ. Bull. 2020, 29, 1436–1442. [Google Scholar]
- Alsmairat, N.; Al-Qudah, T.; El-Assi, N.; Mehyar, G.; Gammoh, I.; Othman, Y.; Araj, S.; Al-Antary, T. Effect of drying process on physical and chemical properties of ‘Medjool’ date palm fruits. Fresenius Environ. Bull. 2019, 28, 1552–1559. [Google Scholar]
- Sarraf, M.; Jemni, M.; Kahramanoğlu, I.; Artés, F.; Shahkoomahally, S.; Namsi, A.; Ihtisham, M.; Brestic, M.; Mohammadi, M.; Rastogi, A. Commercial techniques for preserving date palm (Phoenix dactylifera) fruit quality and safety: A review. Saudi J. Biol. Sci. 2021, 28, 4408–4420. [Google Scholar] [CrossRef]
- Li, M.; Verboven, P.; Buchsbaum, A.; Cantre, D.; Nicolaï, B.; Heyes, J.; Mowat, A.; East, A. Characterising kiwifruit (Actinidia sp.) near skin cellular structures using optical coherence tomography. Postharvest Biol. Technol. 2015, 110, 247–256. [Google Scholar] [CrossRef]
- Si, Y.; Khanal, B.; Knoche, M. Factors affecting cuticle synthesis in apple fruit identified under field conditions. Sci. Hortic. 2021, 290, 110512. [Google Scholar] [CrossRef]
- Al-Hajjaj, H.; Ayad, J. Effect of foliar boron applications on yield and quality of Medjool date palm. J. Appl. Hortic. 2018, 20, 182–189. [Google Scholar] [CrossRef]
- JODA. The Jordanian Dates Association. Available online: https://jodates.org/book/ (accessed on 27 November 2022).
- Kader, A.; Hussein, A. Harvesting and Postharvest Handling of Dates; The International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2009; Volume IV+, p. 15. [Google Scholar]
- Isaid, H.; Bitar, A.; Abu-Qaoud, H. Effect of water stress at fruit maturity stage on production and skin separation phenomenon of date palm cv. Medjool. Hebron Uni. Res. J. 2021, 10, 1–17. Available online: https://digitalcommons.aaru.edu.jo/hujr_a/vol10/iss1/1 (accessed on 27 November 2022).
- Huang, W.; Zhu, N.; Zhu, C.; Wu, D.; Chen, K. Morphology and cell wall composition changes in lignified cells from loquat fruit during postharvest storage. Postharvest Biol. Technol. 2019, 157, 110975. [Google Scholar] [CrossRef]
- Shafiei, M.; Karimi, K.; Taherzadeh, M. Palm date fibers: Analysis and enzymatic hydrolysis. Int. J. Mol. Sci. 2010, 11, 4285–4296. [Google Scholar] [CrossRef]
- Khanal, B.; Knoche, M. Mechanical properties of apple skin are determined by epidermis and hypodermis. J. Am. Soc. Hortic. Sci. 2014, 139, 139–147. [Google Scholar] [CrossRef]
- Gophen, M. Skin separation in Date fruit. Int. J. Plant Res. 2014, 4, 11–16. [Google Scholar]
- Lustig, I.; Bernstein, Z.; Gophen, M. Skin separation in Majhul. Int. J. Plant Res. 2014, 4, 29–35. [Google Scholar]
- Khanal, B.; Imoro, Y.; Chen, Y.; Straube, J.; Knoche, M. Surface moisture increases microcracking and water vapor permeance of apple fruit skin. Plant Biol. 2021, 23, 74–82. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S. Distribution of stone cell in Asian, Chinese, and European pear fruit and its morphological changes. J. Appl. Bot. Food Qual. 2013, 86, 185–189. [Google Scholar]
- Lin, S.; Lin, D.; Wu, B.; Ma, S.; Sun, S.; Zhang, T.; Zhang, W.; Bai, Y.; Wang, Q.; Wu, J. Morphological and developmental features of stone cells in Eriobotrya Fruits. Front. Plant Sci. 2022, 13, 823993. [Google Scholar] [CrossRef]
- Mamat, A.; Tusong, K.; Xu, J.; Yan, P.; Mei, C.; Wang, J. Integrated transcriptomic and proteomic analysis reveals the complex molecular mechanisms underlying stone cell formation in Korla pear. Sci. Rep. 2021, 11, 7688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Xue, C.; Wang, R.; Zhang, M.; Qi, K.; Fan, J.; Hu, H.; Zhang, S.; Wu, J. The variation of stone cell content in 236 germplasms of sand pear (Pyrus pyrifolia) and identification of related candidate genes. Hortic. Plant J. 2021, 7, 108–116. [Google Scholar] [CrossRef]
- George, N.; Antony, A.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Microscopic investigations of silicification and lignification suggest their coexistence in Tracheary phytoliths in date fruits (Phoenix dactylifera L.). Front. Plant Sci. 2020, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yan, C.; Qiu, J.; Zhang, N.; Lin, Y.; Cai, Y. Structural characterization and deposition of stone cell lignin in Dangshan Su pear. Sci. Hortic. 2013, 155, 123–130. [Google Scholar] [CrossRef]
- Nii, N.; Kawahara, T.; Nakao, Y. The development of stone cells in Japanese pear fruit. J. Hortic. Sci. Biotechnol. 2008, 83, 148–153. [Google Scholar] [CrossRef]
- Bauer, P.; Elbaum, R.; Weiss, I. Calcium and silicon mineralization in land plants: Transport, structure and function. Plant Sci. 2011, 180, 746–756. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Fahima, T.; Aharoni, A.; Elbaum, R. Genetic control over silica deposition in wheat awns. Physiol. Plant 2010, 140, 10–20. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Tanimoto, E.; Lux, A.; Luxová, M.; Sugimoto, Y. Silicon induced changes in viscoelastic properties of sorghum root cell walls. Plant Cell Physiol. 2003, 44, 743–749. [Google Scholar] [CrossRef]
- Hossain, M.; Mori, R.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Fujii, S.; Yamamoto, R.; Hoson, T. Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J. Plant Res. 2002, 115, 23–27. [Google Scholar] [CrossRef]
- Fernández-Muñoz, R.; Heredia, A.; Domínguez, E. The role of cuticle in fruit shelf-life. Curr. Opin. Biotechnol. 2022, 78, 102802. [Google Scholar] [CrossRef]
- Khanal, B.; Sangroula, B.; Bhattarai, A.; Almeida, G.; Knoche, M. Pathways of postharvest water loss from banana fruit. Postharvest Biol. Technol. 2022, 191, 111979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).