Abstract
Agriculture has long been the cornerstone of human civilization, providing sustenance and livelihoods for millennia. However, as the global population continues to burgeon, agriculture faces mounting challenges. Soil degradation, nutrient depletion, environmental pollution, and the need for sustainable farming practices are among the pressing issues that require innovative solutions. In this context, nano-biofertilizers have emerged as a groundbreaking technological advancement with the potential to reshape modern agriculture. nano-biofertilizers are innovative agricultural products that leverage the combined principles of nanotechnology and biotechnology to enhance nutrient uptake by plants, improve soil health, and promote sustainable farming practices. These specialized fertilizers consist of nanoscale materials and beneficial microorganisms. These fertilizers are eco-friendly and cost-effective and have shown promising results in various crop plants. In this review, we discuss the recent advances in the development of eco-friendly nano-biofertilizers along with an overview of the various types of nano-biofertilizers, their formulation, synthesis, and mode of application for next-generation agriculture. The importance of the interaction between nanoparticles and bacterial species and its impact on the effectiveness of nano-biofertilizers has also been discussed along with the potential benefits, challenges, and future perspectives of using eco-friendly nano-biofertilizers for sustainable agriculture, ensuring a greener and healthier future for generations to come.
1. Introduction
Agriculture is a major contributor to the GDP and a cornerstone of the economy in developing countries like India, where over 60% of the population depends on it for their livelihood. However, with a growing global population, there is an urgent need to increase crop production using sustainable approaches. Numerous methods have been adapted to enhance crop yield, soil fertility, and sustainability [1]. Modern agriculture, driven by the Green Revolution, has significantly increased crop yields and helped feed a growing world population. However, this success has come at a cost. Conventional farming practices heavily rely on synthetic fertilizers, which primarily comprise inorganic nitrogen, phosphorous, and potassium (NPK) fertilizers, which have inadvertently led to soil degradation, nutrient imbalances, and environmental pollution. Runoff from excess fertilizers contributes to eutrophication in water bodies, threatening aquatic ecosystems (Figure 1). Furthermore, edaphic processes immobilize these elements within the soil, inhibiting their timely and adequate availability for plant uptake. The use of chemical fertilizers increases overall production costs, deteriorates soil fertility and texture, and harms human and environmental health. To surmount these issues, there is an imperative need to develop solutions that protect and enhance crop productivity in terms of quality and quantity without disturbing the agroecosystems. As a solution to this global problem, biofertilizers came into the picture and were proven to effectively increase crop yield and crop production. In addition, they are environmentally friendly and cost-effective. Biofertilizers consist of naturally occurring microbes with plant growth-promoting and/or disease-suppression activity. The use of plant growth-promoting rhizobacteria (PGPR) is a sustainable approach to increasing food production, enhancing soil health, and controlling pathogens [2]. The soil-beneficial bacteria or PGPR when applied in the field may act differently and their plant growth-promoting ability varies with plant species, soil types, cultivar, genotype, and agroclimatic conditions [3]. Therefore, it is necessary to gain a better understanding of native bacterial populations including their function, diversity, and activities in their natural rhizospheric environment. This will result in the incorporation of beneficial microorganisms to take care of the ecological balance by boosting crop productivity and soil health on a sustainable basis. Additionally, soil-beneficial bacteria will reduce the worldwide dependence on hazardous agrochemicals or pesticides that disturb the agroecosystems [4].
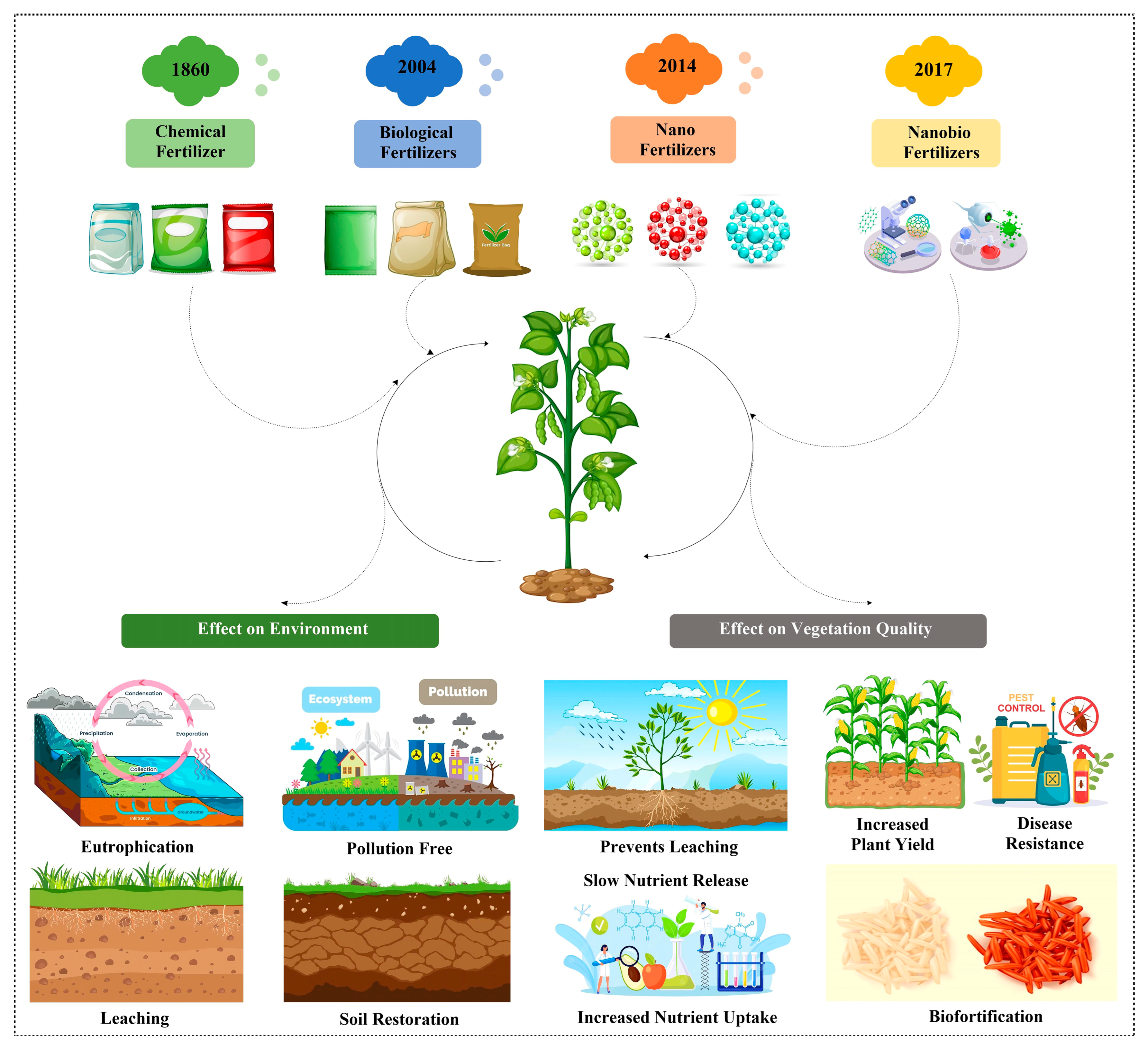
Figure 1.
Timeline showing the evolution of bio-nanofertilizers from chemical fertilizers over a period of time. The figure was designed using resources from Freepick.com (accessed on 25 June 2023).
Nanotechnology, being an emerging and promising area, has introduced another approach to increasing crop production by providing solutions to many glitches in agriculture production through the use of nanoparticles [5]. Recent studies show that nanoscale systems with novel properties are absorbed by plant roots and leaves easily and are assimilated more effectively, which is beneficial for both crops and their niche, and can be utilized to make agricultural systems “smart” and sustainable [6,7,8].
Another approach that is being looked after these days is a combination of biofertilizers with nanoparticles to give rise to nano-biofertilizers. Nano-biofertilizers represent a convergence of nanotechnology and biotechnology in agriculture. At their core, these innovative fertilizers consist of nanoscale materials intricately linked with beneficial microorganisms. The objective is twofold: to enhance nutrient uptake by plants and to reduce the ecological footprint of farming practices. The nano-biofertilizer could be any plant growth-promoting factor entrapped in nanoparticles or it can be bacterial cells adhered on the nanoparticles’ surface. Although nano-biofertilizers have been synthesized using multiple approaches, an in-depth understanding of their interaction with soil microbiota, soil components, plants, and endophytes is lacking, especially at the molecular level [9]. Since nano-biofertilizers are required in small quantities compared to other types of fertilizers, their utility for improving crop productivity, biofortification, and resistance to abiotic and biotic stresses is very promising, considering the goal of sustainable agriculture. The imperative, therefore, is to optimize nutrient management while minimizing environmental harm. In this review, the evolution from chemical fertilizers to the development of nano-biofertilizers (Figure 1) as a sustainable solution for agricultural growth and environmentally friendly technology with reference to their efficiency, use in crop plants, dosages, cost, and as a future fertilizer are discussed.
2. Biofertilizer Formulations
In recent years, sustainable agricultural practices have been endorsed, resulting in biofertilizer formulations containing consortia of living microorganisms like bacteria, fungi, and/or algae that can be used as conventional fertilizers for increasing crop production in addition to other benefits such as maintenance of soil health and microbiota [10]. The choice of biofertilizer formulation depends on the specific needs of the soil and the crops being grown. The effectiveness of a biofertilizer depends on two major factors, the choice of microbial strain and the method of inoculation. Based upon the individual role microorganisms play in the niche of crop plants, they have been classified into several groups, namely nitrogen-fixing microbes, phosphorus-solubilizing and mineral-solubilizing microbes, mycorrhizal biofertilizers, and compost biofertilizers. Here are a few examples of biofertilizer formulations:
Rhizobium Biofertilizer: Rhizobium is a nitrogen-fixing bacteria that forms a symbiotic relationship with legume plants. Rhizobium biofertilizer contains Rhizobium bacteria that help fix atmospheric nitrogen for utilization by plants.
Azotobacter Biofertilizer: Azotobacter is a free-living nitrogen-fixing bacteria capable of fixing atmospheric nitrogen and converting it into a form that plants can use. Azotobacter biofertilizer contains Azotobacter bacteria that help improve soil fertility and promote plant growth by aiding in the solubilization of phosphates, enhancing the production of plant hormones, and suppressing plant pathogens and the degradation of pesticides [11].
Azospirillum Biofertilizer: Azospirillum is a nitrogen-fixing bacteria that can form a symbiotic relationship with non-legume plants. Azospirillum biofertilizer contains Azospirillum bacteria that help improve plant growth and yield by producing antifungal and antimicrobial substances as well as enhancing phytohormone synthesis. Moreover, it also enhances induced systemic resistance in different plants [12].
Phosphorus-Solubilizing Bacteria (PSB) Biofertilizer: Phosphorus is an essential nutrient for plant growth, but it is often present in insoluble forms in the soil. PSB biofertilizer contains bacteria that can solubilize the inorganic and organic forms of insoluble phosphorous by hydrolyzing it and making it available to plants. PSB effectuates this by chelating cations by organic and inorganic acids secreted by these bacteria, lowering the pH of the soil, and mineralization of soil organic phosphate through the production of various phosphatase enzymes [13].
Mycorrhizae Biofertilizer: Mycorrhizae are symbiotic fungi that form a relationship with plant roots, helping them absorb nutrients from the soil. Mycorrhizae biofertilizer contains arbuscular mycorrhizal fungi (AMF) that ameliorate nutritional supply from the soil to the plants as they are thinner and have more accessibility to minerals and nutrients. AMF also help plants to tolerate salinity and drought stress better, confer disease resistance, and alleviate the toxicity of heavy metals [14].
Biofertilizer formulations (BFFs) often contain plant growth-promoting rhizobacteria (PGPR), which are associated with plant roots and thereby contribute to crop yield enhancement. This can be through direct means such as nitrogen fixation, phosphorous solubilization, and the augmented production of plant hormones [15,16], and by indirect methods including the production of a number of compounds such as siderophores, antibiotics, hydrogen cyanide (HCN), lytic enzymes, etc., which provide resistance to plant pathogens [17]. The microorganisms are pooled together to form a BFF with diverse plant growth-promoting (PGP) activities. To market these BFFs, some carrier materials that increase their shelf-life and viability are essential. Carrier materials are the harmless carriage for the transmission of live BFFs from industrial plants to the crop field while providing a shielding niche for live microorganisms. A biofertilizer formulation should comprise viable microorganisms in a stable carrier, along with compounds/materials that maintain their stability and confer protection during storage and transport [18]. Apart from these attributes, the biofertilizer formulation should be easy to handle, easy to apply, delivered to the target in a stable form, and able to protect microorganisms from detrimental factors and augment the activity of microorganisms in the soil.
Based on the state of carriage material used for biofertilizer formulations, biofertilizers can be broadly categorized into two categories: solid and liquid biofertilizers. Biofertilizers in liquid or solid forms with multifunctional properties are desirable [19]. Commonly, different kinds of organic materials (paddy, straw compost, wheat bran, rice bran, seed, and charcoal), soil materials (lignite powder, clay, talc, rock phosphate pellet, soil, and peat) or inert materials (vermiculite, perlite, kaolin, bentonite, and silicates) are used as carrier materials to ensure maximum viability and the extended shelf-life of microorganisms [20]. Peat is a widely used carrier of biofertilizers, mainly for rhizobia inoculants, owing to its availability, cost, and long history of field trials. When supplemented with peat, PGPR uphold metabolic activities and in some instances multiplies in the storage interval, but this can differ with various strains [21]. In liquid-based carriers, materials like carboxymethyl cellulose, glycerol, PVP, trehalose, Fe-EDTA, sucrose, and gum Arabic are widely used, especially for marketing [22].
3. Solid Biofertilizers
Solid biofertilizers are formulated using crop residues (compost) and inert materials (zeolite, vermiculite, etc.). Apart from these, charcoal- and talc-based biogas sludge along with rock phosphate are used as carrier materials [23]. Solid biofertilizer formulations have been made by mixing bacterial consortiums with the carrier [24]. PGPR and Azotobacter count increased during storage (3 months) to 15 log10 cfu/g in different carrier materials. The maximum number of cells of Azotobacter after one month was observed in solid biofertilizers with 5% primary liquid inoculant augmented with 5% zeolite. In another study, biofertilizer was prepared by mixing sawdust with chicken litter, vegetable waste, and sewage sludge in different combinations with Actinomycetes sp. [25]. Composting using a polyethylene (PET) vessel as the bioreactor was used for the trial scale study. Currently, in the area of solid formulation manufacturing, the focus is on polysaccharide-immobilized inoculants [21] and inoculants manufactured in solid-state fermentation (SSF) settings using agro-industrial wastes [26]. SSF can be described as a solid matrix fermentation process conducted without the presence of water yet requiring adequate substrate moisture to facilitate the proliferation of microorganisms. This approach operates under the premise that a substantial substrate quantity can be acquired by positioning the cultivated microorganisms in close proximity to the substrate [27]. The SSF technique has several benefits like co-cultivation of two microorganisms, augmentation with soluble P, and generation of biocontrol activity [19,28]. One of the disadvantages associated with solid BFFs, highlighted by [29], is that microorganisms do not survive when exposed to UV rays and temperatures exceeding 30 °C. The density of the microbes was 108 cfu/mL when the biofertilizer was prepared but decreased to 106 cfu/mL within four months.
4. Liquid Biofertilizer
Liquid biofertilizer formulations (LBFFs) comprise dormant plant-beneficial microorganisms combined with vital nutrients, essential for their optimal growth. Root exudates and soil-residing carbon play a pivotal role in facilitating the emergence of active cell batches from dormant ones upon their arrival in the soil environment [30]. Water, oil, or polymers are added as additives to LBFFs to enhance their viscosity, stability, and dispersion ability [21,22]. LBFFs were prepared by mixing water, yogurt, manure, raw milk, molasses, borax, and rock phosphate and incubated for two months followed by filtration. When used on lettuce and cabbage, LBFFs enhanced their size and yield [31]. The mineral component as well as the metabolite regulators of plant origin, formed during microbial growth in liquid biofertilizer, are responsible for the positive effects of the LBFFs. An optimization study conducted by Gopi et al. [32] on an LBFF mix containing one species each of Azospirillum and Azotobacter chroococcum and two species of Bacillus identified that the treatment with 15 mM trehalose was the best compared to glycerol, polyvinylpyrrolidone, trehalose, and other combinations.
The limitation of LBFFs is their shelf life. With time, the microbial cell count and their metabolic activity drop rapidly during storage and in the soil after their application, particularly if they are not supplemented with the right additives. To overcome this, another approach to using LBFFs is by applying cell-free formulations like fermentation broth filtrates [19,22]. Bacteria secrete several chemicals as their metabolic byproducts, for instance, antibiotics, siderophores, and lytic enzymes, and solubilize phosphate, which promotes plant growth and development [33].
5. Modes of Application of Biofertilizer
Several methods have been evaluated for applying biofertilizers/PGPR, including seed treatment, seedlings treatment, and direct application in the soil [33,34]. Each technique has some beneficial effects as well as disadvantages, which depend on the inoculant type, plant, soil, and environmental conditions, as well as the ability of farmers to use the products as per instructions [35]. The process of seed treatment essentially includes the even application of biofertilizer formulation in a slurry form to the seeds, followed by drying in a shaded area, and subsequently planting the treated seeds within a span of 24 h. The application of biofertilizers through seed coating is highly favored due to its practicality and the need for a smaller quantity of inoculants, especially when covering extensive agricultural fields. To enhance the adhesion of microorganisms to the seeds, various materials, including carboxymethyl cellulose, are utilized as adhesives [20]. This is to ensure adherence of a maximum number of bacteria to seeds and their protection against unfavorable environmental conditions. Seedling treatment, also known as seedling root dipping, involves immersing the seedling roots in a water-based biofertilizer suspension for a specific duration according to the crop variety prior to transplanting them into the soil. Soil inoculation is employed when there is a requirement for a large number/population of microbial strains to be introduced. In this case, granules of peat, perlite, etc., are placed in the soil surrounding the seeds. When liquid formulations are used, seeds are placed in the soil furrows and are sprayed with the biofertilizer. One advantage of inoculation in the soil is the higher probability of seed contact with biofertilizer for an extended time compared to seed treatment. The disadvantages of this method include the requirement of large quantities of biofertilizer and the time required to accomplish the process, which in turn increase the financial burden and skew the cost-benefit ratio of using biofertilizers.
6. Mode of Action of Biofertilizer Formulations and Effect on Crop Plants
The beneficial bacteria, when introduced into the soil as biofertilizers, establish colonies and undergo rapid multiplication. This microbial activity results in the transformation of otherwise insoluble and organic nutrients into soluble forms, facilitating their effortless uptake by plants. The biofertilizers containing nitrogen-fixing bacteria (NFB) such as Rhizobium, Frankia, Xanthomonas, etc., can synthesize the nitrogenase enzyme and thereby prompt the conversion of nitrogen to ammonia (NH3). Phosphate-solubilizing microorganisms such as Bacillus, Aspergillus, and Pseudomonas release some enzymes or organic acids that catalyze the conversion of the insoluble phosphate complex (aluminum and tricalcium phosphates) into soluble and plant-absorbable forms. The PGPR also release growth-promoting factors such as iron, vitamins, and hormones essential for plants. In addition, the connection between PGPR and plants is fortified by cellular communication methods such as quorum sensing. This serves as a signaling mechanism through which the microorganisms gauge their environment and its activity within the rhizosphere [36]. The effect of drying along with different periods of storage of biofertilizer formulations by different methods was investigated in the growth and yield of tomato plants [37]. The viability of bacteria was reduced during storage of biofertilizer, but a significant reduction was not reflected in the improvement of macro and micronutrients and plant growth up to 3 months. The use of food and dairy waste-derived biofertilizers resulted in a significant increase in the production and levels of total and soluble solids of tomatoes compared to the use of chemical fertilizers [38]. Further, Pseudomonas and Bacillus species were shown to act as biocontrol agents against pathogens causing pome fire blight, barley net blotch and leaf stripe, blueberry mummy berry fungus, and lettuce bottom rot [39]. Soil contaminated with arsenic and chromium, when amended with biosludge and biofertilizer, reduced metal uptake and improved the growth of Jatropha curcas, a biodiesel crop [40]. The organic matter in the biosludge chelated metals which was beneficial for planting J. curcas in marginal soils. Mohamed et al. [41] showed that biofertilizer (Azotobacter) combined with sludge and compost enhanced the yield parameters and nutrient content of wheat. Six commercially available liquid biofertilizers, three with Azotobacter and three with phosphate solubilizing bacteria (PSB) with Bacillus, displayed up to 50% yield improvement in chickpeas [42]. In field trials, a consortium of all six liquid biofertilizers increased the total yield of grain by 144%. A liquid biofertilizer with brown marine alga Stoechospermum marginatum enhanced plant length, biomass, photosynthetic pigments, amino acids, and nitrate reductase activity in brinjal plants [43]. The application of liquid biofertilizer with Azotobacter, Azospirillum, and Rhizobium improved the morphological, biochemical, and physiological parameters in Vigna mungo L. [44]. Compared to individual inoculation and control, biofertilizers offer better sustainable development and reduce the use of chemical fertilizers [30]. Table 1 shows various plant growth-promoting microbes and their effects.

Table 1.
Different types of plant growth-promoting microbes used as biofertilizers.
7. Nanofertilizer Formulations
A new approach to stimulating crop growth involves the use of nanofertilizers, which are designed to overcome the limitations of conventional fertilizers and biofertilizer formulations (BFFs). Nanofertilizers encompass nutrients that are encapsulated or coated with nanomaterials, generally exhibiting particle sizes ranging from 1 to 100 nanometers. A significant feature of nanofertilizers is their ability to facilitate a controlled and gradual release of nutrients [57]. The size of nanoparticles plays a vital role in their increased and effective uptake by plants [58]. For optimal plant response, it is essential to provide plants with the appropriate types and forms of nutrients. Nanofertilizers possess crucial attributes, with the expansive surface area of the nanoparticles playing a pivotal role in effectively retaining a surplus of nutrients. Moreover, they facilitate a controlled and gradual nutrient release that aligns with the requirements of the plants. The plants are highly selective in their nutrient uptake, so the use of an appropriate nanoformulation is necessary. Nanofertilizer formulations are typically categorized into three groups.
- (1)
- Nanoscale fertilizer: The powdered solid or liquid fertilizers are reformulated into a nanosize, i.e., the size reduction of fertilizer or any supplement required for plant growth, down to the nanoscale. This input can be achieved by mechanical and chemical methods. Compared to traditional fertilizers, these fertilizers offer advantages such as reduced quantity requirement, extended shelf life, and the added capability to function as multitasking agents, serving as both pesticides and scavengers for heavy metals [59].
- (2)
- Nanoscale additives: The addition of nanoparticles, which can be in the form of a fertilizer, micronutrient, or an additional supplement, into the bulk (>100 nm) macroscale fertilizer input. These nanoparticles may enhance the activity of bulk fertilizers, such as increased water retention properties and pathogen control in plants and soil. The introduction of carbon nanotubes (CNT) at the nanoscale into the media used for the germination of tomato seeds had a beneficial impact on enhancing the rate at which these seeds undergo germination as well as significantly enhancing the overall biomass of the plant [60]. Moreover, CNT promotes the intake of water by the seeds which was measured by the total moisture content of the tomato seeds after their incubation in the CNT-supplemented medium.
- (3)
- Nanoscale coatings for fertilizers: The use of nano-thin films or nanoporous materials such as zeolites, clay, and polymer coatings for controlled release of nutrient input. An example within this classification is nanoclays. They serve as supportive fillers for creating nanocomposite formations, enhancing the overall mechanical robustness and thermal resilience of the bulk materials. They act as a medium for absorption in the case of nanofertilizers.
Though most of the nanofertilizer formulations fall under these three categories, it is not mandatory that a certain type of nanoformulation falls into a specific category [61].
8. Synthesis of Nanofertilizers
The desirable properties (mainly size and physicochemical characteristics) of required nanomaterials are the basis for the selection method for the synthesis of nanofertilizers. There are three types of approaches for the production of nanofertilizers [62], which are described below.
9. Top-Down Approach
In the top-down approach to the synthesis of nanofertilizers, the precursor material is smashed down using physical methods such as grinding, crushing, milling, etching, and other lithographic approaches into nano-range particles. Since these are applied to crop fields, they have been termed nanofertilizers [62]. Through the top-down approach, a bulk amount of nanofertilizers can be produced from any precursor material but since it is a physical method of producing nano-range particles, it is not suitable for designer customized nanoparticles. Therefore, only a certain kind of nanofertilizer can be produced and the production of a desired variety in morphology of nanomaterials/nanofertilizers is often unattainable through this approach [57,62,63].
10. Bottom-Up Approach
The bottom-up approach uses several physical and chemical methods to form nanoparticles from atomic and molecular levels. Physical methods like chemical vapor deposition, flame pyrolysis, electrolysis, etc., are used to synthesize nanoparticles from gaseous and liquid precursors. Chemical methods used for the synthesis of nanofertilizers include hydrothermal, microemulsion, polyol synthesis, and sol-gel methods. The bottom-up approach is considered to be the most up-and-coming method for producing nanofertilizers due to its ability to produce mass-scale nanoparticles with regulated physicochemical properties [57,62].
11. Biological/Green Synthesis Approach
In the biological/green synthesis approach, bacteria, algae, fungi, and many angiosperms are used for the production of nanofertilizers for plant growth and development. Advantages associated with this approach are reduced toxicity and increased eco-friendliness compared with the other two approaches. The extracted nanofertilizer from these biological sources is then stabilized by encapsulation, synthetic carriers, nano-films, nano-tubes, dispersion in an emulsion, polymer coatings, etc., with the main disadvantage being the slow rate of synthesis of nanoparticles [57,64].
12. Mode of Application of Nanofertilizers
The impact and effectiveness of nanofertilizers are predominantly influenced by their mode of application. Therefore, a number of factors have to be controlled which influence their efficiency, half-life, stability, solubility, and side effects in crop plants [65]. Nanofertilizers have two popular modes of application:
13. Foliar Mode of Application
The foliar mode of application of nanofertilizers involves sprinkling nanofertilizers on the leaves so that they are directly absorbed by the stomata present on the leaf’s surface. Different plant nutrients (macro and micro) in various forms have been converted into nano-forms and used as nanofertilizers. Findings regarding foliar application reveal that the cuticle acts as a barrier to the absorption of nutrients through the leaf surface but nanofertilizers enhance their absorption efficiency so that the cuticle no longer acts as a barrier [8]. The proposed mechanism behind nanofertilizer foliar spray absorption is diffusion through the stomata and penetration of vascular bundles moving around the plant by using symplastic and apoplastic pathways [66].
14. Soil Mode of Application
The use of nanofertilizers can provide a dual benefit of improving soil quality and promoting plant growth [64]. Nanofertilizers can be applied to the soil through direct addition or spraying. Once applied, nanofertilizers are absorbed by crop plants in the rhizosphere through endocytosis, plasmodesmata, or with the help of carrier proteins. The nutrients are then transported through symplastic and apoplastic pathways [67].
15. Mode of Action of Nanofertilizers and Their Effect on Crop Plants
The unique characteristics of nanoparticles, such as their ability to exchange electrons, high surface-to-volume ratio, and small size, allow them to interact with plant cells and tissues as demonstrated through various imaging techniques such as transmission electron microscopy and confocal microscopy [68]. When nanoparticles are applied as a foliar spray on plants, they can be taken up by different pathways, including cuticular, lipophilic, hydrophilic, and stomatal pathways. Only nanoparticles that are 0.6–4.8 nm in size can be taken up by cuticular, lipophilic, and hydrophilic pathways, while nanoparticles larger than 20 nm can enter through stomata. This was observed through the use of confocal laser scanning microscopy (CLSM) [69]. The phloem system is responsible for transporting photosynthates, carbohydrates, and macromolecules, including proteins and tiny RNA, from the leaf downwards to the shoots and roots. Conversely, the xylem system transports substances from the roots upwards to the shoots. Therefore, the phloem system is the only feasible pathway for the translocation of nanoparticles from the leaf to the root [70]. The transport of nanoparticles occurs through various mechanisms, such as aquaporins, ion channels, endocytosis, and membrane transporters [71,72]. Nanoparticles with sizes smaller than 100 nm can enter through stomata and be transported to other plant parts. The transport of nanoparticles from the leaves to the stem and to the roots was observed using a transmission electron microscope [73]. Polymer-coated nanoparticles act as smart nanofertilizers, releasing nutrients in a controlled manner [74]. Biodegradable and biocompatible polymers, including both synthetic polymers (polyacrylates, polycaprolactones, and polylactide), and natural polymers (albumin, alginate, and chitosan), are the preferred materials for nanofertilizers, which improve the nutrient use efficiency of plants [75]. For instance, chitosan nanoparticles (78 nm) have been used for the slow release of chemical fertilizers [76] while nano-clay-based fertilizers have demonstrated twice the efficiency of conventional fertilizers in the release of nutrients [77]. Natural zeolites have been demonstrated to bind nutrients and facilitate their slow release in soil [78]. Additionally, urea-hydroxyapatite (HA) nanohybrids have been developed that release nitrogen more slowly than conventional urea fertilizers, with the urea-encapsulated HA nanoparticles releasing over 10 mg of nitrogen after two months compared to only four days with chemical fertilizers [79]. When applying nanofertilizers to plants in soil, a range of factors can affect their efficacy, including particle properties, environmental conditions, plant species, and rhizosphere composition. In this mode of application, nanofertilizers are taken up by roots through apoplastic, symplastic, and transmembrane transport pathways, and particles ranging from 7 to 200 nm can be absorbed [69]. Upon application to the plant rhizosphere, nanofertilizers first become adsorbed onto the root surface, where their positive surface charge interacts with the negatively charged root surface, resulting in absorption and accumulation [80]. In order to be taken up and transported to the shoots through the xylem, NPs must pass through various root barriers [69]. The mode of application plays a critical role in the distribution and accumulation of nanofertilizers in crop plants, ultimately contributing to their growth and development. Table 2 summarizes the effects of various nanofertilizers.

Table 2.
Different types of nanoparticles used as part of nanofertilizers.
16. Nano-Biofertilizer Formulations
The combination of biofertilizers with nanofertilizers to enhance the overall impact and mitigate individual drawbacks is referred to as nano-biofertilizers. The approach to achieving this involves various methods and techniques, depending on the type of nanoparticles that encapsulate biofertilizers or the biofertilizers that adhere to nanoparticles. This innovation leads to improved and gradual nutrient release attributes, coupled with decreased production costs for fertilizers and the potential reduction in the required amount of fertilizer application to plants. The gradual release of nutrients also increases the efficacy of the product. Encapsulation incorporates biofertilizer into the nanomaterial cover. This method involves the use of starch with a non-toxic substance like calcium alginate, which accelerates the growth of bacterial strains. Nanomaterials employed for the purpose of encapsulation can be nano-scale substances such as zeolite, chitosan, and polymers, as well as various metallic and metal-oxide compounds [92,93].
17. Synthesis of Nano-Biofertilizers
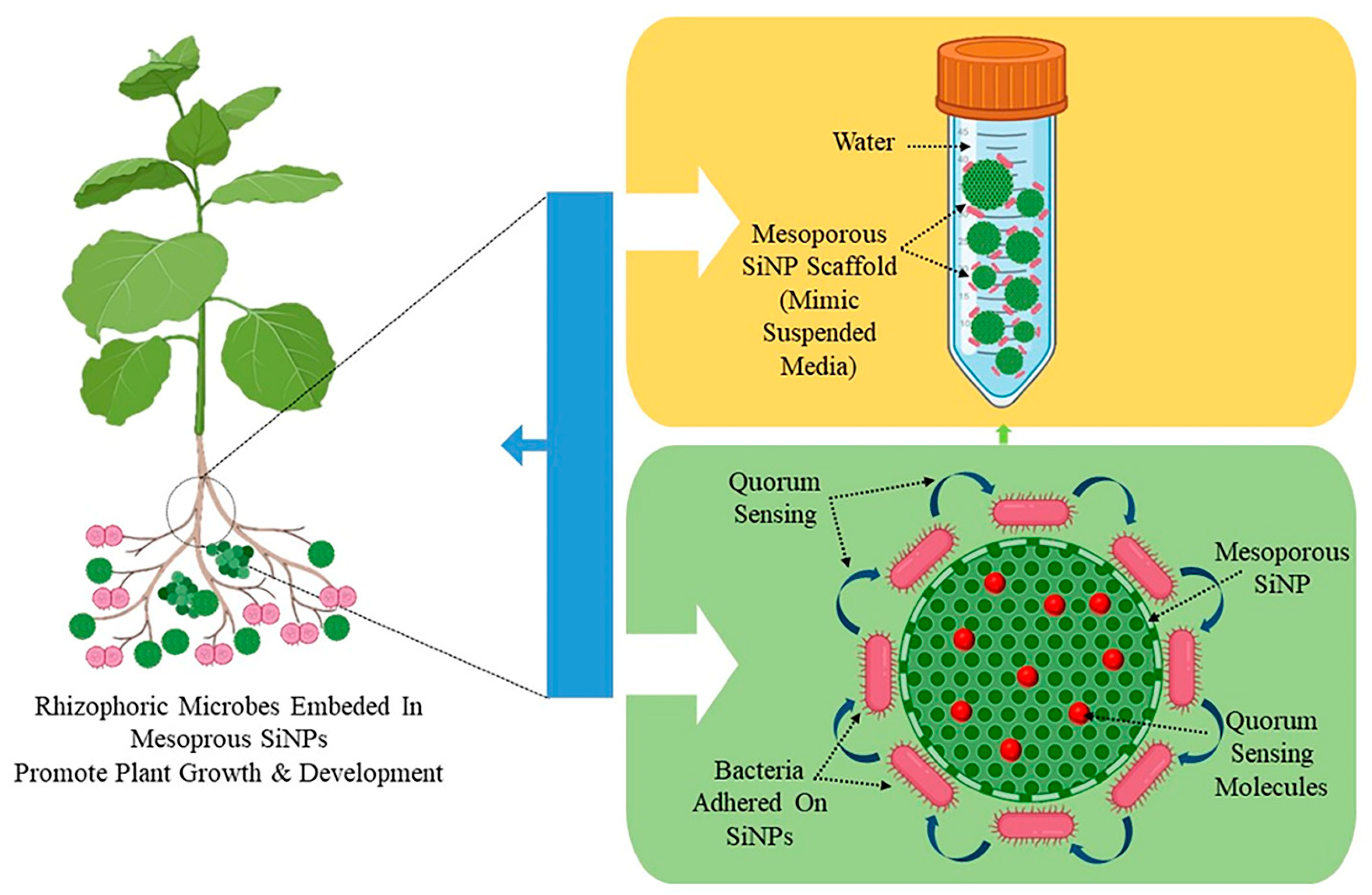
Three crucial steps are involved in the preparation of a nano-biofertilizer: (1) microbial culture preparation, (2) encapsulation with nanoparticles, and (3) testing its efficacy, quality, and shelf life [94]. Its production entails combining PGPR suspension with sodium alginate, starch, and bentonite followed by cross-linking with calcium chloride [95]. Nano-biofertilizers have also been created using salicylic acid and nanoparticles. This approach involves combining the biofertilizer with sodium alginate, ZnONPs, and salicylic acid followed by the addition of calcium chloride [94]. A nanosilica hybrid scaffold is used as part of the nano-biofertilizer for plant growth promotion. A mesoporous nanosilica scaffold (MNS) impregnated with minimal media acts as suspended media for the growth and colonization of bacteria and increases its shelf life. The MNS will decrease steric hindrance and cause an increase in the lag phase of bacterial growth, consequently delaying the exponential phase by the slow release of nutrients to bacterial colonies adhered to them. The MNS not only provides nutrients to bacteria for a longer duration but also provides the bacteria with a surface to adhere to, which in turn starts its colonization by releasing quorum sensing molecules, i.e., acyl-homoserine lactones (HSL) (Figure 2). Quorum sensing is a known phenomenon in bacterial strains used in PGP activity [96]. Quorum-sensing molecules (QSMs) facilitate the expression of genes which are dependent on the cell population compactness in gram-negative bacteria. They help in cell-to-cell communication in a bacterial population and enable cellular adaptation to varying environmental conditions. QSMs help maintain the viability of bacteria in MSS media. The organic waste from plants, animals, and even food, in combination with nanoparticles, can be used to make a potent nano-biofertilizer that improves soil fertility and plant growth. The organic waste is broken down into little bits, rinsed with water to eliminate contaminants, and then either pyrolyzed or allowed to decompose. To create a nano-biofertilizer, this partially degraded or pyrolyzed waste is mixed with nanoparticles [67].
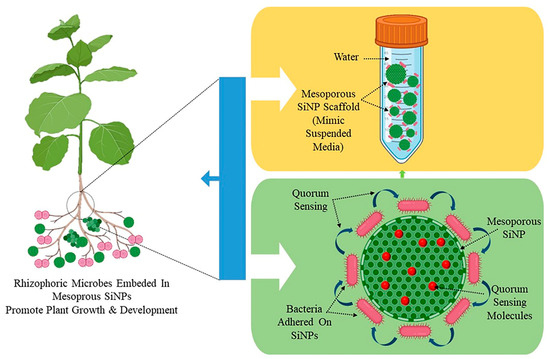
Figure 2.
Scheme to prepare and employ a nano-biofertilizer prepared using mesoporous silica nanoparticles and plant growth-promoting bacteria.
18. Mode of Application of Nano-Biofertilizers
Efforts to improve plant growth-promoting fertilizers are underway due to the inefficient utilization of applied fertilizers by crop plants and the negative impact of over-application on soil quality [59]. However, traditional fertilizer application methods are still not efficient enough and result in a significant amount of fertilizer being lost in the environment without benefiting the plants. Nanomaterials are more efficient than traditional fertilizers, but they must be applied in low quantities to avoid harmful effects on the environment and human health. To address this, a nano-biofertilizer has been developed, combining nanomaterials and PGPR for targeted nutrient delivery to crops over time [97]. The limitation, however, is the large-scale production and execution of nano-biofertilizer formulations due to a lack of comprehensive understanding of the interactions among nanoparticles, biofertilizer microflora, and plant systems [98].
Nanofertilizers can be applied to crop plants in two ways: separately as individual nanofertilizers and biofertilizers or combined as a nano-biofertilizer [97]. Nanoparticles can have direct effects on plant growth, such as improving enzyme activity, seed germination, carbon sequestration, nitrogen fixation, and photosynthesis when used as separate units [60,99]. Multi-walled carbon nanotubes and CuNPs have been used to improve plant growth in tomato, soybean, corn, and pigeon pea [100]. In addition to direct effects, optimal concentrations of nanoparticles have been found to enhance microbial growth. The mechanism behind it is the improved growth rate and cell viability under hostile environments by stimulating the secretion of abiotic stress-reducing enzymes and molecules from microbes, providing increased surface area, greater nodule development, and acting as a shield for the inoculants against dehydration. A dose-dependent improvement in PGPR siderophore production on the application of ZnO-NPs and IAA production on the application of CuNPs was observed [101]. These studies validate the impact of nanoparticles on microbes.
19. Mode of Action of Nano-Biofertilizers
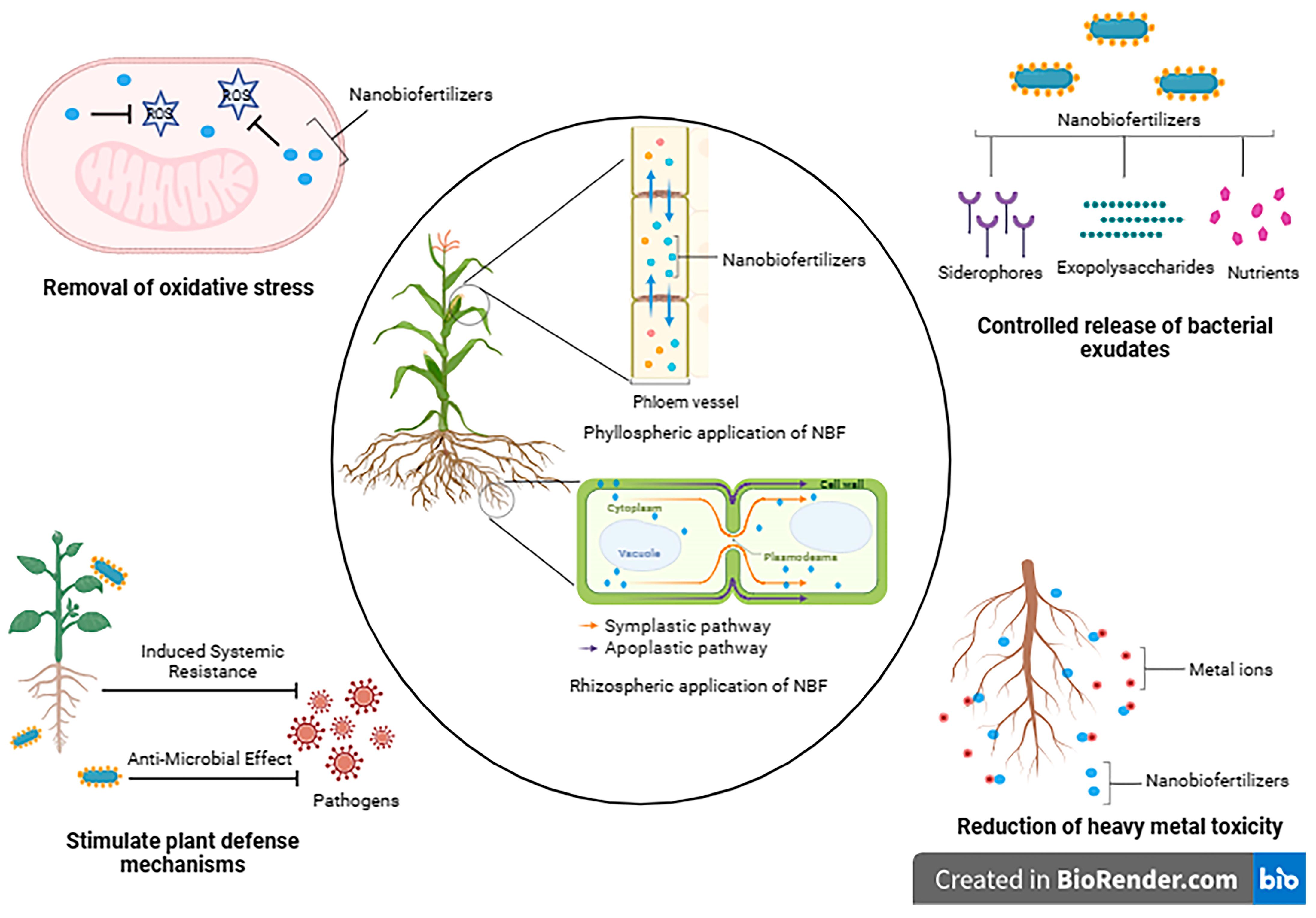
Nano-biofertilizers are a promising solution to addressing the current challenges related to nutrient and environmental safety. Nano-biofertilizers, when applied to the rhizosphere, utilize the apoplastic route to access the vascular tissue, reaching both the core of the root and its central region (Figure 3). Meanwhile, for bypassing the Casparian strip barrier within this pathway, the symplastic pathway is employed. Conversely, when nano-biofertilizers are applied to the leaves in a phyllospheric manner, they follow the path of phloem translocation. The silicon nanocomposites aid in enhancing the photosynthetic ability of the plants by increasing the size of the chloroplast and grana quantity along with chlorophyll content [102]. Abiotic and biotic stress cause adverse effects on plant growth by decreasing chlorophyll content and increasing ROS production, leading to DNA damage. Nano-biofertilizers have the capacity to mimic the functions of antioxidant enzymes like nano-enzymes, which aid in the removal of oxidative stress. In addition, depending upon the surface charge, area, and tiny size, the toxic heavy metals would interact with these particles, leading to their reduction [103]. By combining the benefits of nanofertilizers and biofertilizers, nano-biofertilizers offer improved results and multiple utilities. The concentration of the nanomaterial used is an important factor to consider, as a higher concentration would lead to phytotoxicity. Certain PGPRs can act as a solution to mitigate this impact. For instance, Azotobacter salinestris produces extracellular polymeric substance (EPS) that effectively trap nanoparticles, forming a metal-EPS complex, ensuring their consistent presence in the rhizosphere [104]. As a result, these EPSs help reduce the negative consequences associated with elevated levels of specific nanoparticles. To further enhance the benefits of nano-biofertilizers, efforts can be made to improve nutrient use efficiency, increase bioavailability, and enhance plant growth-promoting attributes. Additionally, nano-biofertilizers can offer protection to microbial inoculants from dehydration, increase cell viability, extend shelf life, and improve the production of PGP substances and secondary metabolites. Table 3 provides an overview of various nano-biofertilizers and their effects.
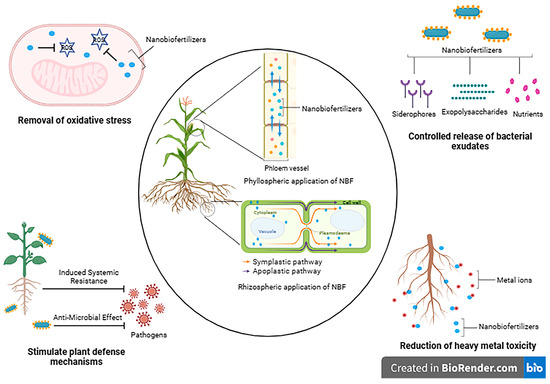
Figure 3.
Different modes of action of nano-biofertilizers. The figure was made using biorender.com (accessed on 17 June 2023).

Table 3.
Different types of nano-biofertilizers.
20. Critical Aspects of Using Nano-Biofertilizers
It is essential to have a well-rounded understanding of any agricultural practice or technology, including biofertilizers, nano-biofertilizers, and pseudo-nano-biofertilizers to make informed decisions. Biofertilizers and nano-biofertilizers have gained attention for their potential to enhance soil fertility and promote sustainable agriculture. However, like any technology, they also have their challenges and limitations. The formulation of nanobiofertilizers utilizing minuscule particles, measuring less than 100 nanometers, stands as a pioneering breakthrough with the potential to revolutionize sustainable agriculture in an environment-friendly manner. These tiny particles, derived from commonly available organic and inorganic sources, exhibit exceptional characteristics that render them highly effective even at low concentrations. They serve the dual purpose of providing essential nutrients to plants while fortifying their resilience against both biotic and abiotic stressors. This underscores their superior efficiency when compared to traditional fertilizers [116].
Nonetheless, nano-biofertilizers do come with certain limitations. Their elevated reactivity levels can pose potential toxicity risks to both plants and animals. As a result, this emerging field holds immense promise but necessitates extensive research and development to fully harness its potential while mitigating the associated challenges discussed below [57].
Effectiveness: The growing interest in nano-biofertilizers aligns with their potential to contribute significantly to sustainable agriculture. Nevertheless, a prevailing challenge in this sphere is the subpar quality of many currently available products, which has eroded farmers’ trust in their efficacy. Formulating a biofertilizer is a complex, multi-step procedure aimed at incorporating one or more strains of microorganisms into a suitable carrier. This carrier serves a vital role by creating a protective environment, shielding the microorganisms from the harsh storage conditions, and ensuring their survival and successful establishment in soil upon application. Quality control emerges as a pivotal concern throughout the entire formulation development and production process, demanding meticulous scrutiny at each stage to guarantee the reliability and effectiveness of the end products [117].
Environmental Impact: Biofertilizers and nano-biofertilizers may not always fully eliminate nutrient runoff, and excess nutrients can still affect water bodies, contributing to issues like eutrophication [118].
Economic Viability: Critics often examine the cost-effectiveness of biofertilizers compared to traditional fertilizers, particularly in terms of immediate yield increases [119].
Regulatory and Quality Control Issues: Ensuring the quality and consistency of biofertilizer products can be challenging, leading to concerns about product reliability [120].
Today, nano-biofertilizers have transitioned from the realm of research and development to practical applications in agriculture. They are poised to revolutionize modern farming practices by increasing crop yields while reducing the environmental impact. Ongoing research aims to finetune formulations, optimize nutrient release, and explore the potential of nano-biofertilizers in addressing specific crop and soil types. There are various types of biofertilizers and nanofertilizers available in the market, each containing different types of microorganisms and nanoparticles, respectively.
21. Conclusions
The journey of nano-biofertilizers from their inception to their current status as a transformative green technology for agriculture is emblematic of human innovation and our collective commitment to addressing the challenges of modern farming sustainably. The widespread problem of excessive reliance on chemical fertilizers to achieve greater crop yields has given rise to numerous environmental challenges. These include soil acidification and elevated emissions of nitrogen oxide (N2O) and carbon dioxide (CO2), which contribute to the intensification of the greenhouse effect, the emergence of blue baby syndrome, and a decline in the organic content of the soil. To address these issues, biofertilizers were developed as an eco-friendly alternative source of plant nutrients that enhance plant productivity and yield while maintaining soil fertility. Biofertilizers decompose natural products to enrich the soil with organic compounds and provide nutrients for crops. They also help plants resist disease and environmental stress. Compared to conventional fertilizers, biofertilizers are safer and more economically viable. Although they have benefits, biofertilizers pose some serious issues such as a short shelf life, resulting in a drop in cell numbers over a period of time, the absence of effective carrier material, the possibility of drying, reduced effectiveness when exposed to high salt conditions, etc., when conventional methods of application to plants are employed [116]. Combining biofertilizers with nanoparticle-based formulations can enhance their shelf life and efficacy. Nanoparticle formulations facilitate the slow release of nutrients that are utilized by microbes over a longer period, resulting in more effective fertilizer usage by plants. Synthesizing and applying nano-biofertilizer is crucial to protect our environment and natural resources while meeting the needs of a growing population. Therefore, in the second approach for the use of nano-biofertilizers, a microbial consortium with the appropriate nanoparticles is applied to plants either by foliar application or by soil application in an appropriate quantity which is generally markedly less than the conventional fertilizers. When a biofertilizer and a nanofertilizer are combined in a single formulation and used on plants, the resulting nano-biofertilizer is more effective and advantageous than those used individually. During their evolution, nano-biofertilizers have undergone significant refinement. Researchers have harnessed the principles of nanotechnology and biotechnology to design and optimize these specialized fertilizers. Nanoparticles, beneficial microorganisms, and precise nutrient delivery mechanisms have all contributed to the effectiveness and efficiency of nano-biofertilizers.
One of the most compelling aspects of nano-biofertilizers is their alignment with the principles of green technology. These fertilizers address the detrimental environmental impacts of traditional fertilizers, such as eutrophication and soil degradation, by reducing nutrient runoff and waste. By fostering soil health and enhancing nutrient use efficiency, nano-biofertilizers not only bolster crop yields but also promote sustainable farming practices. While the evolution of nano-biofertilizers has been remarkable, it is essential to acknowledge that this journey is ongoing. Future work is required for investigating the mechanism of interaction of nanoparticles with the bacterial species and how they support their cell viability by quorum sensing, increased surface areas, and entrapping the nutrients encapsulated in them. Emphasis should be directed towards assessing the enduring stability and potential toxicity of these formulations over extended timeframes, while also optimizing the economically feasible large-scale industrial production of nano-biofertilizers. Researchers continue to refine formulations, optimize nutrient delivery, and explore novel applications in diverse agricultural contexts. Biochar is emerging as a soil amendment for sustainable agriculture [121] and can be used in combination with bio-nanofertilizers as an innovative technology. Challenges remain, including ensuring product quality, addressing potential ecological impacts, and finetuning implementation practices. The evolution of nano-biofertilizers is a testament to our commitment to sustainable agriculture and environmental responsibility. These innovative fertilizers hold the promise of enhancing food security, safeguarding natural ecosystems, and reducing the ecological footprint of farming. As we look to the future, it is clear that nano-biofertilizers will play a pivotal role in shaping a greener, more sustainable agricultural landscape, ensuring that we can feed the world’s growing population while preserving the planet for generations to come.
Author Contributions
C.P. performed the work, was involved in conceptualization, and wrote the initial draft. J.S. was involved in conceptualization and wrote part of the manuscript. A.K. wrote part of the manuscript. W.R. supervised the work, was involved in conceptualization, and prepared the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Rahman, K.M.A.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhao, H.; Wang, P. Recent development in functional nanomaterials for sustainable and smart agricultural chemical technologies. Nano Converg. 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, X.; Guo, X.; Geng, Z.; Wang, G. Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. J. Hazard. Mater. 2016, 314, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Danish, M.; Hussain, T. Nanobiofertilizers in crop production. In Nanotechnology for Agriculture: Crop Production and Protection; Panpatte, D., Jhala, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 107–118. [Google Scholar]
- Thomas, L.; Singh, I. Microbial biofertilizers: Types and applications. In Soil Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar]
- Das, H.K. Azotobacters as biofertilizer. Adv. Appl. Microbiol. 2019, 108, 1–43. [Google Scholar] [PubMed]
- Raffi, M.M.; Charyulu, P. Azospirillum-biofertilizer for sustainable cereal crop production: Current status. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–209. [Google Scholar]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Brahmaprakash, G.P.; Sahu, P.K. Biofertilizers for sustainability. J. Indian Inst. Sci. 2012, 92, 37–62. [Google Scholar]
- Ullah, S.; Adeel, M.; Zain, M.; Rizwan, M.; Irshad, M.K.; Jilani, G.; Hameed, A.; Khan, A.; Arshad, M.; Raza, A.; et al. Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: A full life cycle study. J. Environ. Manag. 2020, 263, 110365. [Google Scholar] [CrossRef] [PubMed]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Xavier, I.J.; Holloway, G.; Leggett, M. Development of rhizobial inoculant formulations. Crop Manag. 2004, 3, 1–6. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R. Superior polymeric formulations and emerging innovative products of bacterial inoculants for sustainable agriculture and the environment. In Agriculturally Important Microorganisms—Commercialization and Regulatory Requirements in Asia; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 15–46. [Google Scholar]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Hindersah, R.; Rahmadina, I.; Harryanto, R.; Suryatmana, P.; Arifin, M. Bacillus and Azotobacter counts in solid biofertilizer with different concentration of zeolite and liquid inoculant. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on Agriculture and Bio-Industry, Banda Aceh, Indonesia, 27–28 October 2020; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 667, p. 012010. [Google Scholar]
- Asadu COInnocent, S.I.; Chijioke, E.O.; Samuel, O.E.; Maxwell, O.; Gordian, O.M.; Chibuzor, N.E. Investigation of the influence of biofertilizer synthesized using microbial inoculums on the growth performance of two agricultural crops. Biotechnol. Rep. 2020, 27, e00493. [Google Scholar]
- Vassilev, N.; Eichler-Löbermann, B.; Flor-Peregrin, E.; Martos, V.; Reyes, A.; Vassileva, M. Production of a potential liquid plant bio-stimulant by immobilized Piriformospora indica in repeated-batch fermentation process. AMB Express 2017, 7, 106. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Mendes, G.O.; Galvez, A.; Vassileva, M.; Vassilev, N. Fermentation liquid containing microbially solubilized P significantly improved plant growth and P uptake in both soil and soilless experiments. Appl. Soil Ecol. 2017, 117–118, 208–211. [Google Scholar] [CrossRef]
- Santhosh, G.P. Formulation and shelf life of liquid biofertilizer inoculants using cell protectants. IJRBA Technol. 2015, 2, 243–247. [Google Scholar]
- Dey, A. Liquid biofertilizers and their applications: An overview. In Environmental and Agricultural Microbiology: Applications for Sustainability; Wiley: Hoboken, NJ, USA, 2021; pp. 275–292. [Google Scholar]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Microbes for sustainable agro-ecosystem. In Microorganisms for Green Revolution; Springer Singapore: Singapore, 2018; Volume 2, 252p. [Google Scholar]
- Gopi, G.K.; Meenakumari, K.S.; Nysanth, N.S.; Subha, P. An optimized standard liquid carrier formulation for extended shelf-life of plant growth promoting bacteria. Rhizosphere 2019, 11, 100160. [Google Scholar] [CrossRef]
- Li, K.; Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef]
- Li, K.; Pidatala, V.R.; Shaik, R.; Datta, R.; Ramakrishna, W. Integrated metabolomic and proteomic approaches dissect the effect of metal resistant bacteria on maize biomass and copper uptake. Environ. Sci. Technol. 2014, 48, 1184–1193. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Sarbani, N.M.M.; Yahaya, N. Advanced development of bio-fertilizer formulations using microorganisms as inoculant for sustainable agriculture and environment–A review. Malays. J. Sci. Health Technol. 2022, 8, 92–101. [Google Scholar] [CrossRef]
- Altuhaish, A.; Hamim, H.; Tjahjoleksono, A. Biofertilizer effects in combination with different drying system and storage period on growth and production of tomato plant under field conditions. Emir. J. Food Agric. 2014, 26, 716. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Verma, K.; Yadav, M.; Varjani, S.; Singh, S.P.; Tong, Y.W. Food waste digestate as biofertilizer and their direct applications in agriculture. Bioresour. Technol. Rep. 2023, 23, 101515. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Juwarkar, A.A.; Yadav, S.K.; Kumar, P.h.a.n.i.; Singh, S.K. Effect of biosludge and biofertilizer amendment on growth of Jatropha curcas in heavy metal contaminated soils. Environ. Monit. Assess. 2008, 145, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Thalooth, A.; Elewa, T.; Ahmed, A. Yield and nutrient status of wheat plants (Triticum aestivum) as affected by sludge, compost, and biofertilizers under newly reclaimed soil. Bull. Natl. Res. Cent. 2019, 43, 31. [Google Scholar] [CrossRef]
- Ansari, M.F.; Tipre, D.R.; Dave, S.R. Efficiency evaluation of commercial liquid biofertilizers for growth of Cicer aeritinum (chickpea) in pot and field study. Biocatal. Agric. Biotechnol. 2015, 4, 17–24. [Google Scholar] [CrossRef]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. Waste Agric. 2015, 4, 167–173. [Google Scholar] [CrossRef]
- Maheswari, N.U.; Elakkiya, T. Effect of liquid biofertilizers on growth and yield of Vigna mungo L. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 42–45. [Google Scholar]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Sarkar, D.; Sankar, A.; Devika, O.S.; Singh, S.; Shikha Parihar, M.; Rakshit, A.; Sayyed, R.Z.; Gafur, A.; Ansari, M.J.; Danish, S.; et al. Optimizing nutrient use efficiency, productivity, energetics, and economics of red cabbage following mineral fertilization and biopriming with compatible rhizosphere microbes. Sci. Rep. 2021, 11, 15680. [Google Scholar] [CrossRef]
- Ji, S.-H.; Kim, J.-S.; Lee, C.-H.; Seo, H.-S.; Chun, S.-C.; Oh, J.; Choi, E.-H.; Park, G. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Naqqash, T.; Imran, A.; Hameed, S.; Shahid, M.; Majeed, A.; Iqbal, J.; Hanif, M.K.; Ejaz, S.; Malik, K.A. First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci. Rep. 2020, 10, 12893. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Wahid, A.; Danish, S.; Khan, M.J.; Fahad, S.; Brtnicky, M.; Hussain, G.S.; Battaglia, M.L.; Datta, R. Compost mixed fruits and vegetable waste biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep. 2021, 11, 6606. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Ahmad, R.; Alam, M.M.; Hannan, A.; Ahmed, W.; Fatima, N.; Inam-ul-Haq, M. Characterization of native plant growth promoting rhizobacteria and their anti-oomycete potential against Phytophthora capsici affecting chilli pepper (Capsicum annum L.). Sci. Rep. 2020, 10, 13859. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Ekprasert, J.; Cooper, J.; Boonlue, S. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci. Rep. 2021, 11, 6501. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Mehta, P.; Walia, A.; Chauhan, A.; Kulshrestha, S.; Shirkot, C.K. Phosphate solubilisation and plant growth promoting potential by stress tolerant Bacillus sp. isolated from rhizosphere of apple orchards in trans Himalayan region of Himachal Pradesh. Ann. Appl. Biol. 2013, 163, 430–443. [Google Scholar] [CrossRef]
- Navya, H.M.; Naveen, J.; Hariprasad, P.; Niranjana, S.R. Beneficial rhizospheric microorganisms mediated plant growth promotion and suppression of aflatoxigenic fungal and aflatoxin contamination in groundnut seeds. Ann. Appl. Biol. 2015, 167, 225–235. [Google Scholar] [CrossRef]
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Ait-Rahou, Y.; Mitsui, T.; Douira, A.; el Modafar, C.; Wahbi, S.; et al. Assemblage of indigenous arbuscular mycorrhizal fungi and green waste compost enhance drought stress tolerance in carob (Ceratonia siliqua L.) trees. Sci. Rep. 2021, 11, 22835. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.P.; Kaur, H. Nanoscale fertilizers: Harnessing boons for enhanced nutrient use efficiency and crop productivity. Nanobiotechnol. Appl. Plant Prot. 2019, 2, 191–208. [Google Scholar]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Mukhopadhyay, A.; Paul, S.; Sarkar, S.; Mukhopadhyay, R. Nanocomposite-based smart fertilizers: A boon to agricultural and environmental sustainability. Sci. Total Environ. 2023, 863, 160859. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered nanomaterials in soil: Their impact on soil microbiome and plant health. Plants 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani ZU, R.; Poczai, P. Improvement of plant responses by nanobiofertilizer: A step towards sustainable agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Lough, T.J.; Lucas, W.J. Integrative plant biology: Role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 2006, 57, 203–232. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, Y.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Abdel-Aziz HM, M.; Soliman, M.I.; Abo Al-Saoud, A.M.; El-Sherbeny, G.A. Waste-derived NPK nanofertilizer enhances growth and productivity of Capsicum annuum L. Plants 2021, 10, 1144. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Mikula, K.; Izydorczyk, G.; Taf, R.; Gersz, A.; Witek-Krowiak, A.; Chojnacka, K. Smart fertilizers—Toward implementation in practice. In Smart Agrochemicals for Sustainable Agriculture; Chojnacka, K., Saeid, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 81–102. [Google Scholar]
- Mejias, J.H.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A cutting-edge approach to increase nitrogen use efficiency in grasslands. Front. Environ. Sci. 2021, 9, 635114. [Google Scholar] [CrossRef]
- Corradini, E. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Sen, J. Nano clay composite and phyto nanotechnology: A new horizon to food security issue in Indian agriculture. J. Glob. Biosci. 2015, 4, 2187–2198. [Google Scholar]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of zeolites in agriculture and other potential uses: A review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Zhou, D.; Jin, S.; Li, L.; Wang, Y.; Weng, N. Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. J. Environ. Sci. 2011, 23, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, K.; Verma, P.; Singh, O.; Panwar, A.; Singh, T.; Kumar, Y.; Raliya, R. Effect of nitrogen and zinc nanofertilizer with the organic farming practices on cereal and oil seed crops. Sci. Rep. 2022, 12, 6938. [Google Scholar] [CrossRef] [PubMed]
- Afify, R.R.; El-Nwehy, S.S.; Bakry, A.B.; Add El-Aziz, A.M. Response of peanut (Arachis hypogaea L.) crop grown on newly reclaimed sandy soil to foliar application of potassium nano-fertilizer. Middle East J. Appl. Sci. 2019, 9, 78–85. [Google Scholar]
- McKnight, M.M.; Qu, Z.; Copeland, J.K.; Guttman, D.S.; Walker, V.K. A practical assessment of nano-phosphate on soybean (Glycine max) growth and microbiome establishment. Sci. Rep. 2020, 10, 9151. [Google Scholar] [CrossRef]
- Kornarzyński, K.; Sujak, A.; Czernel, G.; Wiącek, D. Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Sci. Rep. 2020, 10, 8068. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar] [CrossRef] [PubMed]
- Shalev, O.; Karasov, T.L.; Lundberg, D.S.; Ashkenazy, H.; Pramoj Na Ayutthaya, P.; Weigel, D. Commensal Pseudomonas strains facilitate protective response against pathogens in the host plant. Nat. Ecol. Evol. 2022, 6, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Liu, J.; Wolfe, K.; Potter, P.M.; Cobb, G.P. Distribution and speciation of copper and arsenic in rice plants (Oryza sativa japonica ‘Koshihikari’) treated with copper oxide nanoparticles and arsenic during a life cycle. Environ. Sci. Technol. 2019, 53, 4988–4996. [Google Scholar] [CrossRef]
- Kandil, E.E.; Abdelsalam, N.R.; el Aziz, A.A.A.; Ali, H.M.; Siddiqui, M.H. Efficacy of nanofertilizer, fulvic acid and boron fertilizer on sugar beet (Beta vulgaris L.) yield and quality. Sugar. Technol. 2020, 22, 782–791. [Google Scholar] [CrossRef]
- Kumar, P.; Pahal, V.; Gupta, A.; Vadhan, R.; Chandra, H.; Dubey, R.C. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep. 2020, 10, 20409. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, A.; Sharma, M.; Bhalla, N.; Estrela, P.; Jain, A.; Thakur, P.; Thakur, A. Nanomaterial fungicides: In vitro and in vivo antimycotic activity of cobalt and nickel nanoferrites on phytopathogenic fungi. Glob. Chall. 2017, 1, 1700041. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorization of food waste into biofertiliser and its field application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, D.P. Nano-biofertilizer: An emerging eco-friendly approach for sustainable agriculture. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 733–741. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef]
- Saberi-Rise, R.; Moradi-Pour, M. The effect of Bacillus subtilis Vru1 encapsulated in alginate—Bentonite coating enriched with titanium nanoparticles against Rhizoctonia solani on bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Jung, B.K.; Khan, A.R.; Hong, S.-J.; Park, G.-S.; Park, Y.-J.; Kim, H.-J.; Jeon, H.-J.; Khan, M.A.; Waqas, M.; Lee, I.-J.; et al. Quorum sensing activity of the plant growth-promoting rhizobacterium Serratia glossinae GS2 isolated from the sesame (Sesamum indicum L.) rhizosphere. Ann. Microbiol. 2017, 67, 623–632. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, H. Nano-biofertilizers: Harnessing dual benefits of nano-nutrient bio-fertilizers for enhanced nutrient use efficiency sustainable productivity. In Nanoscience for Sustainable Agriculture; Pudake, R., Chauhan, N., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 57–73. [Google Scholar]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Haris, Z.; Ahmad, I. Impact of metal oxide nanoparticles on beneficial soil microorganisms and their secondary metabolites. Int. J. Life-Sci. Sci. Res. 2017, 3, 1020–1030. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Ahmed, B.; Syed, A.; Rizvi, A.; Shahid, M.; Bahkali, A.H.; Khan, M.S.; Musarrat, J. Impact of metal-oxide nanoparticles on growth, physiology and yield of tomato (Solanum lycopersicum L.) modulated by Azotobacter salinestris strain ASM. Environ. Pollut. 2021, 269, 116218. [Google Scholar] [CrossRef]
- Eliaspour, S.; Seyed Sharifi, R.; Shirkhani, A.; Farzaneh, S. Effects of biofertilizers and iron nano-oxide on maize yield and physiological properties under optimal irrigation and drought stress conditions. Food Sci. Nutr. 2020, 8, 5985–5998. [Google Scholar] [CrossRef]
- Panichikkal, J.; Mohanan, D.P.; Koramkulam, S.; Krishnankutty, R.E. Chitosan nanoparticles augmented indole-3-acetic acid production by rhizospheric Pseudomonas monteilii. J. Basic Microbiol. 2022, 62, 1467–1474. [Google Scholar] [CrossRef]
- Rajak, J.; Bawaskar, M.; Rathod, D.; Agarkar, G.; Nagaonkar, D.; Gade, A.; Rai, M. Interaction of copper nanoparticles and an endophytic growth promoter Piriformospora indica with Cajanus cajan. J. Sci. Food Agric. 2017, 97, 4562–4570. [Google Scholar] [CrossRef]
- Tahir, M.; Imran, M.; Nawaz, F.; Shahid, M.; Naeem, M.A.; Ahmad, I.; Akram, M.; Khalid, U.; Farooq, A.B.U.; Bakhat, H.F.; et al. Effects of Bacillus sp. MR-1/2 and magnetite nanoparticles on yield improvement of rice by urea fertilizer under different watering regimes. J. Appl. Microbiol. 2021, 131, 2433–2447. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Bejai, S.; Meijer, J.; Seisenbaeva, G.A.; Kessler, V.G. Nano titania aided clustering and adhesion of beneficial bacteria to plant roots to enhance crop growth and stress management. Sci. Rep. 2015, 5, 10146. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.E.; Abdelsalam, N.R.; Mansour, M.A.; Ali, H.M.; Siddiqui, M.H. Potentials of organic manure and potassium forms on maize (Zea mays L.) growth and production. Sci. Rep. 2020, 10, 8752. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, B.R.; Naqvi, A.H.; Singh, H.B. Potential of biosynthesized silver nanoparticles using Stenotrophomonas sp. BHU-S7 (MbTCC 5978) for management of soil-borne and foliar phytopathogens. Sci. Rep. 2017, 7, 45154. [Google Scholar] [CrossRef] [PubMed]
- Merinero, M.; Alcudia, A.; Begines, B.; Martínez, G.; Martín-Valero, M.J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Navarro-Torre, S.; Torres, Y.; et al. Assessing the biofortification of wheat plants by combining a plant growth-promoting rhizobacterium (PGPR) and polymeric Fe-nanoparticles: Allies or Enemies? Agronomy 2022, 12, 228. [Google Scholar] [CrossRef]
- Moradi Pour, M.; Saberi Riseh, R.; Skorik, Y.A. Sodium alginate and gelatin nanoformulations for encapsulation of Bacillus velezensis and their use for biological control of Pistachio Gummosis. Materials 2022, 15, 2114. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Carvajal-Muñoz, J.S.; Carmona-Garcia, C.E. Benefits and limitations of biofertilization in agricultural practices. Livest. Res. Rural Dev. 2012, 24, 43. [Google Scholar]
- Biswal, D. Use of Nanofertilizers in Agriculture: Advantages, Disadvantages, and Future Implications. In Implications of Nanoecotoxicology on Environmental Sustainability; IGI Global: Harrisburg, PA, USA, 2023; pp. 102–133. [Google Scholar]
- Raimi, A.; Adeleke, R.; Roopnarain, A. Soil fertility challenges and Biofertiliser as a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric. 2017, 3, 1400933. [Google Scholar] [CrossRef]
- El-Ghamry, A.; Mosa, A.A.; Alshaal, T.; El-Ramady, H. Nanofertilizers vs. biofertilizers: New insights. Environ. Biodivers. Soil Secur. 2018, 2, 51–72. [Google Scholar]
- Herrmann, L.; Lesueur, D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]
- Yadav, R.; Ramakrishna, W. Biochar as an environment-friendly alternative for multiple applications. Sustainability 2023, 15, 13421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).