Pre-Harvest Bagging of Table Grapes Reduces Accumulations of Agrochemical Residues and Increases Fruit Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bagging Treatments
2.3. Agrochemical Treatments
2.4. Morphological, Colorimetric and Texture Analyses
2.5. Chemical Analyses
2.6. Agrochemical Residual Analyses
2.7. Statistical Analyses
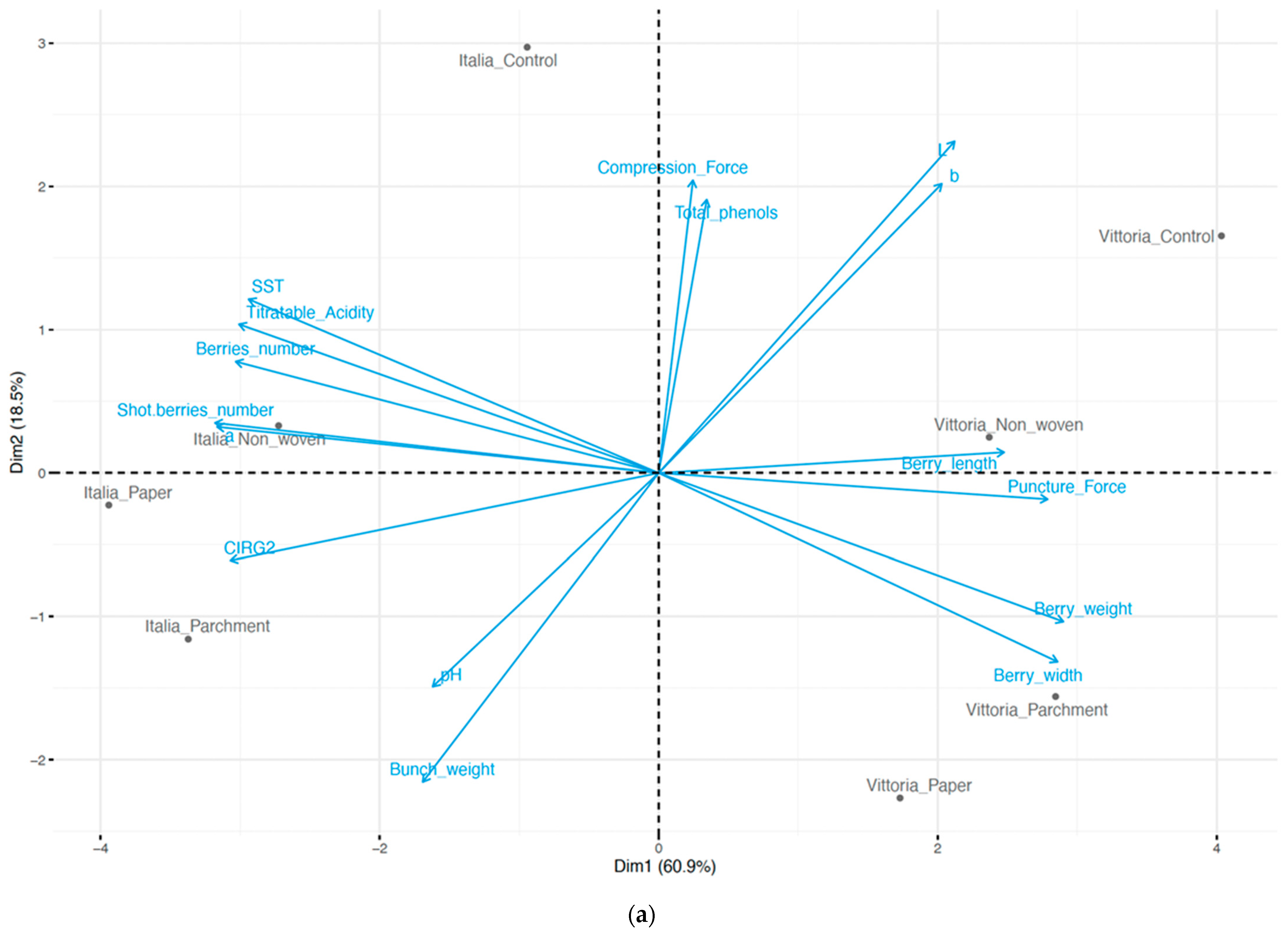
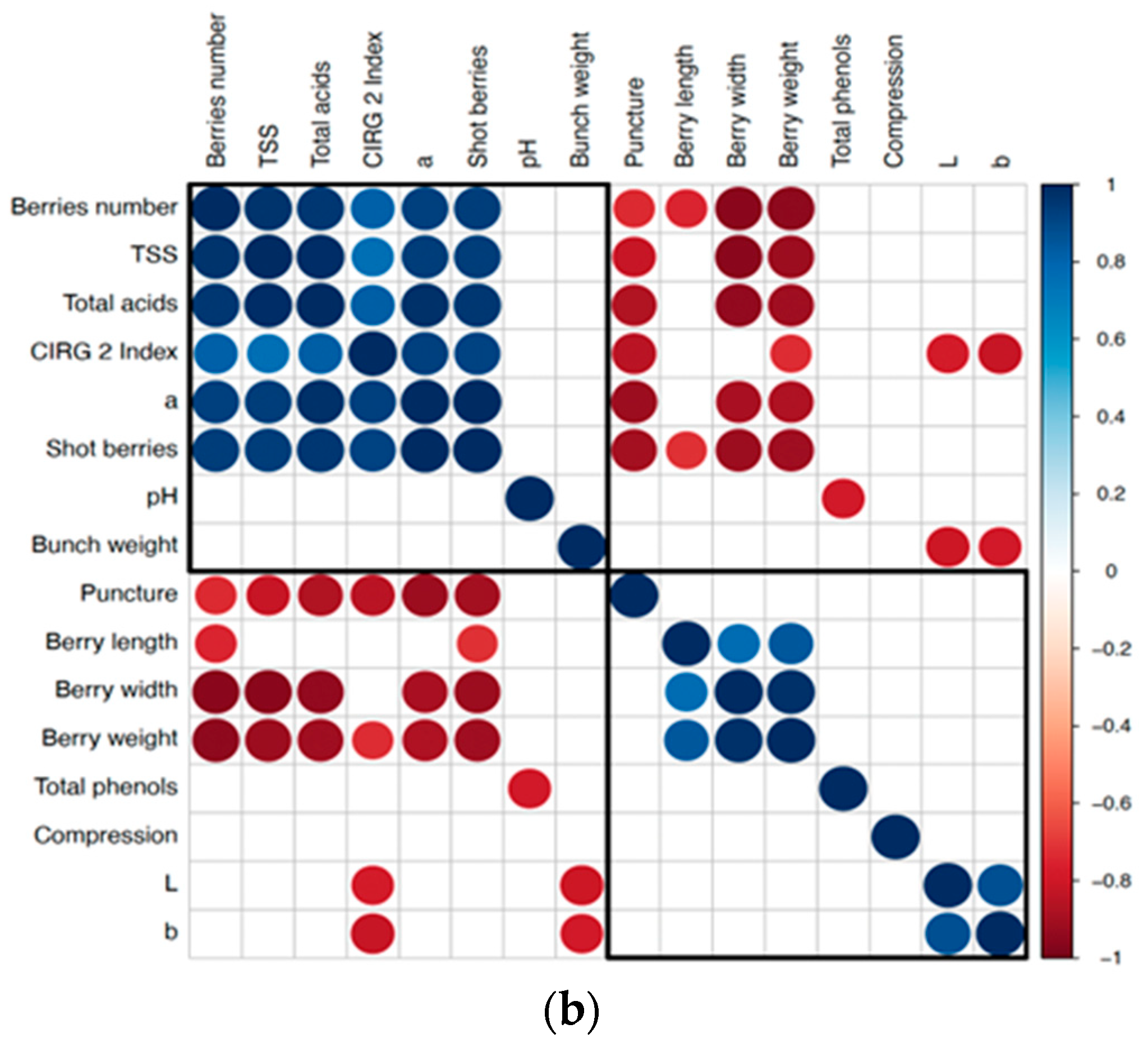
3. Results
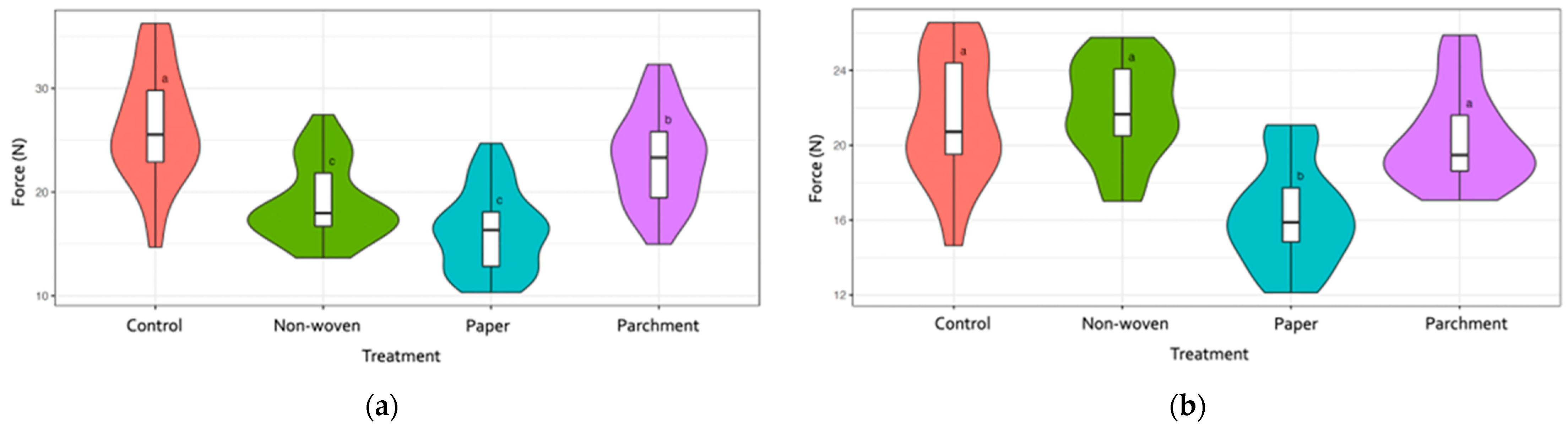
3.1. Berry Quality Traits
3.2. Agrochemical Residue Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV). 2019 Statistical Report on World Vitiviniculture; International Organisation of Vine and Wine (OIV): Dijon, France, 2019. [Google Scholar]
- This, P.; Lacombe, T.; Thomas, M.R. Historical Origins and Genetic Diversity of Wine Grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Limier, B.; Ivorra, S.; Bouby, L.; Figueiral, I.; Chabal, L.; Cabanis, M.; Ater, M.; Lacombe, T.; Ros, J.; Brémond, L.; et al. Documenting the History of the Grapevine and Viticulture: A Quantitative Eco-Anatomical Perspective Applied to Modern and Archaeological Charcoal. J. Archaeol. Sci. 2018, 100, 45–61. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Annual Review of Environment and Resources Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of Agrochemicals on Soil Health. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–187. [Google Scholar]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Hoppin, J.A.; Kamel, F. Health Effects of Chronic Pesticide Exposure: Cancer and Neurotoxicity. Annu. Rev. Public. Health 2004, 25, 155–197. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public. Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Xu, F.; Baker, R.C.; Whitaker, T.B.; Luo, H.; Zhao, Y.; Stevenson, A.; Boesch, C.J.; Zhang, G. Review of Good Agricultural Practices for Smallholder Maize Farmers to Minimise Aflatoxin Contamination. World Mycotoxin J. 2022, 15, 171–186. [Google Scholar] [CrossRef]
- Bennici, S.; Di Guardo, M.; Distefano, G.; La Malfa, S.; Puglisi, D.; Arcidiacono, F.; Ferlito, F.; Deng, Z.; Gentile, A.; Nicolosi, E. Influence of the Genetic Background on the Performance of Molecular Markers Linked to Seedlessness in Table Grapes. Sci. Hortic. 2019, 252, 316–323. [Google Scholar] [CrossRef]
- Ilnitskaya, E.; Makarkina, M.; Petrov, V. Potential of Genetic Resistance of New Table Grape Hybrids to Fungal Pathogens. BIO Web Conf. 2021, 34, 02001. [Google Scholar] [CrossRef]
- Puglisi, D.; Las Casas, G.; Ferlito, F.; Nicolosi, E.; Di Guardo, M.; Scollo, F.; Saitta, G.; La Malfa, S.; Gentile, A.; Distefano, G. Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs. Agriculture 2022, 12, 1096. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Jhalegar, M.J. Pre-Harvest Fruit Bagging: A Useful Approach for Plant Protection and Improved Post-Harvest Fruit Quality—A Review. J. Hortic. Sci. Biotechnol. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Pal, R.K.; Asrey, R.; Sagar, V.R.; Dhiman, M.R.; Rana, M.R. Pre-Harvest Fruit Bagging Influences Fruit Color and Quality of Apple Cv. Delicious. Agric. Sci. 2013, 4, 443–448. [Google Scholar] [CrossRef]
- Jaroenkit, T.; Maichoo, S.; Manochai, P.; Ratanamano, S. Effect of fruit bagging on chemical compositions and skin pigments of fresh longan (Dimocarpus longan Lour.). Acta Hortic. 2010, 863, 397–402. [Google Scholar] [CrossRef]
- Fang, H.; Lee, W.; Huang, C.; Liang, Y. Effect of Bagging Materials on Pericarp Coloration and Pesticide Residues of “Yu-Her-Pao” Litchi (Litchi chinensis Sonn.) Fruit. J. Taiwan Agric. Res. 2016, 65, 184–193. [Google Scholar]
- Sang, X.; Li, Z.; Zhang, W.; Tang, J.; Jue, D. Effects of Bagging Treatment on Fruit Quality and Pesticide Residues of ‘Donghong’ Kiwifruit. Not. Bot. Horti Agrobot. Cluj Napoca 2022, 50, 12987. [Google Scholar] [CrossRef]
- Xu, G.; Nie, J.; Wu, Y.; Yan, Z.; Ye, M. The Effects of Fruit Bagging on Residue Behavior and Dietary Risk for Four Pesticides in Apple. Sci. Rep. 2018, 8, 14348. [Google Scholar] [CrossRef] [PubMed]
- Karajeh, M.R. Pre-Harvest Bagging of Grape Clusters as a Non-Chemical Physical Control Measure against Certain Pests and Diseases of Grapevines. Org. Agric. 2018, 8, 259–264. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.S.; Akter, M.M.; Hasan, M.N.; Uddin, M.S. Influence of Pre-Harvest Bagging on Fruit Quality of Mango (Mangifera indica L.) Cv. Langra. Asian J. Agric. Hortic. Res. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Pisciotta, A.; Planeta, D.; Giacosa, S.; Paissoni, M.A.; Di Lorenzo, R.; Rolle, L. Quality of Grapes Grown inside Paper Bags in Mediterranean Area. Agronomy 2020, 10, 792. [Google Scholar] [CrossRef]
- Shah, G.; Chand, S.; Srivastava, R.; Kumar, R.; Sharma, R. Effect of Pre Harvest Fruit Bagging on the Physico-Chemical Properties of Litchi (Litchi chinensis Sonn.) CV. Rose Scented. J. Pharmacogn. Phytochem. 2020, 9, 1812–1819. [Google Scholar]
- Nicolosi, E.; Scollo, F.; Distefano, G.; Ferlito, F.; Luca, L.; Seminara, S.; Inzirillo, I.; La Malfa, S.; Gentile, A. Determination of Yield Qualitative and Hygienic Traits in Table Grapes Protected with Bags. Acta Hortic. 2022, 1354, 355–360. [Google Scholar] [CrossRef]
- Rolle, L.; Giacosa, S.; Gerbi, V.; Novello, V. Comparative Study of Texture Properties, Color Characteristics, and Chemical Composition of Ten White Table-Grape Varieties. Am. J. Enol. Vitic. 2011, 62, 49–56. [Google Scholar] [CrossRef]
- Rolle, L.; Siret, R.; Segade, S.R.S.; Maury, C.; Gerbi, V.; Jourjon, F. Instrumental Texture Analysis Parameters as Markers of Table-Grape and Winegrape Quality: A Review. Am. J. Enol. Vitic. 2012, 63, 11–28. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- BS EN 15661:2018; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-Method. European Standards. Available online: https://www.en-standard.eu/bs-en-15662-2018-foods-of-plant-origin-multimethod-for-the-determination-of-pesticide-residues-using-gc-and-lc-based-analysis-following-acetonitrile-extraction-partitioning-and-clean-up-by-dispersive-spe-modular-quechers-method. (accessed on 1 June 2023).
- Kumar, M.; Jat, R.; Ahamad, S.; Kumar, V.; Singh, V.P. Pre-Harvest Fruit Bagging for Quality Improvement in Fruit Crops: A Review. Pharma Innov. J. 2021, 10, 530–541. [Google Scholar]
- Hyun, W.J.; Secor, E.B.; Rojas, G.A.; Hersam, M.C.; Francis, L.F.; Frisbie, C.D. All-Printed, Foldable Organic Thin-Film Transistors on Glassine Paper. Adv. Mater. 2015, 27, 7058–7064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Guo, J.Y.; Ma, R.J.; Cai, Z.X.; Yan, J.; Zhang, C.H. Relationship between the Bagging Microenvironment and Fruit Quality in ‘Guibao’ Peach [Prunus persica (L.) Batsch]. J. Hortic. Sci. Biotechnol. 2015, 90, 303–310. [Google Scholar] [CrossRef]
- Tu, K.; Nicolai, B.; De Baerdemaeker, J. Effects of Relative Humidity on Apple Quality under Simulated Shelf Temperature Storage. Sci. Hortic. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Pitam, S.; Mukherjee, I.; Kumar, A. Evaluation of Environmental Fate of Acetamiprid in the Laboratory. Environ. Monit. Assess. 2013, 185, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Shanker, A. Persistence of Acetamiprid in Tea and Its Transfer from Made Tea to Infusion. Food Chem. 2008, 111, 805–810. [Google Scholar] [CrossRef]
- Nicol, E.; Varga, Z.; Vujovic, S.; Bouchonnet, S. Laboratory Scale UV–Visible Degradation of Acetamiprid in Aqueous Marketed Mixtures—Structural Elucidation of Photoproducts and Toxicological Consequences. Chemosphere 2020, 248, 126040. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gajbhiye, V.T.; Gupta, R.K. Effect of Light on the Degradation of Two Neonicotinoids Viz Acetamiprid and Thiacloprid in Soil. Bull. Environ. Contam. Toxicol. 2008, 81, 185–189. [Google Scholar] [CrossRef]
| Commercial Name | Active Compound | Target Organism | Action Modes | PHI (Days) | Rate per Hectare (600 L of Water) | Italia 2021 | Vittoria 2021 | Italia 2022 | Vittoria 2022 |
|---|---|---|---|---|---|---|---|---|---|
| Vertimec EC | Abamectin (1.84%) | Insecticide Acaricide Nematocide | L.S. | 4 | 1 L | 16 July | 16 July | Not used | Not used |
| Vitene Ultra SC | Cymoxanil (20.83%) | Plasmopara viticola | S. | 28 | 1 L | Not used | Not used | 13 August 15 September 29 September | Not used |
| Topas 10 EC | Penconazole (10.1%) | Fungicide | S. | 14 | 0.6 L | 16 June 30 June | 16 June 30 June | Not used | Not used |
| Epik SL | Acetamiprid (46.7%) | Insecticide Acaricide | S. | 14 | 2 L | 12 June 28 July | 12 June 28 July | 13 August 2 September | 13 August 2 September |
| Karathane Star | Meptyldinocap (35.71%) | Erysiphe necator | C. | 21 | 0.5 L | 12 June 30 June | 12 June 30 June | Not used | Not used |
| Reboot | Cymoxanil (33%) Zoxamide (33%) | Plasmopara viticola | L.S. | 28 | 0.5 kg | 12 June | 12 June | 21 August | Not used |
| Cymbal | Cymoxanil (44%) | Plasmopara viticola | L.S. | 28 | 0.5 kg | 30 June 16 July 28 July 12 August | 30 June 16 July 28 July 12 August | 16 June 13 July | 16 June |
| Radiant pro | Spinetoram (12%) | Insetticide | C. | 7 | 0.25 L | 30 June | 30 June | 13 July 15 September | Not used |
| Prosper 300 CS | Spiroxamine (30.6%) | Erysiphe necator | S. | 35 | 1.2 L | 16 July 28 July 12 August | 16 July 28 July 12 August | 16 June 29 June 13 July 28 July 21 August 15 September 29 September | Not used |
| Topas 200EW | Penconazole (19%) | Erysiphe necator | S. | 14 | 0.25 L | 28 July 12 August | 28 July 12 August | 16 June 29 June 13 July 28 July 13 August 21 August 2 September 15 September | 16 June 29 June 21 August |
| CoStar WG | Bacillus thuringiensis (18%) | Lobesia botrana Eupoecilia ambiguella Cryptoblabes gnidiella | C. | / | 0.75 kg | 12 August | 12 August | 16 June 29 June 28 July 21 August 29 September | 16 June 29 June 21 August |
| Enviromite FL | Bifenazate (43.55%) | Acaricide | C. | 14 | 0.35 L | Not used | Not used | 22 April 8 June 28 July | Not used |
| Tiovit Jet | Sulfur (80%) | Erysiphe necator | C. | / | 2 kg | Not used | Not used | 22 April | Not used |
| Kop-Twin | Copper Hydroxide (8.9%) Tribasic Copper Sulphate (13.3%) | Plasmopara viticola Guignardia bidwellii | C. | 21 | 1 L | Not used | Not used | 2 September | Not used |
| Sercadis SC | Fluxapyroxad (26.6%) | Erysiphe necator | S. | 35 | 0.15 L | Not used | Not used | 2 September | Not used |
| Switch | Cyprodinil (37.5%) Fludioxonil (25%) | Botrytis cinerea Aspergillus spp. Penicillium spp. | S. | 7 | 1 kg | Not used | Not used | 15 September | Not used |
| Cultivar | Treatment | Bunch Weight [g] | Berry Weight [g] | Berry Length [mm] | Berry Width [mm] | Berries Number | Shot Berries Number |
|---|---|---|---|---|---|---|---|
| Italia | Control | 894.26 ± 241.54 | 7.82 b ± 1.52 | 25.5 b ± 2.2 | 21.6 a ± 1.63 | 159.4 ± 42.79 | 30.5 ± 18.4 |
| Non-woven | 949.45 ± 259.73 | 7.64 b ± 1.17 | 25.7 ab ± 1.85 | 20.9 b ± 1.59 | 149.5 ± 41.83 | 40.4 ± 40.9 | |
| Paper | 975.2 ± 250.94 | 6.65 c ± 1.45 | 25.2 b ± 2.07 | 19.9 c ± 1.72 | 161.6 ± 37.02 | 41.4 ± 30.5 | |
| Parchment | 1067.5 ± 251.02 | 8.25 a ± 1.44 | 26.2 a ± 2.6 | 21.6 a ± 1.84 | 155.3 ± 46.30 | 41.4 ± 37.2 | |
| Significance | N.S. | *** | *** | *** | N.S. | N.S. | |
| Vittoria | Control | 908.35 ± 244 | 10.6 b ± 2.79 | 30 b ± 3.33 | 22.7 a ± 2.32 | 101.5 ± 27.1 | 7.45 ± 8.65 |
| Non-woven | 938.7 ± 167 | 9.58 c ± 1.86 | 28.2 c ± 2.74 | 21.4 b ± 1.74 | 117.3 ± 27.2 | 13.8 ± 20.7 | |
| Paper | 972.7 ± 237 | 10.1 bc ± 2.25 | 30.1 b ± 3.08 | 21.5 b ± 2.07 | 120.9 ± 30.5 | 11.4 ± 11.4 | |
| Parchment | 946.75 ± 144 | 11.5 a ± 3.41 | 31.2 a ± 4.04 | 22.7 a ± 2.57 | 98.8 ± 22.9 | 10.8 ± 16.1 | |
| Significance | N.S. | *** | *** | *** | N.S. | N.S. |
| Cultivar | Treatment | L* | a* | b* | CIRG2 |
|---|---|---|---|---|---|
| Italia | Control | 43.4 a ± 2.79 | −4.56 a ± 2.09 | 14.9 a ± 3.47 | 0.115 c ± 0.03 |
| Non-woven | 42 b ± 2.62 | −3.64 a ± 1.62 | 13.1 b ± 2.72 | 0.135 b ± 0.03 | |
| Paper | 40.8 c ± 2.7 | −3.51 a ± 1.5 | 12.5 b ± 2.4 | 0.146 a ± 0.03 | |
| Parchment | 41 c ± 2.71 | −3.44 b ± 1.38 | 12.4 b ± 2.53 | 0.147 a ± 0.04 | |
| Significance | *** | *** | *** | *** | |
| Vittoria | Control | 43.9 a ± 3.63 | −7.38 a ± 1.38 | 14.3 b ± 2.45 | 0.092 c ± 0.02 |
| Non-woven | 42.9 b ± 3.18 | −7.25 ab ± 1.63 | 15.1 a ± 3.2 | 0.095 bc ± 0.02 | |
| Paper | 41.2 b± 3.82 | −6.82 b ± 1.92 | 13.1 c ± 2.83 | 0.11 a ± 0.03 | |
| Parchment | 42.5 c ± 2.79 | −6.88 b ± 1.45 | 13.7 bc ± 2.26 | 0.10 b ± 0.02 | |
| Significance | *** | ** | *** | *** |
| Cultivar | Treatment | TSS [°Bx] | pH [pH] | Titratable Acidity [mg/L] | Total Phenols [mg GAE/L] |
|---|---|---|---|---|---|
| Italia | Control | 18 ± 0.83 | 4.23 ± 0.15 | 3.73 ± 2.47 | 23.3ab ± 4.77 |
| Non-woven | 17.2 ± 4.11 | 4.3 ± 0.05 | 3.75 ± 2.55 | 24.3ab ± 1.87 | |
| Paper | 17.7 ± 4.05 | 4.28 ± 0.03 | 3.73 ± 2.49 | 28.6a ± 3.98 | |
| Parchment | 17.3 ± 4.03 | 4.35 ± 0.79 | 3.69 ± 2.52 | 19.6b ± 3.33 | |
| Significance | N.S. | N.S. | N.S. | ** | |
| Vittoria | Control | 14.2 ± 0.32 | 4b± 0.11 | 3.97 ± 2.69 | 36.9 ± 9.93 |
| Non-woven | 14.9 ± 2.32 | 4.3a ± 0.12 | 3.79 ± 2.68 | 21.3 ± 6.65 | |
| Paper | 14.3 ± 0.11 | 4.21ab ± 0.02 | 3.76 ± 2.55 | 18.1 ± 3.33 | |
| Parchment | 14.3 ± 0.49 | 4.35a ± 0.19 | 3.61 ± 2.42 | 18 ± 4 | |
| Significance | N.S. | ** | N.S. | N.S. |
| Agrochemicals Detected | M.R.L | Italia 2021 | Italia 2022 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | Non-Woven | Paper | Parchment | Control | Non-Woven | Paper | Parchment | Control | |
| Acetamiprid | 0.5 | 0.014 | 0.005 | N.D. | 0.33 | 0.014 | 0.003 | 0.001 | 0.161 |
| Ametoctradin | 6 | N.D. | N.D. | N.D. | 0.008 | N.D. | N.D. | N.D. | N.D. |
| Cymoxanil | 0.05 | 0.006 | 0.007 | 0.006 | 0.006 | N.D. | N.D. | N.D. | 0.01 |
| Fenhexamid | 15 | 0.011 | N.D. | N.D. | 0.017 | N.D. | N.D. | N.D. | N.D. |
| Meptyldinocap | 1 | N.D. | N.D. | N.D. | 0.005 | N.D. | N.D. | N.D. | N.D. |
| Metrafenone | 7 | N.D. | N.D. | N.D. | 0.009 | N.D. | N.D. | 0.016 | N.D. |
| Penconazole | 0.5 | 0.026 | 0.022 | N.D. | 0.14 | N.D. | 0.001 | N.D. | N.D. |
| Spinosad | 0.5 | N.D. | 0.012 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Spiroxamine | 0.6 | 0.01 | N.D. | N.D. | 0.14 | 0.009 | 0.0038 | 0.003 | 0.039 |
| Zoxamide | 5 | 0.005 | N.D. | N.D. | 0.078 | N.D. | N.D. | N.D. | N.D. |
| Total | 0.072 | 0.046 | 0.006 | 0.733 | 0.023 | 0.008 | 0.02 | 0.201 | |
| Agrochemicals Detected | M.R. L | Vittoria 2021 | Vittoria 2022 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | Non-Woven | Paper | Parchment | Control | Non-Woven | Paper | Parchment | Control | |
| Acetamiprid | 0.5 | 0.131 | 0.07 | 0.179 | 0.19 | 0.046 | 0.068 | 0.044 | 0.074 |
| Cymoxanil | 0.05 | N.D. | N.D. | N.D. | 0.002 | N.D. | N.D. | N.D. | 0.001 |
| Metrafenone | 7 | N.D. | N.D. | N.D. | 0.012 | N.D. | N.D. | N.D. | N.D. |
| Penconazole | 0.5 | N.D. | N.D. | N.D. | N.D. | 0.02 | N.D. | 0.001 | N.D. |
| Spiroxamine | 0.6 | N.D. | N.D. | N.D. | N.D. | 0.004 | 0.001 | 0.001 | 0.002 |
| Zoxamide | 5 | 0.001 | 0.001 | 0.001 | 0.002 | N.D. | N.D. | N.D. | N.D. |
| Total | 0.132 | 0.071 | 0.18 | 0.206 | 0.07 | 0.069 | 0.046 | 0.077 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, L.P.; Scollo, F.; Distefano, G.; Ferlito, F.; Bennici, S.; Inzirillo, I.; Gentile, A.; La Malfa, S.; Nicolosi, E. Pre-Harvest Bagging of Table Grapes Reduces Accumulations of Agrochemical Residues and Increases Fruit Quality. Agriculture 2023, 13, 1933. https://doi.org/10.3390/agriculture13101933
Luca LP, Scollo F, Distefano G, Ferlito F, Bennici S, Inzirillo I, Gentile A, La Malfa S, Nicolosi E. Pre-Harvest Bagging of Table Grapes Reduces Accumulations of Agrochemical Residues and Increases Fruit Quality. Agriculture. 2023; 13(10):1933. https://doi.org/10.3390/agriculture13101933
Chicago/Turabian StyleLuca, Leonardo Paul, Francesco Scollo, Gaetano Distefano, Filippo Ferlito, Stefania Bennici, Ilaria Inzirillo, Alessandra Gentile, Stefano La Malfa, and Elisabetta Nicolosi. 2023. "Pre-Harvest Bagging of Table Grapes Reduces Accumulations of Agrochemical Residues and Increases Fruit Quality" Agriculture 13, no. 10: 1933. https://doi.org/10.3390/agriculture13101933
APA StyleLuca, L. P., Scollo, F., Distefano, G., Ferlito, F., Bennici, S., Inzirillo, I., Gentile, A., La Malfa, S., & Nicolosi, E. (2023). Pre-Harvest Bagging of Table Grapes Reduces Accumulations of Agrochemical Residues and Increases Fruit Quality. Agriculture, 13(10), 1933. https://doi.org/10.3390/agriculture13101933











