Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions
Abstract
:1. Introduction
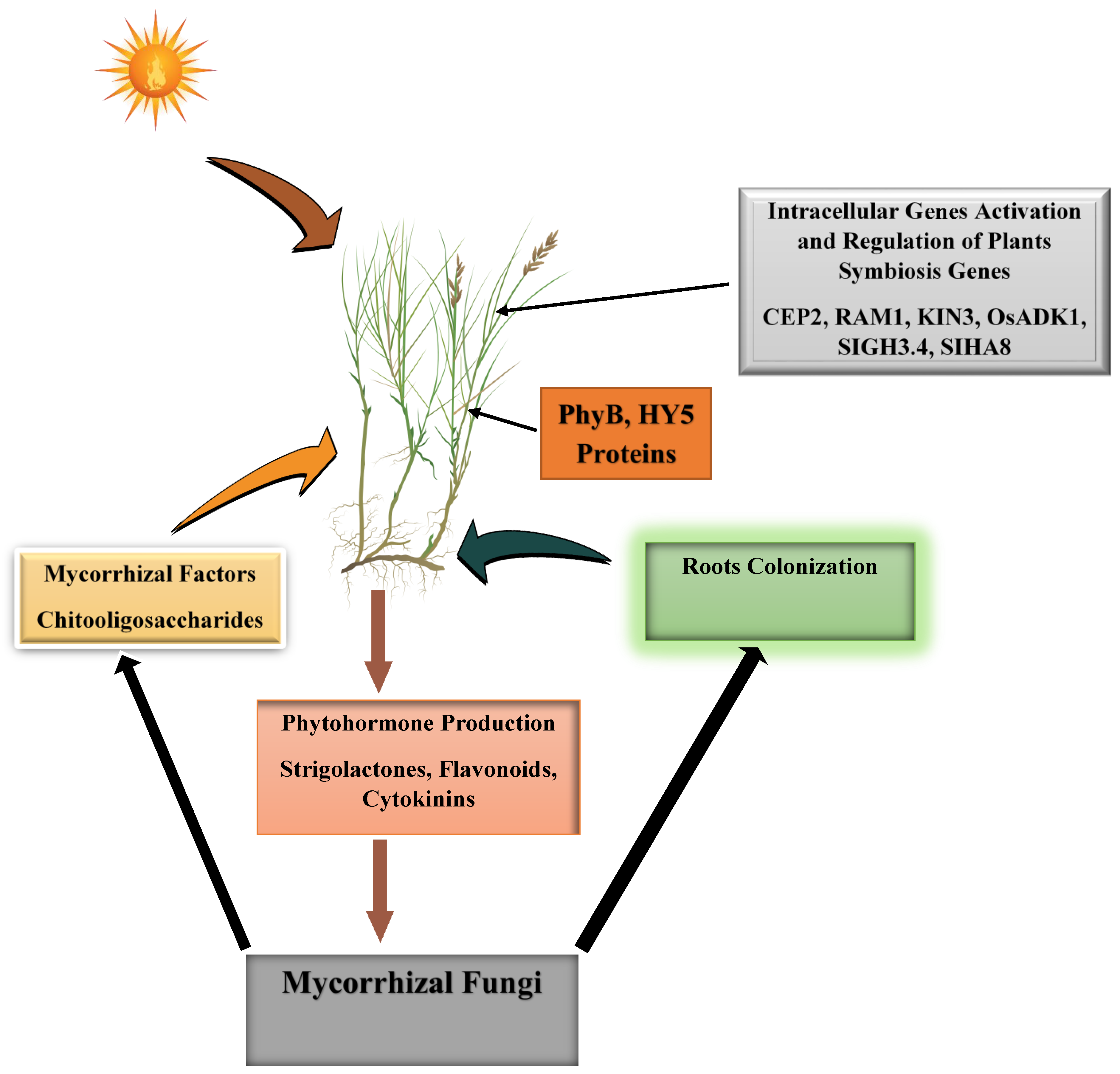
2. Arbuscular Mycorrhizal Fungi Recognition and Colonization of Plants
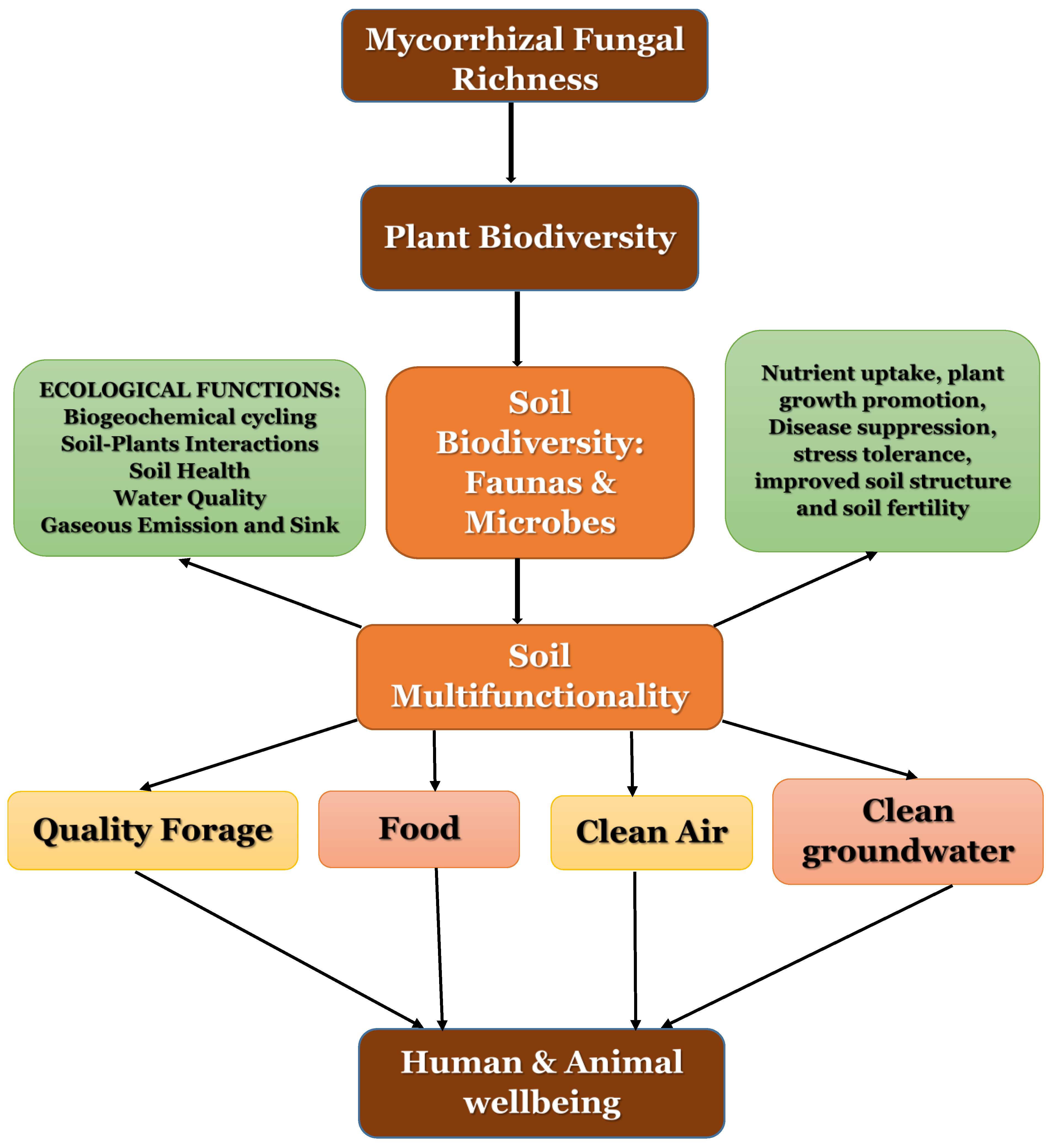
3. Arbuscular Mycorrhizal Fungal Richness, Plant Biodiversity and Ecological Functions
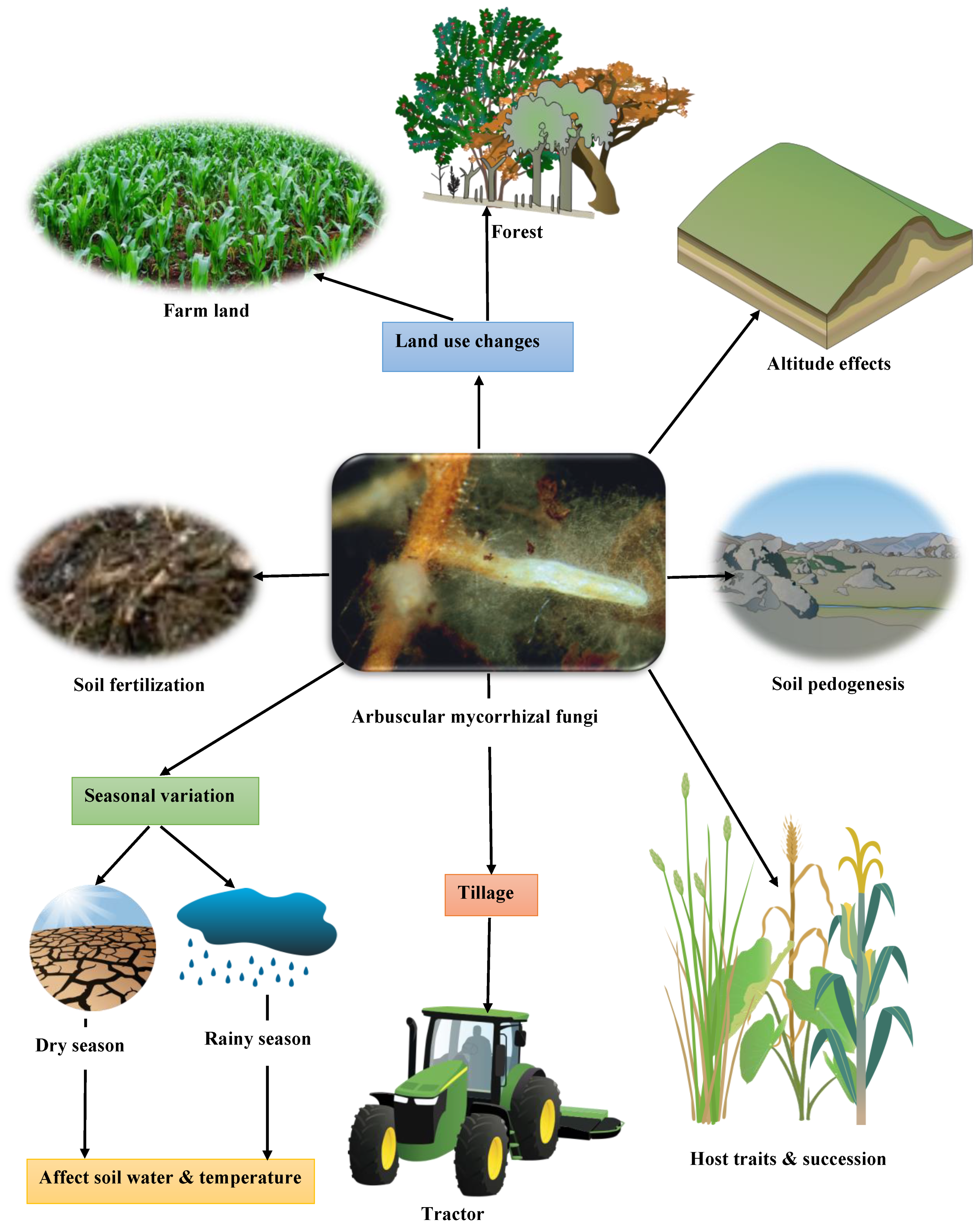
4. Factors Affecting Mycorrhizal Fungal Richness and Function in the Soil
4.1. Host Traits and Succession
4.2. Soil Pedogenesis
4.3. Altitude Effects
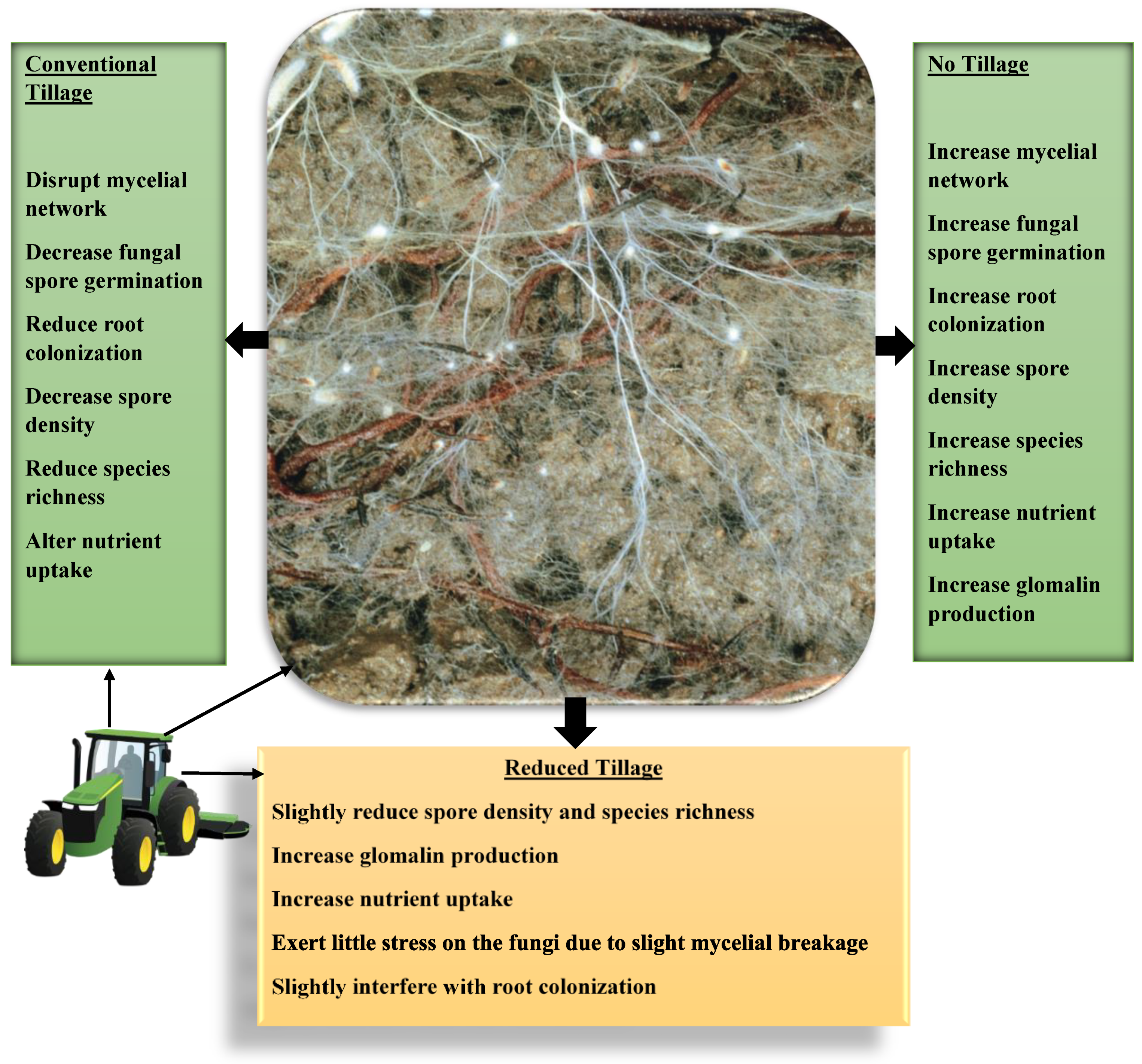
4.4. Tillage Practice
4.5. Land Use Changes
4.6. Seasonal Variation
4.7. Effects of Soil Pollutants
4.7.1. Cadmium (Cd)
4.7.2. Lead (Pb)
4.7.3. Silver (Ag)
4.7.4. Copper (Cu)
4.7.5. Nickel (Ni)
4.7.6. Mercury (Hg)
4.7.7. Arsenic (As) and Antimony (Sb)
4.7.8. Microplastics
5. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Gomes, S.I.; Fortuna, M.A.; Bascompte, J.; Merckx, V.S. Mycoheterotrophic plants preferentially target arbuscular mycorrhizal fungi that are highly connected to autotrophic plants. New Phytol. 2022, 235, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Liu, C.-C.; Zhu, A.-Q.; Niu, K.-X.; Guo, R.; Tian, L.; Wu, Y.-N.; Sun, B.; Wang, B. OsRAM2 function in lipid biosynthesis is required for arbuscular mycorrhizal symbiosis in rice. Mol. Plant-Microbe Interact. 2022, 35, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Bever, J.D. Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 2007, 88, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 2015, 7, plv050. [Google Scholar] [CrossRef]
- Fujita, M.; Kusajima, M.; Fukagawa, M.; Okumura, Y.; Nakajima, M.; Akiyama, K.; Asami, T.; Yoneyama, K.; Kato, H.; Nakashita, H. Response of tomatoes primed by mycorrhizal colonization to virulent and avirulent bacterial pathogens. Sci. Rep. 2022, 12, 4686. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2021, 41, 1429–1444. [Google Scholar] [CrossRef]
- Pickles, B.J.; Wilhelm, R.; Asay, A.K.; Hahn, A.S.; Simard, S.W.; Mohn, W.W. Transfer of 13C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. New Phytol. 2017, 214, 400–411. [Google Scholar] [CrossRef]
- Mali, S.; Naik, S.; Jha, B.; Singh, A.; Bhatt, B. Planting geometry and growth stage linked fertigation patterns: Impact on yield, nutrient uptake and water productivity of Chilli pepper in hot and sub-humid climate. Sci. Hortic. 2019, 249, 289–298. [Google Scholar] [CrossRef]
- Ge, S.; He, L.; Jin, L.; Xia, X.; Li, L.; Ahammed, G.J.; Qi, Z.; Yu, J.; Zhou, Y. Light-dependent activation of HY5 promotes mycorrhizal symbiosis in tomato by systemically regulating strigolactone biosynthesis. New Phytol. 2022, 233, 1900–1914. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Stirnemann, M.; Gübeli, C.; Egloff, S.; Courty, P.-E.; Aubry, S.; Vandenbussche, M.; Morel, P.; Reinhardt, D.; Martinoia, E. Strigolactones play an important role in shaping exodermal morphology via a KAI2-dependent pathway. IScience 2019, 17, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.K.; Schorderet, M.; Reinhardt, D. The role of the cell wall compartment in mutualistic symbioses of plants. Front. Plant Sci. 2014, 5, 238. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011, 155, 974–987. [Google Scholar] [CrossRef]
- Mayzlish-Gati, E.; De-Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012, 160, 1329–1341. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; De Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef]
- Mohammad, I. Mycorrhizae’s role in plant nutrition and protection from pathogens. Curr. Investig. Agric. Curr. Res. 2019, 8, 1037–1045. [Google Scholar]
- Hsieh, Y.H.; Wei, Y.H.; Lo, J.C.; Pan, H.Y.; Yang, S.Y. Arbuscular mycorrhizal symbiosis enhances tomato lateral root formation by modulating CEP2 peptide expression. New Phytol. 2022, 235, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.B.; Chakraborty, S.; Ivanov, S.; Schultze, M.; Mysore, K.S.; Harrison, M.J.; Ané, J.M. KIN3 impacts arbuscular mycorrhizal symbiosis and promotes fungal colonisation in Medicago truncatula. Plant J. 2022, 110, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wu, Y.N.; Liu, C.C.; Liu, Y.N.; Tian, L.; Cheng, J.F.; Pan, Z.; Wang, D.; Wang, B. OsADK1, a novel kinase regulating arbuscular mycorrhizal symbiosis in rice. New Phytol. 2022, 234, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Yan, A.; Zhao, Q.; Xie, K.; Li, Y. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3. 4 in tomato. Plant Cell Environ. 2022, 45, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Goh, D.M.; Cosme, M.; Kisiala, A.B.; Mulholland, S.; Said, Z.M.; Spíchal, L.; Emery, R.N.; Declerck, S.; Guinel, F.C. A stimulatory role for cytokinin in the arbuscular mycorrhizal symbiosis of pea. Front. Plant Sci. 2019, 10, 262. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Xie, K.; Tian, Y.; Yan, A.; Liu, J.; Huang, Y.; Wang, S.; Zhu, Y.; Chen, A. A mycorrhiza-specific H+-ATPase is essential for arbuscule development and symbiotic phosphate and nitrogen uptake. Plant Cell Environ. 2020, 43, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Vangelisti, A.; Natali, L.; Bernardi, R.; Sbrana, C.; Turrini, A.; Hassani-Pak, K.; Hughes, D.; Cavallini, A.; Giovannetti, M.; Giordani, T. Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci. Rep. 2018, 8, 4. [Google Scholar] [CrossRef]
- Banasiak, J.; Jamruszka, T.; Murray, J.D.; Jasiński, M. A roadmap of plant membrane transporters in arbuscular mycorrhizal and legume–rhizobium symbioses. Plant Physiol. 2021, 187, 2071–2091. [Google Scholar] [CrossRef]
- Hooper, D.U.; Vitousek, P.M. The effects of plant composition and diversity on ecosystem processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Mooney, H.A. Biodiversity and Ecosystem Function; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Aarssen, L.W. Ecological combining ability and competitive combining ability in plants: Toward a general evolutionary theory of coexistence in systems of competition. Am. Nat. 1983, 122, 707–731. [Google Scholar] [CrossRef]
- Brown, V.; Gange, A. Herbivory by soil-dwelling insects depresses plant species richness. Funct. Ecol. 1989, 3, 667–671. [Google Scholar] [CrossRef]
- Dobson, A.; Crawley, M. Pathogens and the structure of plant communities. Trends Ecol. Evol. 1994, 9, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Environmental heterogeneity and plant species diversity: A hypothesis. Am. Nat. 1977, 111, 376–381. [Google Scholar] [CrossRef]
- Grime, J.; Mackey, J.; Hillier, S.; Read, D. Floristic diversity in a model system using experimental microcosms. Nature 1987, 328, 420–422. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G. Arbuscular mycorrhizal fungi as a determinant of plant diversity: In search of underlying mechanisms and general principles. In Mycorrhizal Ecology; Springer: Berlin, Heidelberg, 2003; pp. 243–265. [Google Scholar]
- Van der Heijden, M.G.; Boller, T.; Wiemken, A.; Sanders, I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 1998, 79, 2082–2091. [Google Scholar] [CrossRef]
- Bartelt-Ryser, J.; Joshi, J.; Schmid, B.; Brandl, H.; Balser, T. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 27–49. [Google Scholar] [CrossRef]
- Han, X.-M.; Wang, R.-Q.; Jian, L.; Wang, M.-C.; Juan, Z.; Guo, W.-H. Effects of vegetation type on soil microbial community structure and catabolic diversity assessed by polyphasic methods in North China. J. Environ. Sci. 2007, 19, 1228–1234. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef]
- Dirks, I.; Navon, Y.; Kanas, D.; Dumbur, R.; Gruenzweig, J.M. Atmospheric water vapor as driver of litter decomposition in Mediterranean shrubland and grassland during rainless seasons. Glob. Chang. Biol. 2010, 16, 2799–2812. [Google Scholar] [CrossRef]
- Matias, L.; Castro, J.; Zamora, R. Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob. Chang. Biol. 2011, 17, 1646–1657. [Google Scholar] [CrossRef]
- Barea, J.; Palenzuela, J.; Cornejo, P.; Sánchez-Castro, I.; Navarro-Fernández, C.; Lopéz-García, A.; Estrada, B.; Azcón, R.; Ferrol, N.; Azcón-Aguilar, C. Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J. Arid Environ. 2011, 75, 1292–1301. [Google Scholar] [CrossRef]
- Martínez-García, L.B.; de Dios Miranda, J.; Pugnaire, F.I. Impacts of changing rainfall patterns on mycorrhizal status of a shrub from arid environments. Eur. J. Soil Biol. 2012, 50, 64–67. [Google Scholar] [CrossRef]
- Tkacz, A.; Bestion, E.; Bo, Z.; Hortala, M.; Poole, P.S. Influence of plant fraction, soil, and plant species on microbiota: A multikingdom comparison. MBio 2020, 11, e02719–e02785. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Schmid, M.W.; van Moorsel, S.J.; Hahl, T.; De Luca, E.; De Deyn, G.B.; Wagg, C.; Niklaus, P.A.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
- Carney, K.M.; Matson, P.A. Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. Functional diversity of bacterial communities in the rhizosphere of maize grown on a soil under organic and inorganic fertilization. Sci. Afr. 2022, 16, e01212. [Google Scholar] [CrossRef]
- Nwokolo, N.L.; Enebe, M.C.; Chigor, C.B.; Chigor, V.N.; Dada, O.A. The contributions of biotic lines of defence to improving plant disease suppression in soils: A review. Rhizosphere 2021, 19, 100372. [Google Scholar] [CrossRef]
- Egli, S.; Brunner, I. Mykorrhiza: Eine Faszinierende Lebensgemeinschaft im Wald; WSL: Zürich, Switzerland, 2011. [Google Scholar]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root metabolome of plant–arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Susceptibility and plant immune control—A case of mycorrhizal strategy for plant colonization, symbiosis, and plant immune suppression. Front. Microbiol. 2023, 14, 1178258. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Janos, D.P. Mycorrhizae influence tropical succession. Biotropica 1980, 12, 56–64. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. AMF, phylogeny, and succession: Specificity of response to mycorrhizal fungi increases for late-successional plants. Ecosphere 2016, 7, e01555. [Google Scholar] [CrossRef]
- Almario, J.; Fabiańska, I.; Saridis, G.; Bucher, M. Unearthing the plant–microbe quid pro quo in root associations with beneficial fungi. New Phytol. 2022, 234, 1967–1976. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Honnay, O.; Helsen, K.; Van Geel, M. Plant community reassembly on restored semi-natural grasslands lags behind the assembly of the arbuscular mycorrhizal fungal communities. Biol. Conserv. 2017, 212, 196–208. [Google Scholar] [CrossRef]
- Hannula, S.E.; Heinen, R.; Huberty, M.; Steinauer, K.; De Long, J.R.; Jongen, R.; Bezemer, T.M. Persistence of plant-mediated microbial soil legacy effects in soil and inside roots. Nat. Commun. 2021, 12, 5686. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of plant developmental stage on microbial community structure and activity in the rhizosphere of three field crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- López-García, Á.; Varela-Cervero, S.; Vasar, M.; Öpik, M.; Barea, J.M.; Azcón-Aguilar, C. Plant traits determine the phylogenetic structure of arbuscular mycorrhizal fungal communities. Mol. Ecol. 2017, 26, 6948–6959. [Google Scholar] [CrossRef]
- López-García, Á.; Azcón-Aguilar, C.; Barea, J.M. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 2014, 176, 1075–1086. [Google Scholar] [CrossRef]
- Cobb, A.B.; Wilson, G.W.; Goad, C.L.; Bean, S.R.; Kaufman, R.C.; Herald, T.J.; Wilson, J.D. The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: Enhancing sustainability through plant-microbial partnership. Agric. Ecosyst. Environ. 2016, 233, 432–440. [Google Scholar] [CrossRef]
- Kabouw, P.; van Dam, N.M.; van der Putten, W.H.; Biere, A. How genetic modification of roots affects rhizosphere processes and plant performance. J. Exp. Bot. 2012, 63, 3475–3483. [Google Scholar] [CrossRef]
- Liu, W. Do genetically modified plants impact arbuscular mycorrhizal fungi? Ecotoxicology 2010, 19, 229–238. [Google Scholar] [CrossRef]
- Ciccolini, V.; Ercoli, L.; Davison, J.; Vasar, M.; Öpik, M.; Pellegrino, E. Land-use intensity and host plant simultaneously shape the composition of arbuscular mycorrhizal fungal communities in a Mediterranean drained peatland. FEMS Microbiol. Ecol. 2016, 92, fiw186. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Wilson, G.W.; Rinella, M.J. Predicting plant responses to mycorrhizae: Integrating evolutionary history and plant traits. Ecol. Lett. 2012, 15, 689–695. [Google Scholar] [CrossRef]
- Tahiri, A.-i.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Dierks, J.; Blaser-Hart, W.J.; Gamper, H.A.; Six, J. Mycorrhizal fungi-mediated uptake of tree-derived nitrogen by maize in smallholder farms. Nat. Sustain. 2022, 5, 64–70. [Google Scholar] [CrossRef]
- Qin, Z.; Peng, Y.; Yang, G.; Feng, G.; Christie, P.; Zhou, J.; Zhang, J.; Li, X.; Gai, J. Relationship between phosphorus uptake via indigenous arbuscular mycorrhizal fungi and crop response: A 32P-labeling study. Appl. Soil Ecol. 2022, 180, 104624. [Google Scholar] [CrossRef]
- Massa, N.; Cesaro, P.; Todeschini, V.; Capraro, J.; Scarafoni, A.; Cantamessa, S.; Copetta, A.; Anastasia, F.; Gamalero, E.; Lingua, G. Selected autochthonous rhizobia, applied in combination with AM fungi, improve seed quality of common bean cultivated in reduced fertilization condition. Appl. Soil Ecol. 2020, 148, 103507. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, C.; Wang, C. Vermicompost assisted arbuscular mycorrhizal fungi to transfer 15 N from crop residues to lettuce. Plant Soil 2020, 456, 175–187. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular mycorrhizal fungi modulate the crop performance and metabolic profile of saffron in soilless cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Yield and quality of inflorescences in the Zantedeschia albomaculata (Hook.) Baill. ‘Albomaculata’ after the treatment with AMF and GA3. Agronomy 2021, 11, 644. [Google Scholar] [CrossRef]
- Deja-Sikora, E.; Werner, K.; Hrynkiewicz, K. AMF species do matter: Rhizophagus irregularis and Funneliformis mosseae affect healthy and PVY-infected Solanum tuberosum L. in a different way. Front. Microbiol. 2023, 14, 1127278. [Google Scholar] [CrossRef]
- Marro, N.; Cofre, N.; Grilli, G.; Alvarez, C.; Labuckas, D.; Maestri, D.; Urcelay, C. Soybean yield, protein content and oil quality in response to interaction of arbuscular mycorrhizal fungi and native microbial populations from mono-and rotation-cropped soils. Appl. Soil Ecol. 2020, 152, 103575. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.H.u.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Feng, G.; Li, Z.; Zhang, Y.; Cao, N. Arbuscular mycorrhizal enhancement of phosphorus uptake and yields of maize under high planting density in the black soil region of China. Sci. Rep. 2021, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Yang, D.; Yao, Y.; Guo, N. Effects of Rhizophagus intraradices on soybean yield and the composition of microbial communities in the rhizosphere soil of continuous cropping soybean. Sci. Rep. 2022, 12, 17390. [Google Scholar] [CrossRef] [PubMed]
- Trisilawati, O.; Hartoyo, B.; Bermawie, N.; Pribadi, E. Application of AMF (Arbuscular Mycorrhizal Fungi) and organic fertilizer to increase the growth, biomass and bioactive content of Centella. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Saint Petersburg, Russia, 17–18 April 2019; p. 012067. [Google Scholar]
- Dickie, I.A.; Martínez-García, L.B.; Koele, N.; Grelet, G.-A.; Tylianakis, J.M.; Peltzer, D.A.; Richardson, S.J. Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil 2013, 367, 11–39. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, L.B.; Richardson, S.J.; Tylianakis, J.M.; Peltzer, D.A.; Dickie, I.A. Host identity is a dominant driver of mycorrhizal fungal community composition during ecosystem development. New Phytol. 2015, 205, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Courty, P.; Buee, M.; Tech, J.; Brulé, D.; Colin, Y.; Leveau, J.; Uroz, S. Impact of soil pedogenesis on the diversity and composition of fungal communities across the California soil chronosequence of Mendocino. Mycorrhiza 2018, 28, 343–356. [Google Scholar] [CrossRef]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Waud, M.; Merckx, V.S.; Lievens, B.; Brys, R. Mycorrhizal diversity, seed germination and long-term changes in population size across nine populations of the terrestrial orchid Neottia ovata. Mol. Ecol. 2015, 24, 3269–3280. [Google Scholar] [CrossRef]
- Johnson, N.C. Can fertilization of soil select less mutualistic mycorrhizae? Ecol. Appl. 1993, 3, 749–757. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Promotion of utilization of arbuscular mycorrhiza through reduced P fertilization 1. Bioassays in a growth chamber. Plant Soil 2000, 227, 191–206. [Google Scholar] [CrossRef]
- Ma, M.; Ongena, M.; Wang, Q.; Guan, D.; Cao, F.; Jiang, X.; Li, J. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. AMB Express 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.A.; Ward, V.; Jones, M.D. Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J. 2014, 8, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, R.; Bai, S.; Bai, Y.; Wang, J. Slope aspect influences arbuscular mycorrhizal fungus communities in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 2017, 27, 189–200. [Google Scholar] [CrossRef]
- Begum, F.; Bajracharya, R.M.; Sitaula, B.K.; Sharma, S. Seasonal dynamics, slope aspect and land use effects on soil mesofauna density in the mid-hills of Nepal. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 290–297. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Vašutová, M.; Edwards-Jonášová, M.; Baldrian, P.; Čermák, M.; Cudlín, P. Distinct environmental variables drive the community composition of mycorrhizal and saprotrophic fungi at the alpine treeline ecotone. Fungal Ecol. 2017, 27, 116–124. [Google Scholar] [CrossRef]
- Argüelles-Moyao, A.; Garibay-Orijel, R. Ectomycorrhizal fungal communities in high mountain conifer forests in central Mexico and their potential use in the assisted migration of Abies religiosa. Mycorrhiza 2018, 28, 509–521. [Google Scholar] [CrossRef]
- Looby, C.I.; Maltz, M.R.; Treseder, K.K. Belowground responses to elevation in a changing cloud forest. Ecol. Evol. 2016, 6, 1996–2009. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- Egan, C.P.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal fungal communities along an elevation gradient. Mycorrhiza 2017, 27, 273–282. [Google Scholar] [CrossRef]
- Liu, L.; Hart, M.M.; Zhang, J.; Cai, X.; Gai, J.; Christie, P.; Li, X.; Klironomos, J.N. Altitudinal distribution patterns of AM fungal assemblages in a Tibetan alpine grassland. FEMS Microbiol. Ecol. 2015, 91, fiv078. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Klironomos, J.N. Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS ONE 2012, 7, e36695. [Google Scholar] [CrossRef] [PubMed]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Sun, X.; Jia, S.; Zhang, X.; Liang, W. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.J.; Sparkes, D.; Fraser, W.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Indoria, A.; Rao, C.S.; Sharma, K.; Reddy, K.S. Conservation agriculture–a panacea to improve soil physical health. Curr. Sci. 2017, 112, 52–61. [Google Scholar] [CrossRef]
- Lohan, S.K.; Jat, H.; Yadav, A.K.; Sidhu, H.; Jat, M.; Choudhary, M.; Peter, J.K.; Sharma, P. Burning issues of paddy residue management in north-west states of India. Renew. Sustain. Energy Rev. 2018, 81, 693–706. [Google Scholar] [CrossRef]
- Giller, K.E.; Andersson, J.A.; Corbeels, M.; Kirkegaard, J.; Mortensen, D.; Erenstein, O.; Vanlauwe, B. Beyond conservation agriculture. Front. Plant Sci. 2015, 6, 870. [Google Scholar] [CrossRef]
- de Pontes, J.S.; Oehl, F.; Pereira, C.D.; de Toledo Machado, C.T.; Coyne, D.; da Silva, D.K.A.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in the Brazilian’s Cerrado and in soybean under conservation and conventional tillage. Appl. Soil Ecol. 2017, 117, 178–189. [Google Scholar] [CrossRef]
- Hu, J.; Yang, A.; Wang, J.; Zhu, A.; Dai, J.; Wong, M.H.; Lin, X. Arbuscular mycorrhizal fungal species composition, propagule density, and soil alkaline phosphatase activity in response to continuous and alternate no-tillage in Northern China. Catena 2015, 133, 215–220. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Do arbuscular mycorrhizal fungi recover from soil disturbance differently? Trop. Ecol. 2004, 45, 97–112. [Google Scholar]
- Hui, N.; Jumpponen, A.; Francini, G.; Kotze, D.J.; Liu, X.; Romantschuk, M.; Strömmer, R.; Setälä, H. Soil microbial communities are shaped by vegetation type and park age in cities under cold climate. Environ. Microbiol. 2017, 19, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Kerfahi, D.; Tripathi, B.M.; Lee, J.; Edwards, D.P.; Adams, J.M. The impact of selective-logging and forest clearance for oil palm on fungal communities in Borneo. PLoS ONE 2014, 9, e111525. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Schubert, M.G.; Nguyen, N.H.; Bruns, T.D. Measuring ectomycorrhizal fungal dispersal: Macroecological patterns driven by microscopic propagules. Mol. Ecol. 2012, 21, 4122–4136. [Google Scholar] [CrossRef]
- Verbruggen, E.; Röling, W.F.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef]
- Ishaq, L.; Barber, P.A.; Hardy, G.E.S.J.; Dell, B. Diversity of fungi associated with roots of Eucalyptus gomphocephala seedlings grown in soil from healthy and declining sites. Australas. Plant Pathol. 2018, 47, 155–162. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Hui, N.; Liu, X.; Jumpponen, A.; Setälä, H.; Kotze, D.J.; Biktasheva, L.; Romantschuk, M. Over twenty years farmland reforestation decreases fungal diversity of soils, but stimulates the return of ectomycorrhizal fungal communities. Plant Soil 2018, 427, 231–244. [Google Scholar] [CrossRef]
- Moora, M.; Davison, J.; Öpik, M.; Metsis, M.; Saks, Ü.; Jairus, T.; Vasar, M.; Zobel, M. Anthropogenic land use shapes the composition and phylogenetic structure of soil arbuscular mycorrhizal fungal communities. FEMS Microbiol. Ecol. 2014, 90, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Martinová, V.; Van Geel, M.; Lievens, B.; Honnay, O. Strong differences in Quercus robur-associated ectomycorrhizal fungal communities along a forest-city soil sealing gradient. Fungal Ecol. 2016, 20, 88–96. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: Is there a role for stochastic processes? J. Ecol. 2010, 98, 419–428. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Maeder, P.; Wiemken, A.; Boller, T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 2009, 134, 257–268. [Google Scholar] [CrossRef]
- Gavito, M.E.; Schweiger, P.; Jakobsen, I. P uptake by arbuscular mycorrhizal hyphae: Effect of soil temperature and atmospheric CO2 enrichment. Glob. Chang. Biol. 2003, 9, 106–116. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Vázquez-Santos, Y.; Castillo-Argüero, S.; Martínez-Orea, Y.; Sánchez-Gallen, I.; Vega-Frutis, R.; Camargo-Ricalde, S.L.; Hernández-Cuevas, L.V. The reproductive phenology of Acaena elongata and its relation with arbuscular mycorrhizal fungi. Symbiosis 2019, 79, 129–140. [Google Scholar] [CrossRef]
- Vega-Frutis, R.; Guevara, R. Greater mycorrhizal colonization of unisexual morphs than of hermaphroditic morphs of Jacaratia mexicana during flowering and fruiting in central Mexico. Symbiosis 2013, 59, 173–181. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Dalpé, Y.; Sahraoui, A.L.-H. Soil and seasons affect arbuscular mycorrhizal fungi associated with Tamarix rhizosphere in arid and semi-arid steppes. Appl. Soil Ecol. 2016, 107, 182–190. [Google Scholar] [CrossRef]
- Hewins, C.R.; Carrino-Kyker, S.R.; Burke, D.J. Seasonal variation in mycorrhizal fungi colonizing roots of Allium tricoccum (wild leek) in a mature mixed hardwood forest. Mycorrhiza 2015, 25, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Rawlik, M.; Jagodziński, A.M. Seasonal dynamics of shoot biomass of dominant clonal herb species in an oak–hornbeam forest herb layer. Plant Ecol. 2020, 221, 1133–1142. [Google Scholar] [CrossRef]
- Zubek, S.; Rola, K.; Rożek, K.; Błaszkowski, J.; Stanek, M.; Chmolowska, D.; Chowaniec, K.; Zalewska-Gałosz, J.; Stefanowicz, A.M. Experimental assessment of forest floor geophyte and hemicryptophyte impact on arbuscular mycorrhizal fungi communities. Plant Soil 2022, 480, 651–673. [Google Scholar] [CrossRef]
- Saini, I.; Aggarwal, A.; Kaushik, P. Inoculation with mycorrhizal fungi and other microbes to improve the morpho-physiological and floral traits of Gazania rigens (L.) Gaertn. Agriculture 2019, 9, 51. [Google Scholar] [CrossRef]
- Tognon, G.B.; Sanmartín, C.; Alcolea, V.; Cuquel, F.L.; Goicoechea, N. Mycorrhizal inoculation and/or selenium application affect post-harvest performance of snapdragon flowers. Plant Growth Regul. 2016, 78, 389–400. [Google Scholar] [CrossRef]
- Lazzara, S.; Militello, M.; Carrubba, A.; Napoli, E.; Saia, S. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contrasting P availability in a highly organic substrate. Mycorrhiza 2017, 27, 345–354. [Google Scholar] [CrossRef]
- Xie, M.-M.; Wu, Q.-S. Arbuscular mycorrhizal fungi regulate flowering of hyacinths orientalis l. Anna marie. Emir. J. Food Agric. 2018, 30, 144–149. [Google Scholar]
- Liu, S.; Guo, H.; Xu, J.; Song, Z.; Song, S.; Tang, J.; Chen, X. Arbuscular mycorrhizal fungi differ in affecting the flowering of a host plant under two soil phosphorus conditions. J. Plant Ecol. 2018, 11, 623–631. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Y. Silver nanoparticles and arbuscular mycorrhizal fungi influence Trifolium repen root-associated AMF community structure and its co-occurrence pattern. Sci. Hortic. 2023, 320, 112232. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Fan, Z.; Fang, M.; Xu, Z.; Ban, Y. The function and community structure of arbuscular mycorrhizal fungi in ecological floating beds used for remediation of Pb contaminated wastewater. Sci. Total Environ. 2023, 872, 162233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X.; Wang, Z.; Su, X.; Liu, F.; Tian, X.; Ye, Y.; Shao, Y.; Yuan, Z. Cd contamination determined assembly processes and network stability of AM fungal communities in an urban green space ecosystem. Sci. Total Environ. 2023, 899, 166372. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, B.; Zhang, G.; Chen, W.; Wei, G. Stochastic community assembly decreases soil fungal richness in arid ecosystems. Mol. Ecol. 2021, 30, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, G.; Xing, S.; Fu, W.; Liu, X.; Wu, H.; Zhou, X.; Ma, Y.; Zhang, X.; Chen, B. Structure and diversity of fungal communities in long-term copper-contaminated agricultural soil. Sci. Total Environ. 2022, 806, 151302. [Google Scholar] [CrossRef]
- Betancur-Agudelo, M.; Meyer, E.; Lovato, P.E. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 2021, 17, 100307. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcon-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Staddon, P.L.; Thompson, K.; Jakobsen, I.; Grime, J.P.; Askew, A.P.; Fitter, A.H. Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob. Chang. Biol. 2003, 9, 186–194. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Kim, C.-G.; Subramanian, P.; Kim, K.-Y.; Selvakumar, G.; Sa, T.-M. Arbuscular mycorrhizal fungi community structure, abundance and species richness changes in soil by different levels of heavy metal and metalloid concentration. PLoS ONE 2015, 10, e0128784. [Google Scholar] [CrossRef] [PubMed]
- Long, L.K.; Yao, Q.; Guo, J.; Yang, R.H.; Huang, Y.H.; Zhu, H.H. Molecular community analysis of arbuscular mycorrhizal fungi associated with five selected plant species from heavy metal polluted soils. Eur. J. Soil Biol. 2010, 46, 288–294. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Yin, R.; Xing, S.; Fu, W.; Wu, H.; Hao, Z.; Ma, Y.; Zhang, X. Long-term nickel contamination increased soil fungal diversity and altered fungal community structure and co-occurrence patterns in agricultural soils. J. Hazard. Mater. 2022, 436, 129113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, G.; Zhang, Q.; Cui, W.; Li, Z.; Zhang, S. Potential hazards of novel waste-derived sorbents for efficient removal of mercury from coal combustion flue gas. J. Hazard. Mater. 2021, 412, 125226. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, Z.; Zhang, M.; Rinklebe, J.; Ma, Y.; Hou, D. Effect of production temperature and particle size of rice husk biochar on mercury immobilization and erosion prevention of a mercury contaminated soil. J. Hazard. Mater. 2021, 420, 126646. [Google Scholar] [CrossRef]
- Mi, Y.; Bai, X.; Li, X.; Zhou, M.; Liu, X.; Wang, F.; Su, H.; Chen, H.; Wei, Y. Soil Mercury Pollution Changes Soil Arbuscular Mycorrhizal Fungal Community Composition. J. Fungi 2023, 9, 395. [Google Scholar] [CrossRef]
- Ban, Y.; Jiang, Y.; Li, M.; Zhang, X.; Zhang, S.; Wu, Y.; Xu, Z. Homogenous stands of a wetland grass living in heavy metal polluted wetlands harbor diverse consortia of arbuscular mycorrhizal fungi. Chemosphere 2017, 181, 699–709. [Google Scholar] [CrossRef]
- Mi, Y.; Xu, C.; Li, X.; Zhou, M.; Cao, K.; Dong, C.; Li, X.; Ji, N.; Wang, F.; Su, H. Arbuscular mycorrhizal fungi community analysis revealed the significant impact of arsenic in antimony-and arsenic-contaminated soil in three Guizhou regions. Front. Microbiol. 2023, 14, 1189400. [Google Scholar] [CrossRef]
- Schneider, J.; Stürmer, S.L.; Guilherme, L.R.G.; de Souza Moreira, F.M.; de Sousa Soares, C.R.F. Arbuscular mycorrhizal fungi in arsenic-contaminated areas in Brazil. J. Hazard. Mater. 2013, 262, 1105–1115. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Langaas, S.; Futter, M. Pollution: Do microplastics spill on to farm soils? Nature 2016, 537, 488. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Giambalvo, D.; Amato, G.; Ingraffia, R.; Porto, A.L.; Mirabile, G.; Ruisi, P.; Torta, L.; Frenda, A.S. Nitrogen fertilization and arbuscular mycorrhizal fungi do not mitigate the adverse effects of soil contamination with polypropylene microfibers on maize growth. Environ. Pollut. 2023, 334, 122146. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.; Yu, H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Brigido, C.; Van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef]

| Plants | Treatment | Plant Performance | References |
|---|---|---|---|
| Tomato | AMF and compost manure | Application of the AMF and compost significantly increased the tomato shoot biomass by 156%, fruit yield by 111% and fruit quality | [73] |
| Maize | AMF | AMF enhanced the uptake of about 35 kg ha−1 tree-derived N by maize plants | [74] |
| Maize and wheat | Rhizophagus sp. | At low soil phosphorus content, AMF significantly increased the maize P content by 81.8% and wheat by 75.8%. But at high soil P, AMF contribution to the plants’ phosphorus contents decreased by 40.6% for maize and 9.1% for wheat plants | [75] |
| Common bean (Phaseolus vulgaris cv. Billò) | AMF and Rhizobium leguminosarum | The seed protein, starch contents and nutrient contents of the bean seeds were increased significantly in the presence of the inocula | [76] |
| Lettuce | AMF and vermicompost | Lettuce yield and nitrogen uptake were enhanced by fungi and organic fertilizer treatment | [77] |
| Saffron (Crocus sativus L.) | Rhizophagus intraradices and Funneliformis mosseae | AMF significantly increased the yield, water uptake, nutritional contents and antioxidant activity of saffron spice plants | [78] |
| Calla lilies (Zantedeschia albomaculata) | AMF | It increased the plants’ yield, length of spathes and leaves nutritional quality | [79] |
| Potato (Solanum tuberosum L.) | Rhizophagus irregularis and Funneliformis mosseae | AMF, especially R. irregularis, positively increased the potato fresh and dry weights in healthy and virus-infected plants | [80] |
| Soybean | AMF | The fungi enhanced the soybean seed protein content by 12 to 14% and oleic acid by 21 to 25%, as well as its overall yield | [81] |
| Cotton (Gossypium hirsutum L.) | AMF (Rhizophagus irregularis CD1) | The presence of the fungi in association with the plants enhanced their percentage yield (28.54%), P content, boll number per plant, growth, maturation of cotton fibre, and quality | [82] |
| Maize | AMF | Inoculation of the plants with AMF significantly increased stomatal conductance, photosynthesis rate, antioxidant enzyme activity and nutrient uptake by the maize plants | [83] |
| Soybean (Glycine max L.) | Rhizophagus clarus | The fungi increased the overall performance of the plant in the face of drought stress by increasing water and nutrient uptake, plant growth and tolerance to the water stress | [84] |
| Maize (Zea mays L.) | AMF | It enhanced the efficiency of plants’ phosphorus acquisition from the soil and increased maize yield and productivity | [85] |
| Soybean (Glycine max L.) | Rhizophagus intraradices | It increased soybean yield, plants’ vegetative growth properties as well as its rhizosphere microbial communities | [86] |
| Centella (Centella asiatica L. Urban) | AMF and organic fertilizer | The treatment collectively increased the NPK content of the plant, growth parameters, biomass and leaves’ asiaticoside content | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enebe, M.C.; Erasmus, M. Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions. Agriculture 2023, 13, 1899. https://doi.org/10.3390/agriculture13101899
Enebe MC, Erasmus M. Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions. Agriculture. 2023; 13(10):1899. https://doi.org/10.3390/agriculture13101899
Chicago/Turabian StyleEnebe, Matthew Chekwube, and Mariana Erasmus. 2023. "Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions" Agriculture 13, no. 10: 1899. https://doi.org/10.3390/agriculture13101899
APA StyleEnebe, M. C., & Erasmus, M. (2023). Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions. Agriculture, 13(10), 1899. https://doi.org/10.3390/agriculture13101899






