Myristic Acid Regulates Triglyceride Production in Bovine Mammary Epithelial Cells through the Ubiquitination Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Addition of Myristic Acid Solution
2.3. Total Protein Extraction and Western Blotting
2.4. Proteasome Activity Assay
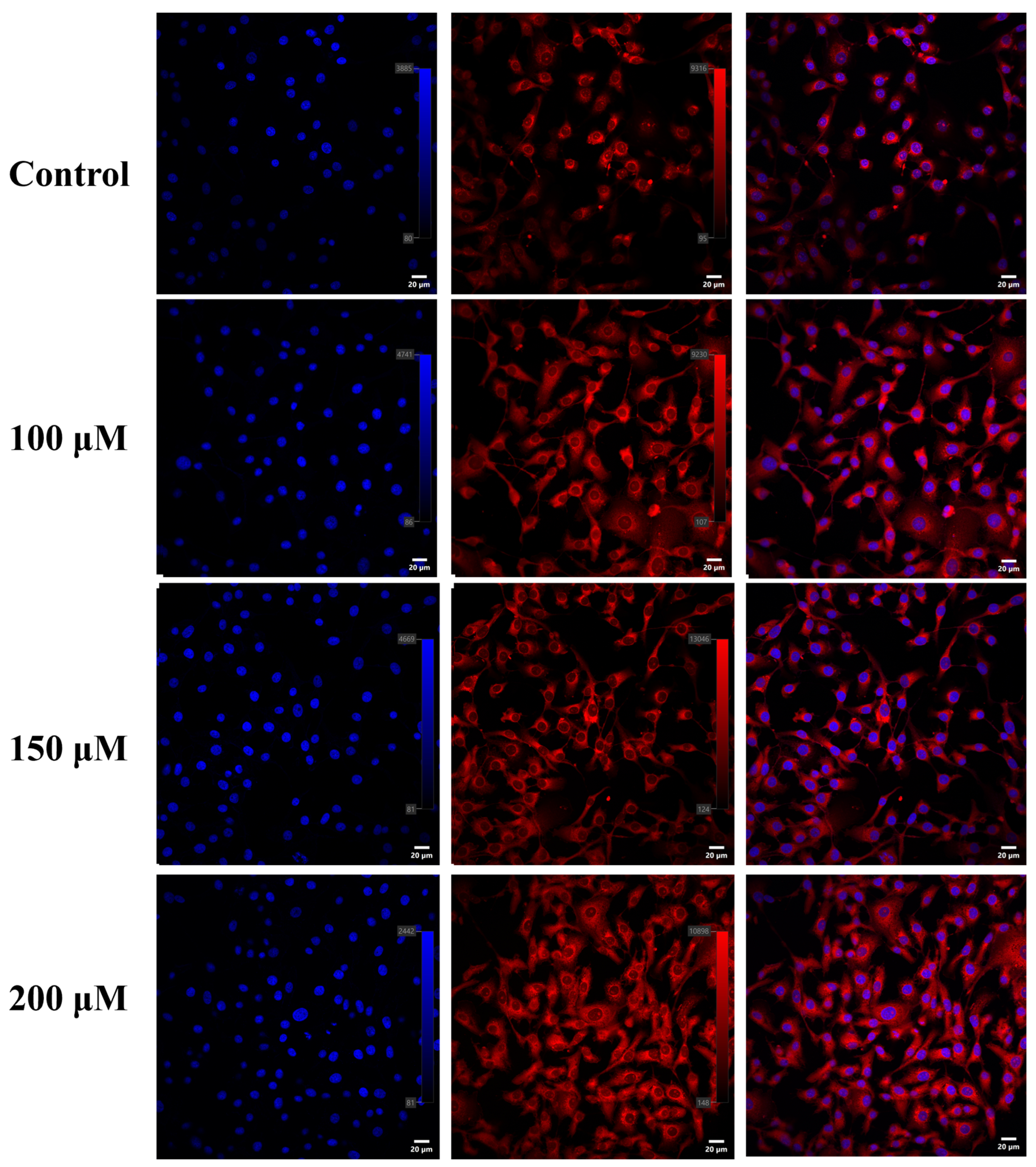
2.5. Nile Red Staining
2.6. Triglyceride Content Assay
2.7. Cell Viability Assay
2.8. Detection of mRNA Expression Levels of Relevant Genes
2.9. Data Analysis
3. Results
3.1. Effects of Different Concentrations of Myristic Acid on the Expression of Lipid-Synthesis-Related Proteins and Ubiquitin Proteins in MAC-T Cells
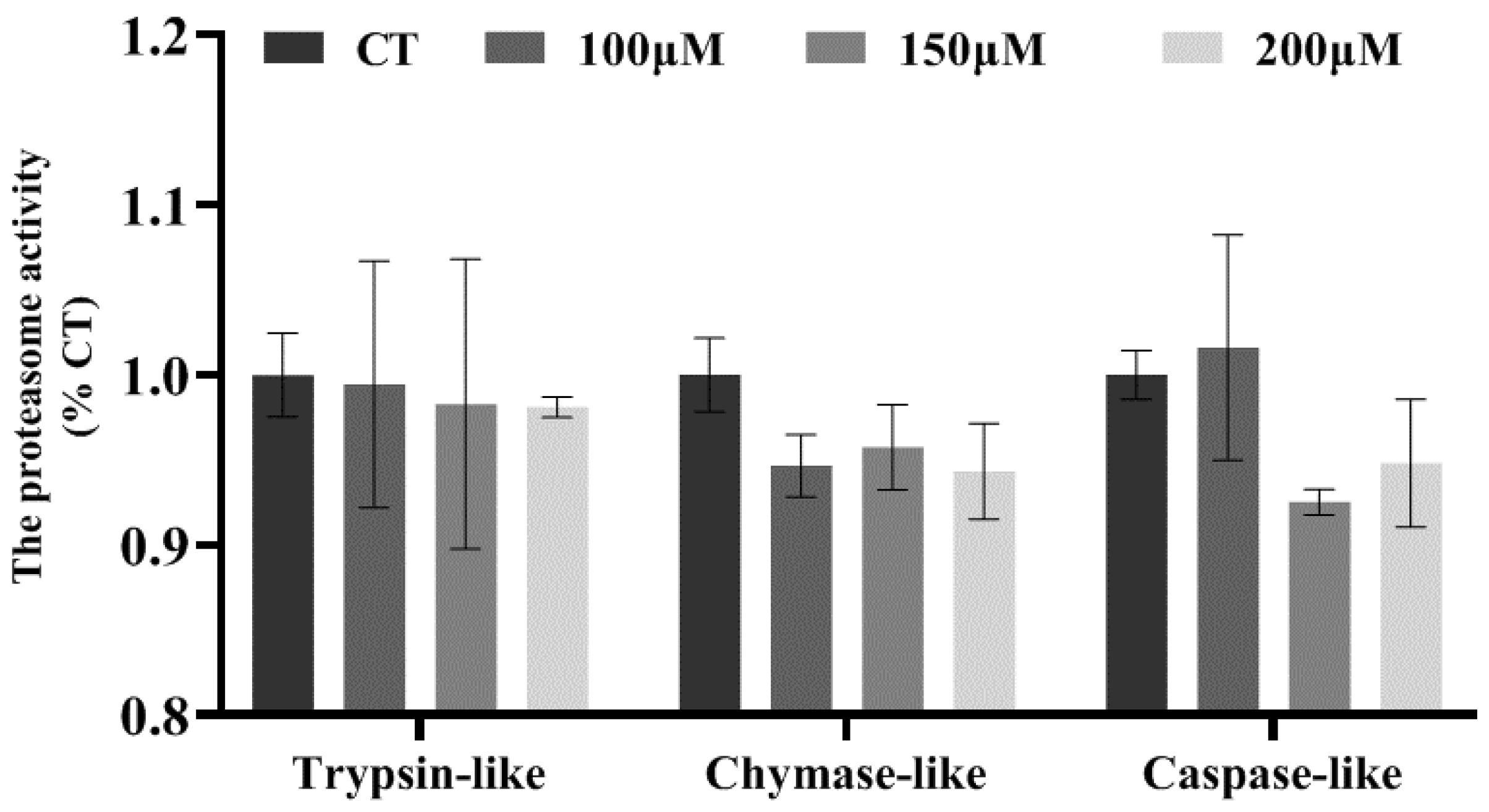
3.2. Effects of Different Concentrations of Myristic Acid on Proteasome Activity in MAC-T Cells
3.3. Effects of Different Concentrations of Myristic Acid on Cell Viability and Triglyceride Synthesis in MAC-T Cells
3.4. Regulation of Relevant Genes in MAC-T Cells by Myristic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oepen, K.; Ozbek, H.; Schuffler, A.; Liermann, J.C.; Thines, E.; Schneider, D. Myristic Acid Inhibits the Activity of the Bacterial ABC Transporter BmrA. Int. J. Mol. Sci. 2021, 22, 13565. [Google Scholar] [PubMed]
- Liu, L.; Wu, P.; Chen, F.; Zhou, J.; Guo, A.; Shi, K.; Zhang, Q. Multi-omics analyses reveal that the gut microbiome and its metabolites promote milk fat synthesis in Zhongdian yak cows. PeerJ 2022, 10, e14444. [Google Scholar]
- Liu, L.; Zhang, Q. Comparative proteome analysis reveals VPS28 regulates milk fat synthesis through ubiquitylation in bovine mammary epithelial cells. PeerJ 2020, 8, e9542. [Google Scholar] [PubMed]
- Ogunnaike, M.; Wang, H.; Zempleni, J. Bovine mammary alveolar MAC-T cells afford a tool for studies of bovine milk exosomes in drug delivery. Int. J. Pharm. 2021, 610, 121263. [Google Scholar] [PubMed]
- Zhao, Y.; Guo, X.; Yan, S.; Shi, B.; Sheng, R. Acetate regulates milk fat synthesis through the mammalian target of rapamycin/eukaryotic initiation factor 4E signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 337–345. [Google Scholar] [PubMed]
- Thering, B.J.; Graugnard, D.E.; Piantoni, P.; Loor, J.J. Adipose tissue lipogenic gene networks due to lipid feeding and milk fat depression in lactating cows. J. Dairy Sci. 2009, 92, 4290–4300. [Google Scholar]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Hristov, A.N.; Lee, C.; Cassidy, T.; Long, M.; Heyler, K.; Corl, B.; Forster, R. Effects of lauric and myristic acids on ruminal fermentation, production, and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2011, 94, 382–395. [Google Scholar]
- Odongo, N.E.; Or-Rashid, M.M.; Kebreab, E.; France, J.; McBride, B.W. Effect of supplementing myristic acid in dairy cow rations on ruminal methanogenesis and fatty acid profile in milk. J. Dairy Sci. 2007, 90, 1851–1858. [Google Scholar]
- Machmuller, A.; Kreuzer, M. Influence of myristic acid supplementation on energy, fatty acid and calcium metabolism of sheep as affected by dietary calcium and forage: Concentrate ratio. J. Anim. Physiol. Anim. Nutr. 2005, 89, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Kummrow, E.; Hussain, M.M.; Pan, M.; Marsh, J.B.; Fisher, E.A. Myristic acid increases dense lipoprotein secretion by inhibiting apoB degradation and triglyceride recruitment. J. Lipid Res. 2002, 43, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Kumar, N.; Ai, W.; Gopal, T.; Bhatt, S.; Harris, E.N.; Talmon, G.A.; Desouza, C.V. Myristic Acid Supplementation Aggravates High Fat Diet-Induced Adipose Inflammation and Systemic Insulin Resistance in Mice. Biomolecules 2022, 12, 739. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jia, H.; Liu, Y.; Yin, P.; Wei, Y. Insulin-Sensitizing Activity of Sub-Nanoscaled Polyalkoxyvanadate Clusters. Adv. Biosyst. 2020, 4, e1900281. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Wang, J.; Qin, D.; Wang, M.; Chai, L.; Fu, Y.; Zhao, C.; Gao, C.; Jia, J.; et al. Myristic acid as a checkpoint to regulate STING-dependent autophagy and interferon responses by promoting N-myristoylation. Nat. Commun. 2023, 14, 660. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Choi, M.; Ahn, Y.; Pak, Y. N-myristoylation regulates insulin-induced phosphorylation and ubiquitination of Caveolin-2 for insulin signaling. Biochem. Biophys. Res. Commun. 2020, 532, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chou, C.A.; Chen, W.Y.; Yang, J.L.; Lee, W.C.; Chen, J.B.; Lee, C.T.; Li, L.C. Empagliflozin Ameliorates Free Fatty Acid Induced-Lipotoxicity in Renal Proximal Tubular Cells via the PPARgamma/CD36 Pathway in Obese Mice. Int. J. Mol. Sci. 2021, 22, 12408. [Google Scholar] [CrossRef]
- Hoosdally, S.J.; Andress, E.J.; Wooding, C.; Martin, C.A.; Linton, K.J. The Human Scavenger Receptor CD36, glycosylation status and its role in trafficking and function. J. Biol. Chem. 2009, 284, 16277–16288. [Google Scholar] [CrossRef]
- Chu, L.Y.; Silverstein, R.L. CD36 ectodomain phosphorylation blocks thrombospondin-1 binding: Structure-function relationships and regulation by protein kinase C. Arter. Thromb. Vasc. Biol. 2012, 32, 760–767. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid beta-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef]
- Han, L.; Yang, Q.; Li, J.; Cheng, F.; Zhang, Y.; Li, Y.; Wang, M. Protocatechuic Acid-Ameliorated Endothelial Oxidative Stress through Regulating Acetylation Level via CD36/AMPK Pathway. J. Agric. Food Chem. 2019, 67, 7060–7072. [Google Scholar] [CrossRef]
- Fang, Y.; Shen, Z.Y.; Zhan, Y.Z.; Feng, X.C.; Chen, K.L.; Li, Y.S.; Deng, H.J.; Pan, S.M.; Wu, D.H.; Ding, Y. CD36 inhibits beta-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 2019, 10, 3981. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, S.; Li, W.; Silverstein, R.L.; McIntyre, T.M. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J. Thromb. Haemost. 2014, 12, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.; Pickering, A.M. The proteasome: A key modulator of nervous system function, brain aging, and neurodegenerative disease. Front. Cell Dev. Biol. 2023, 11, 1124907. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qi, L.; Zeng, Y.; Yang, Y.; Bi, Y.; Shi, X.; Zhu, H.; Zhou, Z.; Sha, J. Transient scrotal hyperthermia induces lipid droplet accumulation and reveals a different ADFP expression pattern between the testes and liver in mice. PLoS ONE 2012, 7, e45694. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.H.; Chan, L. Regulation of Triglyceride Metabolism, I.I.I. Emerging role of lipid droplet protein ADFP in health and disease. Am. J. Physiol. Gastrointest. Liver. Physiol. 2007, 292, G1465–G1468. [Google Scholar] [CrossRef] [PubMed]


| Gene | Name | Primer (5′→3′) | Relative Expression |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | AGATGGTGAAGGTCGGAGTG | / |
| CGTTCTCTGCCTTGACTGTG | |||
| LPL | Lipoprotein lipase | AGCTCCAAGTCGCCTTTCTC | 1.076 |
| TCCTGGTTGGAAAGTGCCTC | |||
| LDLR | Low-density lipoprotein receptor | TGTTGGACACACGTACCCAG | 0.834 ** |
| AAGGTCGCGACTTGTCTCAG | |||
| CD36 | Platelet glycoprotein 4 | GACGGATGTACAGCGGTGAT | 1.192 * |
| GAAAAAGTGCAAGGCCACCA | |||
| ACSL1 | Acyl-CoA synthetase long-chain family member 1 | AGCCGCATTTCACTTTTACTGC | 1.568 ** |
| AGCTCTTTAGGGCAAACCCC | |||
| FABP3 | Fatty-acid-binding protein 3 | ACGCGTTCTCTGTCGTCTTT | 0.488 ** |
| AACCGACACCGAGTGACTTC | |||
| ACACA | Acetyl-coa carboxylase/biotin carboxylase 1 | ACGGCTGACTGGAGTTGAAG | 0.914 |
| AACGTCTGCTTGTCCGTCTT | |||
| ACBP | Aacyl-coa-binding protein | TGGAATCTTTGCAACACCGC | 0.955 * |
| TGTCACCCACAGTTGCTTGT | |||
| FASN | Fatty acid synthase | CCTCAAGATGAAGGTGGTGCT | 0.742 |
| GGCCCTGGGTTATATCGAGC | |||
| SCD | Stearoyl-CoA desaturase | TCCTGATCATTGGCAACACCA | 1.05 |
| CCAACCCACGTGAGAGAAGAA | |||
| DGAT1 | Diacylglycerol o-acyltransferase 1 | TACCCCGACAACCTGACCTA | 1.05 |
| GGGAAGTTGAGCTCGTAGCA | |||
| LPIN1 | Lipin 1 | CTTCGATTCCCAAACCGGGA | 0.262 |
| TCACAGTGACGAACACCTGG | |||
| ADFP | Perilipin-2 | GCGTCTGCTGGCTGATTTC | 2.03 ** |
| (PLIN2) | AGCCGAGGAGACCAGATCATA | ||
| APOE | Apolipoprotein E | CGGTTTCTGGAGGCGAAGAA | 0.796 |
| CTCCATATCCGCCTGGCATC | |||
| PRKCA | Calcium-activated, phospholipid- and diacylglycerol-dependent serine | GACTTCGGGATGTGCAAGGA | 1.005 |
| CGTACGGCTGATAGGCGATT | |||
| MAPK1 | Mitogen-activated protein kinase 1 | AACAAAGTCCGAGTCGCCAT | 0.854 |
| CGATGGTCGGTGCTCGAATA | |||
| ARF6 | ADP-ribosylation factor 6 | AACTGGTATGTGTCAGCCCTC | 0.921 |
| GAAAGAGGTGATGGTGGCGA | |||
| STAM1 | Signal transducing adapter molecule 1 | CCTGGTACTGCGGCTAACAA | 1.277 * |
| ACGAACTTTCCGGCCTTCAT | |||
| EEA1 | Early endosome antigen 1 isoform X4x4 | CAGGCCCAGGACAGCTTAAA | 0.91 * |
| GCAAGTTCCTGTGCTGCTTG | |||
| HERC3 | HECT- and RLD-domain-containing E3 ubiquitin protein ligase 3 | CTCGAGGGCCTAGCTGTCT | 1.163 ** |
| TTTGTCAGAAGGGTCTGGCG | |||
| VPS45 | Vacuolar protein sorting 45 homolog | CCCCAAAGATGCTGTGGCTA | 0.922 * |
| AGTGTGCTGGGGCCTAGATA | |||
| CHMP2B | Charged multivesicular body protein 2b | ACGAGGTACACAGAGGGCTA | 0.868 ** |
| AGCTGTTTGGCTAAAACTCTGC | |||
| CHMP3 | Charged multivesicular body protein 3 | GTTTGAAATCACCGCAGGGG | 0.75 ** |
| CTAAAGGTTCAGGCTCCGGG | |||
| PIP5K | Phosphatidylinositol-4-phosphate 5-kinase, putative | CTCAGCACCTGGAAGAGCAA | 1.623 * |
| TTCTTCTTTCCCCGAGCCAC | |||
| CYHR1 | Cysteine and histidine rich 1 | GCCAACCTGCTTTTGGGAAG | 1.006 |
| GGTTGTGAAAACGGCCACAA | |||
| CP | Ubiquitin-like domain-containing CTD phosphatase 1 | CATGGTGGCCAAAGGTGTTG | 1.156 ** |
| CATCTGCTGGAGATTTTTGGCA | |||
| PSMC1 | Proteasome 26S subunit, ATPase 1 | GGTACGACTCCAACTCAGGC | 0.893 ** |
| ATCCGGTTTGTGGCCATGAT | |||
| PSMC3 | 26S protease regulatory subunit 6A | TGAACAAGACGCTGCCGTAT | 0.89 |
| TGCCGCGTAGAGGTTTTGAT | |||
| PSMC5 | 26S proteasome regulatory subunit 8 | CTCTGCACAAGATCCTGCCT | 0.828 ** |
| ATGCTTCACAGGCAGCTCAA | |||
| PSMD12 | 26S proteasome non-ATPase regulatory subunit 12 | ATACGTCAGGCATCTCGCAG | 0.966 |
| GGCCATGTTGTAGGGGACAA | |||
| UBC | Ubiquitin-C | GGGAGGTGTTTTAAGTTCTCCCT | 1.682 ** |
| TTGAACTCTAACCCACCCCTAAC | |||
| UBA52 | Ubiquitin-60S ribosomal protein L40 | GCCCAGTGACACCATTGAGA | 0.832 ** |
| GCAGGGTGGACTCTTTCTGG | |||
| UBA7 | Ubiquitin-like modifier-activating enzyme 7 | TCAGCAGGATGGTCTGAGGA | 1.3 * |
| AGTTCCAATACCAGCACCCG | |||
| TUBA | Tubulin beta chain | GTCTACTCCTGTTGCCTGC | 1.024 |
| AGGCATTGCCGATCTGGAC | |||
| ISG15 | Ubiquitin-like protein ISG15 | CCATCCTGGTGAGGAACGAC | 1.496 ** |
| GTCTGCTTGTACACGCTCCT | |||
| MX1 | Interferon-induced GTP-binding protein Mx1 | TGCCAACTAGTCAGCACTACATT | 0.639 ** |
| TGTACAGGTTGCTCTTGGACTC | |||
| SPP1 | Secreted phosphoprotein 1 | TCCGCCCTTCCAGTTAAACC | 1.945 ** |
| GCTTCTGAGATGGGTCAGGC | |||
| RPS27A | Ribosomal protein S27a | TTTCGTGAAGACCCTGACGG | 0.819 * |
| GTCTTTGCTGGTCAGGAGGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Wu, P.; Guo, A.; Liu, L. Myristic Acid Regulates Triglyceride Production in Bovine Mammary Epithelial Cells through the Ubiquitination Pathway. Agriculture 2023, 13, 1870. https://doi.org/10.3390/agriculture13101870
Hu M, Wu P, Guo A, Liu L. Myristic Acid Regulates Triglyceride Production in Bovine Mammary Epithelial Cells through the Ubiquitination Pathway. Agriculture. 2023; 13(10):1870. https://doi.org/10.3390/agriculture13101870
Chicago/Turabian StyleHu, Mengxue, Peifu Wu, Aiwei Guo, and Lily Liu. 2023. "Myristic Acid Regulates Triglyceride Production in Bovine Mammary Epithelial Cells through the Ubiquitination Pathway" Agriculture 13, no. 10: 1870. https://doi.org/10.3390/agriculture13101870





