Synthetic Carvacrol Derivatives for the Management of Solenopsis Ants: Toxicity, Sublethal Effects, and Horizontal Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Synthesis of Carvacrol Derivatives
2.3. Bioassays
2.3.1. Acute Toxicity
2.3.2. Individual and Collective Behavior
2.3.3. Walking Behavior
2.3.4. Horizontal Transfer of Compounds
2.4. Statistical Analysis
3. Results
3.1. Acute Toxicity
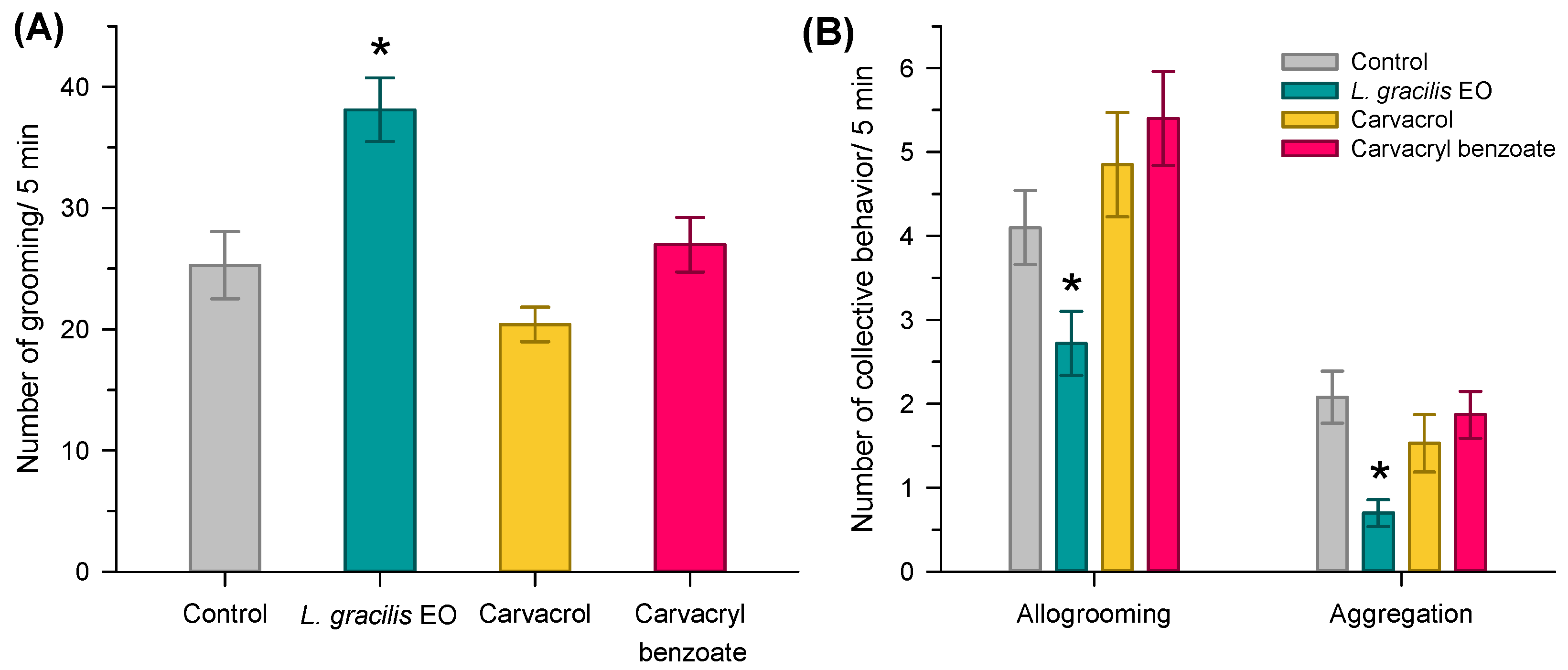
3.2. Individual and Collective Behavior
3.3. Walking Behavior
3.4. Horizontal Transfer of Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, J.P.; Mchugh, J.V.; Ross, K.G. Cladistic analysis of the fire ants of the Solenopsis saevissima species-group (Hymenoptera: Formicidae). Zool. Scr. 2005, 34, 493–505. [Google Scholar] [CrossRef]
- Chan, K.H.; Guénard, B. Ecological and socio-economic impacts of the red import fire ant, Solenopsis invicta (Hymenoptera: Formicidae), on urban agricultural ecosystems. Urban Ecosyst. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Wylie, F.R.; Janssen-May, S. Red imported fire ant in Australia: What if we lose the war? Ecol. Manag. Restor. 2017, 18, 32–44. [Google Scholar] [CrossRef]
- Lyu, D.P.; Lee, H.S. The red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae: Myrmicinae) discovered in Busan sea port, Korea. Korean J. Appl. Entomol. 2017, 56, 437–438. [Google Scholar] [CrossRef]
- Xu, Y.; Vargo, E.L.; Tsuji, K.; Wylie, R. Exotic Ants of the Asia-Pacific: Invasion, National Response, and Ongoing Needs. Annu. Rev. Entomol. 2022, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bolton, B. AntCat: An Online Catalog of the Ants of the World. 2012. Available online: http://antcat.org (accessed on 21 September 2023).
- Fernández, F.; Sendoya, S. List of Neotropical ants (Hymenoptera: Formicidae). Biota Colombiana. 2004, 5, 3–93. [Google Scholar]
- LeBrun, E.G.; Plowes, R.M.; Gilbert, L.E. Imported fire ants near the edge of their range: Disturbance and moisture determine prevalence and impact of an invasive social insect. J. Anim. Ecol. 2012, 81, 884–895. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The Fire Ants; Harvard University Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Drees, B.M.; Calixto, A.A.; Nester, P.R. Integrated pest management concepts for red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). Insect Sci. 2013, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Lard, C.F.; Schmidt, J.; Morris, B.; Estes, L.; Ryan, C.; Bergquist, D. An Economic Impact of Imported Fire Ants in the United States of America; Texas A&M University, Department of Agricultural Economics, Texas Agricultural Experiment Station: College Station, TX, USA, 2006. [Google Scholar]
- Xu, C.; Su, J.; Qu, X.; Zhou, A. Ant-mealybug mutualism modulates the performance of co-occurring herbivores. Sci. Rep. 2019, 9, 13004. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.A.M.; Janssen-May, S.; Santoro, D.; Cooling, M.; Wylie, R. Predicting socio-economic and biodiversity impacts of invasive species: Red Imported Fire Ant in the developing western Pacific. Ecol. Manag. Restor. 2021, 22, 89–99. [Google Scholar] [CrossRef]
- Park, Y.; Vatanparast, M.; Sajjadian, S.M. Pathogenicity of Beauveria bassiana ANU1 to the red imported fire ant, Solenopsis invicta workers in Korea. J. Asia-Pac. Entomol. 2022, 25, 101913. [Google Scholar] [CrossRef]
- Angulo, E.; Hoffman, B.D.; Ballesteros-Mejia, L.; Taheri, A.; Balzani, P.; Bang, A.; Renault, D.; Cordonnier, M.; Bellard, C.; Diagne, C.; et al. Economic costs of invasive alien ants worldwide. Biol. Invasions 2022, 24, 2041–2060. [Google Scholar] [CrossRef]
- Xu, T.; Xu, M.; Lu, Y.; Zhang, W.; Sun, J.; Zeng, R.; Turlings, T.C.J.; Chen, L. A trail pheromone mediates the mutualism between ants and aphids. Curr. Biol. 2021, 31, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.P.; Vanderwoude, C.; Lintermans, M.; Lynch, A.J.J. The Little Fire Ant (Hymenoptera: Formicidae): A Global Perspective. Ann. Entomol. Soc. Am. 2022, 115, 427–448. [Google Scholar] [CrossRef]
- Chen, J.; OI, D.H. Naturally occurring compounds/materials as alternatives to synthetic chemical insecticides for use in fire ant management. Insects 2020, 11, 758. [Google Scholar] [CrossRef]
- Guillet, C.; Martin, O.Y.; Meincke, C.; Joerg, L.; Schmid-Grendelmeier, P. Part I: Insect stings and bites—Beyond the realm of bee and wasp allergies. Allergo J. Int. 2022, 31, 183–193. [Google Scholar] [CrossRef]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef]
- Fu, J.T.; Tang, L.; Li, W.S.; Wang, K.; Cheng, D.M.; Zhang, Z.X. Fumigant toxicity and repellence activity of camphor essential oil from Cinnamonum camphora Siebold against Solenopsis invicta workers (Hymenoptera: Formicidae). J. Insect Sci. 2015, 15, 129. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Yu, S.; Huang, J.; Wang, Z.; Zeng, Y.; Wu, X.; Han, K.; Zhou, H.; Wang, G.; Yu, Z. Study of behavioral, electrophysiological response, and the active compounds of the essential oils from six kinds of flowers against Solenopsis invicta Buren (Hymenoptera: Formicidae). Ind. Crops Prod. 2022, 188, 115603. [Google Scholar] [CrossRef]
- Wen, C.; Chen, J.; Qin, W.Q.; Chen, X.; Cai, J.C.; Wen, J.B.; Wen, X.J.; Wang, C. 2021. Red imported fire ants (Hymenoptera: Formicidae) cover inaccessible surfaces with particles to facilitate food search and transportation. Insect Sci. 2021, 28, 1816–1828. [Google Scholar] [CrossRef]
- Tsai, C.C.; Hung, S.H.; Lin, X.R.; Huang, R.N. Herbal plants as alternatives for the management of the red imported fire ant, Solenopsis invicta. J. Appl. Entomol. 2022, 146, 975–989. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Y.; Li, C.; Tan, Y.; Wu, J.; Zhang, Z. Fumigant toxicity and behavioral inhibition of garlic against red imported fire ants (Solenopsis invicta). Environ. Sci. Pollut. Res. 2022, 30, 1889–1897. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Lee, K.Y. Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives. Agriculture 2022, 12, 378. [Google Scholar] [CrossRef]
- Cruz, E.M.O.; Costa-Junior, L.M.; Pinto, J.A.O.; Santos, D.A.; de Araujo, S.A.; Arrigoni-Blank, M.F.; Bacci, L.; Alves, P.B.; Cavalcanti, S.C.H.; Blank, A.F. Acaricidal activity of Lippia gracilis essential oil and its major constituents on the tick Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2013, 195, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; de Oliveira, B.M.S.; Melo, C.R.; Lima, A.P.S.; Santana, E.D.R.; Blank, A.F.; Picanço, M.C.; Araújo, A.P.A.; Cristaldo, P.F.; Bacci, L. Sub-lethal effects of essential oil of Lippia sidoides on drywood termite Cryptotermes brevis (Blattodea: Termitoidea). Ecotoxicol. Environ. Saf. 2017, 145, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.R.; Picanço, M.C.; Santos, A.A.; Santos, I.B.; Pimentel, M.F.; Santos, A.C.C.; Blank, A.F.; Araújo, A.P.A.; Cristaldo, P.F.; Bacci, L. Toxicity of essential oils of Lippia gracilis chemotypes and their major compounds on Diaphania hyalinata and non-target species. Crop Prot. 2018, 104, 47–51. [Google Scholar] [CrossRef]
- Silva, V.B.; Travassos, D.L.; Nepel, A.; Barison, A.; Costa, E.V.; Scotti, L.; Scotti, M.T.; Mendonça-Junior, F.J.B.; Santos, R.L.C.; Cavalcanti, S.C.H. Synthesis and chemometrics of thymol and carvacrol derivatives as larvicides against Aedes aegypti. J. Arthropod Borne Dis. 2017, 11, 315–330. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; the International Natural Product Sciences Taskforc; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lazarevic, J.S.; Markovic, A.; Smelcerovic, A.; Stojanovic, G.; Ciuffreda, P.; Santaniello, E. Carvacrol derivatives as antifungal agents: Synthesis, antimicrobial activity and in silico studies on carvacryl eters. Acta Chim. Slov. 2022, 69, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Feitosa-Alcantara, R.B.; Bacci, L.; Blank, A.F.; Alves, P.B.; Silva, I.M.A.; Soares, C.A.; Sampaio, T.S.; Nogueira, P.C.L.; Arrigoni-Blank, M.F. Essential oils of Hyptis pectinata chemotypes: Isolation, binary mixtures and acute toxicity on leaf-cutting ants. Molecules 2017, 22, 621. [Google Scholar] [CrossRef]
- Costa, A.C.F.; Cavalcanti, S.C.H.; Santana, A.S.; Lima, A.P.S.; Santana, E.D.R.; Brito, T.B.; Oliveira, R.R.B.; Macêdo, N.A.; Cristaldo, P.F.; Bacci, L. Formicidal activity of indole derivatives on Atta opaciceps (Borgmeier): Lethal, behavioral and locomotive effects. J. Appl. Entomol. 2019, 143, 58–68. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Melo, C.R.; Lima, A.P.S.; Santana, E.D.R.; Oliveira, B.M.S.; Oliviera, J.W.S.; Vieira, J.S.; Blank, A.F.; et al. Apis mellifera (Insecta: Hymenoptera) in the target of neonicotinoids: A one-way ticket? Bioinsecticides can be an alternative. Ecotoxicol. Environ. Saf. 2018, 163, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.G.; Oliveira, B.M.S.; Melo, C.R.; Sampaio, T.S.; Blank, A.F.; Lima, A.D.; Nunes, R.S.; Araújo, A.P.A.; Cristaldo, P.F.; Bacci, L. Lethal effect and behavioral responses of leaf-cutting ants to essential oil of Pogostemon cablin (Lamiaceae) and its nanoformulation. Neotrop. Entomol. 2018, 47, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.M.S.; Melo, C.R.; Santos, A.C.C.; Nascimento, L.F.A.; Nízio, D.A.C.; Cristaldo, P.F.; Blank, A.F.; Bacci, L. Essential oils from Varronia curassavica (Cordiaceae) accessions and their compounds (E)-caryophyllene and α-humulene as an alternative to control Dorymyrmex thoracius (Formicidae: Dolichoderinae). Environ. Sci. Pollut. Res. 2019, 26, 6602–6612. [Google Scholar] [CrossRef]
- Della Lucia, T.M.C.; Amaral, K.D. Past and current strategies for the control of leaf-cutting ants in Brazil. In Forest Pest and Disease Management in Latin America: Modern Perspectives in Natural Forests and Exotic Plantations; Springer: Berlin, Germany, 2020; pp. 31–43. [Google Scholar] [CrossRef]
- Diez, L.; Lejeune, P.; Detrain, C. Keep the nest clean: Survival advantages of corpse removal in ants. Biol. Lett. 2014, 10, 20140306. [Google Scholar] [CrossRef]
- Liang, M.R.; Shuang, Y.M.; Deng, J.F.; Peng, L.Y.; Zhang, S.Q.; Zhang, C.; Xu, Y.J.; Lu, Y.Y.; Wang, L. Toxicity and horizontal transfer of bifenthrin and dimefluthrin against the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae), and the efficacy of their dust applications in the field. J. Integr. Agric. 2023, 22, 1465–1467. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Chen, J.; Zhang, J.; He, Y.; Lu, Y.; Cai, J.; Chen, X.; Wen, X.; Xu, Z.; et al. Toxicity, horizontal transfer, and physiological and behavioral effects of cycloxaprid against Solenopsis invicta (Hymenoptera: Formicidae). Pest Manag. Sci. 2022, 78, 2228–2239. [Google Scholar] [CrossRef]
- Pitts, J.P.; Camacho, G.P.; Gotzek, D.; Mchugh, J.V.; Ross, K.G. Revision of the fre ants of the Solenopsis saevissima species-group (Hymenoptera: Formicidae). Proc. Entomol. Soc. Washington 2018, 120, 308–411. [Google Scholar] [CrossRef]
- Ross, K.G.; Gotzek, D.; Ascunce, M.S.; Shoemaker, D.D. Species delimitation: A case study in a problematic ant taxon. Syst. Biol. 2010, 59, 162–184. [Google Scholar] [CrossRef]
- Mackay, W.; Mackay, E. The Ants of New Mexico (Hymenoptera: Formicidae); The Edwin Mellen Press: Lewiston, NY, USA, 2002. [Google Scholar]
- Coolen, H.K.A.C.; Meeuwis, J.A.M.; Vanleeuwen, P.W.N.M.; Nolte, R.J.M. Substrate selective catalysis by rhodium metal-lohosts. J. Am. Chem. Soc. 1995, 117, 11906–11913. [Google Scholar] [CrossRef][Green Version]
- Dolly, B.B.; Barba, F. Cathodic reduction of hydroxycarbonyl compound trichloroacetyl esters. Tetrahedron 2003, 59, 9161–9165. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; Moura, A.D.A.N.; Sales, A.D.; Rodrigues, A.P.R.; Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.P.; Pinto, J.A.O.; dos Santos, C.A.; Cruz, E.M.O.; Arrigoni-Blank, M.F.; Andrade, T.M.; Santos, D.A.; Alves, P.B.; Blank, A.F. Harvest time and geographical origin affect the essential oil of Lippia gracilis Schauer. Ind. Crops Prod. 2016, 79, 205–210. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, J.E.D.; Santos, A.C.C.; Dantas, J.D.O.; Tavares, S.R.S.A.; Andrade, V.S.; Oliveira, S.D.S.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Bioactivity of essential oils from Croton grewioides and its major compounds: Toxicity to soybean looper Chrysodeixis includens and selectivity to the predatory stink bug Podisus nigrispinus. Environ. Sci. Pollut. Res. 2022, 30, 18798–18809. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.L.; Lima, J.K.; Souza, F.H.; Silva, I.M.; Santos, A.A.; Araújo, A.P.A.; Blank, A.F.; Lima, R.F.; Alves, P.B.; Bacci, L. Insecticidal and repellence activity of the essential oil of Pogostemon cablin against urban ants species. Acta Trop. 2013, 127, 181–186. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl—Uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Oladipupo, S.O.; Hu, X.P.; Appel, A.G. Essential oils in urban insect management-A review. J. Econ. Entomol. 2022, 115, 1375–1408. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Synergistic toxicity interactions between plant essential oil components against the common bed bug (Cimex lectularius L.). Insects 2020, 11, 133. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Rao, Y.; Hong, L.; Zhang, D. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: Muscidae). Environ. Sci. Pollut. Res. 2019, 26, 23824–23831. [Google Scholar] [CrossRef]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Gong, X.; Ren, Y. Larvicidal and ovicidal activity of carvacrol, p-cymene, and γ-terpinene from Origanum vulgare essential oil against the cotton bollworm, Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. 2020, 27, 18708–18716. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Coats, J.R. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol. 2012, 102, 124–128. [Google Scholar] [CrossRef]
- Lu, X.; Weng, H.; Li, C.; He, J.; Zhang, X.; Ma, Z. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020, 147, 112237. [Google Scholar] [CrossRef]
- Natal, C.M.; Fernandes, M.J.G.; Pinto, N.F.; Pereira, R.B.; Vieira, T.F.; Rodrigues, A.R.O.; Pereira, D.M.; Souza, S.F.; Fortes, A.G.; Castanheira, E.M.S.; et al. New carvacrol and thymol derivatives as potential insecticides: Synthesis, biological activity, computational studies and nanoencapsulation. RSC Adv. 2021, 11, 34024–34035. [Google Scholar] [CrossRef]
- Kafle, L.; Shih, C.J. Toxicity and repellency of compounds from clove (Syzygium aromaticum) to red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2013, 106, 131–135. [Google Scholar] [CrossRef]
- Buczkowski, G. Trap–treat–release: Horizontal transfer of fipronil in field colonies of black carpenter ants, Camponotus pennsylvanicus. Pest Manag. Sci. 2019, 75, 2195–2201. [Google Scholar] [CrossRef]
- Adams, C.; Schenker, J.; Weston, P.; Gut, L.; Miller, J. Path meander of male codling moths (Cydia pomonella) foraging for sex pheromone plumes: Field validation of a novel method for quantifying path meander of random movers developed using computer simulations. Insects 2020, 11, 549. [Google Scholar] [CrossRef]
- Melo, C.R.; Blank, A.F.; Oliveira, B.M.S.; Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Bacci, L. Formicidal activity of essential oils of Myrcia lundiana chemotypes on Acromyrmex balzani. Crop Prot. 2021, 139, 105343. [Google Scholar] [CrossRef]





| Compounds | n 1 | LD30 (CI 95%) 2 | LD50 (CI 95%) 2 | LD90 (CI 95%) 2 | Slope ±SE 3 | χ2 | p 4 |
|---|---|---|---|---|---|---|---|
| μg/mg | |||||||
| Essential oil of Lippia gracilis | 336 | 3.42 (2.66–4.14) | 6.11 (5.16–7.20) | 25.2 (19.0–38.3) | 2.08 ±0.23 | 7.41 | 0.12 |
| Carvacrol | 336 | 2.76 (1.81–3.75) | 6.79 (5.19–8.71) | 60.9 (39.2–118) | 1.35 ±0.16 | 0.76 | 0.94 |
| Carvacryl acetate | 392 | 4.09 (3.17–4.94) | 6.92 (5.86–7.97) | 24.9 (20.2–33.5) | 2.30 ±0.24 | 6.39 | 0.27 |
| Carvacryl benzoate | 448 | 2.21 (1.92–2.49) | 3.20 (2.86–3.57) | 7.97 (6.90–9.53) | 3.24 ±0.24 | 4.81 | 0.57 |
| Carvacryl butyrate | 392 | 3.73 (2.67–4.75) | 7.50 (6.09–8.99) | 41.2 (30.5–64.0) | 1.73 ±0.19 | 8.62 | 0.13 |
| Carvacryl ethyl ether | 448 | 3.81 (3.12–4.51) | 6.87 (5.88–8.04) | 29.1 (22.7–40.3) | 2.05 ±0.17 | 7.96 | 0.24 |
| Carvacryl hexanoate | 336 | 6.25 (5.02–7.39) | 10.5 (8.99–12.2) | 37.1 (28.9–53.3) | 2.33 ±0.25 | 6.46 | 0.17 |
| Carvacryl isobutyrate | 336 | 3.46 (2.45–4.40) | 6.54 (5.28–7.84) | 30.9 (23.3–47.4) | 1.90 ±0.22 | 3.54 | 0.47 |
| Carvacryl isovalerate | 392 | 6.16 (3.79–8.18) | 10.8 (8.10–14.5) | 42.3 (26.8–112) | 2.16 ±0.33 | 9.92 | 0.08 |
| Carvacryl pivaloate | 392 | 3.05 (2.41–3.70) | 5.85 (4.88–7.03) | 28.7 (21.4–43.0) | 1.85 ±0.17 | 6.80 | 0.24 |
| Carvacryl trichloroacetate | 336 | 4.05 (3.00–5.12) | 7.92 (6.39–9.66) | 40.7 (30.4–60.7) | 1.80 ±0.18 | 5.99 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas, J.O.; Cavalcanti, S.C.H.; Araújo, A.P.A.; Blank, A.F.; Silva, J.E.; Picanço, M.C.; Lima, E.; Andrade, V.S.; Brito, T.B.; Bacci, L. Synthetic Carvacrol Derivatives for the Management of Solenopsis Ants: Toxicity, Sublethal Effects, and Horizontal Transfer. Agriculture 2023, 13, 1988. https://doi.org/10.3390/agriculture13101988
Dantas JO, Cavalcanti SCH, Araújo APA, Blank AF, Silva JE, Picanço MC, Lima E, Andrade VS, Brito TB, Bacci L. Synthetic Carvacrol Derivatives for the Management of Solenopsis Ants: Toxicity, Sublethal Effects, and Horizontal Transfer. Agriculture. 2023; 13(10):1988. https://doi.org/10.3390/agriculture13101988
Chicago/Turabian StyleDantas, Jaciele O., Sócrates C. H. Cavalcanti, Ana Paula A. Araújo, Arie F. Blank, Jefferson E. Silva, Marcelo C. Picanço, Eraldo Lima, Valfran S. Andrade, Thaysnara B. Brito, and Leandro Bacci. 2023. "Synthetic Carvacrol Derivatives for the Management of Solenopsis Ants: Toxicity, Sublethal Effects, and Horizontal Transfer" Agriculture 13, no. 10: 1988. https://doi.org/10.3390/agriculture13101988
APA StyleDantas, J. O., Cavalcanti, S. C. H., Araújo, A. P. A., Blank, A. F., Silva, J. E., Picanço, M. C., Lima, E., Andrade, V. S., Brito, T. B., & Bacci, L. (2023). Synthetic Carvacrol Derivatives for the Management of Solenopsis Ants: Toxicity, Sublethal Effects, and Horizontal Transfer. Agriculture, 13(10), 1988. https://doi.org/10.3390/agriculture13101988







