Electrophysiological Responses of Curculio elephas (Coleoptera: Curculionidae) to Chestnut Plant Volatiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Volatile Collection of Plant Tissues and Chemical Analysis
2.2.1. Plant Tissues
2.2.2. Chemicals
2.2.3. Volatile Collection
2.2.4. Gas Chromatography–Mass Spectrometry (GC–MS)
2.3. Electrophysiological Responses
2.3.1. Antenna Preparation
2.3.2. Gas Chromatography–Flame Ionization–Electroantennographic Detection (GC–FID–EAD)
2.4. Statistical Analysis
3. Results
3.1. Volatile Analysis
3.1.1. Catkins
3.1.2. Burs
3.1.3. Nuts
3.2. GC-EAD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonal, R.; Munoz, A. Seed growth suppression constrains the growth of seed parasites: Premature acorn abscission reduces Curculio elephas larval size. Environ. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]
- Paparatti, A.; Sperantza, B. Biological control of chestnut weevil (Curculio elephas Gyll. Coleoptera, Curculionidae) with the entomopathogen fungus Beauveria bassiana (Balsam) Vuill. (Deuteromycotina, Hyphomycetes). Acta Hort. 1999, 494, 459–464. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Perlerou, C.; Diamandis, S. Geographic distribution of chestnut feeding insects in Greece. J. Pest Sci. 2013, 86, 185–191. [Google Scholar] [CrossRef]
- Menu, F. Strategies of emergence in the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 96, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Venette, R.C.; Davis, E.E.; Heisler, H.; Larson, M. Mini Risk Assessment Chestnut Weevil, Curculio elephas (Gyllenhal) (Coleoptera: Curculionidae), USDA-APHIS CAPS Pest Risk Assessment. 2003. Available online: https://download.ceris.purdue.edu/file/336 (accessed on 20 April 2023).
- Debouzie, D.; Heizmann, A.; Desouhant, E.; Menu, F. Interference at several temporal and spatial scales between two chestnut insects. Oecologia 1996, 108, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch size manipulations in the chestnut weevil, Curculio elephas: Fitness of oviposition strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef]
- Soula, B.; Menu, F. Variability in diapause duration in the chestnut weevil: Mixed ESS, genetic polymorphism or bet-hedging? Oikos 2003, 100, 574–580. [Google Scholar] [CrossRef]
- Keesey, I.W.; Barrett, B.A.; Lin, C.-H.; Lerch, R.N. Electroantennographic responses of the small chestnut weevil Curculio sayi (Coleoptera: Curculionidae) to volatile organic compounds identified from chestnut reproductive plant tissue. Environ. Entomol. 2012, 4, 933–940. [Google Scholar] [CrossRef]
- Alma, A.; Quacchia, A. Chesnut pests in Chestnut (Castanea sativa): A Multipurpose European Tree. In Proceedings of the Workshop Proceedings, Bruxelles, Belgium, 30 September–1 October 2010. [Google Scholar]
- García-García, C.R.; Parrón, T.; Requena, M.; Alarcón, R.; Tsatsakis, A.M.; Herdández, A.F. Occupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical level. Life Sci. 2016, 145, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.J.; Bartelt, R.J.; Shasha, B.S.; Schuster, D.J.; Riley, D.G.; Stansly, P.A.; Mueller, T.F.; Shuler, K.D.; Hohnson, B.; Davis, J.H.; et al. Aggregation pheromone for the pepper weevil, Anthonomus eugenii cano (Coleoptera: Curculionidae): Identification and field activity. J. Chem. Ecol. 1994, 20, 1537–1555. [Google Scholar] [CrossRef]
- Desouhant, E. Selection of fruits for oviposition by the chestnut weevil, Curculio elephas. Entomol. Exp. Appl. 1998, 86, 71–78. [Google Scholar] [CrossRef]
- Van Tol, R.W.H.M.; Visser, J.H. Olfactory antennal responses of the vine weevil Otiorhynchus sulcatus to plant volatiles. Entomol. Exp. Appl. 2002, 102, 49–64. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, Z.; Malo, E.; Stelinski, L.; Rodriquez-Saona, C. Response of cranberry weevil (Coleoptera: Curculionidae) to host plant volatiles. Environ. Entomol. 2009, 38, 861–869. [Google Scholar] [CrossRef]
- Keesey, I.W.; Barrett, B.A. Behavioral and Electroantennographic Responses of the Lesser Chestnut Weevil, Curculio sayi (Coleoptera: Curculionidae), to Odors Emanating from Different Chestnut Plant Tissues. J. Kans. Entomol. Soc. 2012, 85, 145–154. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Willet, D.S. Phenology and Monitoring of the Lesser Chestnut Weevil. Insects 2022, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, E.; Drizou, F.; Milonas, P.G. Electrophysiological and oviposition responses of Tuta absoluta females to herbivore-induced volatiles in tomato plants. J. Chem. Ecol. 2018, 44, 288–298. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas-Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST Standard Reference Data. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 23 January 2023).
- Keesey, I.W. The Chemical Ecology of the Lesser Chestnut Weevil: Behavioral and Electrophysiological Responses of Curculio sayi (Coleoptera: Curculionidae) to Host-Plant Volatile Organic Compounds. Ph.D. Dissertation, University of Missouri, Columbia, MO, USA, May 2011. [Google Scholar]
- Pinto-Zevallos, D.M.; Strapasson, P.; Zarbin, P.H.G. Herbivore-induced volatile organic compounds emitted by maize: Electrophysiological responses in Spodoptera frugiperda females. Phytochem. Lett. 2016, 16, 70–74. [Google Scholar] [CrossRef]
- El Chami, M.A.; Tourvas, N.; Kazakis, P.; Aravanopoulos, F.A. Genetic characterization of chestnut cultivars in Crete. Forests 2021, 12, 1659. [Google Scholar] [CrossRef]
- Allisandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C. Investigation of organic extractives from unifloral chestnut (Castanea sativa L.) and eucalyptus (Eucalyptus globulus Labill.) honeys and flowers to identification of botanical marker compounds. LWT-Food Sci. Technol. 2011, 44, 1042–1051. [Google Scholar] [CrossRef]
- Machado, A.M.; Antunes, M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Volatile Profile of Portuguese Monofloral Honeys: Significance in Botanical Origin Determination. Molecules 2021, 26, 4970. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Battesti, M.-J.; Djabou, N.; Muselli, A.; Paolini, J.; Tomi, P.; Costa, J. Melissopalynological origin determination and volatile composition analysis of Corsican “chestnut grove” honeys. Food Chem. 2012, 132, 2144–2154. [Google Scholar] [CrossRef]
- Mujić, I.; Zivković, J.; Savić, V.; Alibabić, V.; Staver, M.; Jug, T.; Franić, M.; Damijanić, K. Analysis of volatile compounds in chestnut using solid-phase microextraction coupled with GC-MS. Acta Hortic. 2018, 1220, 203–208. [Google Scholar] [CrossRef]
- Cirlini, M.; Dall’Asta, C.; Silvanini, A.; Beghè, D.; Fabbri, A.; Galaverna, G.; Ganini, T. Volatile fingerprinting of chestnut flours from traditional Emilia Romagna (Italy) cultivars. Food Chem. 2012, 134, 662–668. [Google Scholar] [CrossRef]
- Moure, A.; Cone, E.; Falqué, E.; Domínguez, H.; Parajó, J.C. Production of nutraceutics from chestnut burs by hydrolytic treatment. Food Res. Int. 2014, 65, 359–366. [Google Scholar] [CrossRef]
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile compound analysis of SPME headspace and extract samples from roasted Italian chestnuts (Castanea sativa Mill.) using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473. [Google Scholar] [CrossRef]
- Barlet, E.; Blight, M.M.; Plane, P.; Willimas, I.H. The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol. Exp. Appl. 1997, 85, 257–262. [Google Scholar] [CrossRef]
- Pawlowski, S.P.; Sweeney, J.D.; Hillier, N.K. Electrophysiological Responses of the Beech Leaf-Mining Weevil, Orchestes fagi, to Seasonally-Variant Volatile Organic Compounds Emitted by American Beech, Fagus grandifolia. J. Chem. Ecol. 2020, 46, 935–946. [Google Scholar] [CrossRef]
- Toshova, T.B.; Velchev, D.I.; Subchev, M.A.; Toth, M.; Vuts, J.; Picjett, J.A.; Dewhirst, S.Y. Electrophysiological responses and field attraction of the grey corn weevil, Tanymecus (Episomecus) dilaticollis Gyllenhal (Coleoptera: Curculionidae) to synthetic plant volatiles. Chemoecology 2010, 20, 199–206. [Google Scholar] [CrossRef]
- Wee, S.-L.; El-Sayes, A.M.; Gibb, A.R.; Mitchell, V.; Suckling, D.M. Behavioural and electrophysiological responses of Pantomorus cervinus (Boheman) (Coleoptera: Curculionidae) to host plant volatiles. Aust. J. Entomol. 2008, 47, 24–31. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed]
- Germinara, G.; De Cristofaro, A.; Rotundo, G. Chemical cues for host location by chestnut gall wasp, Dryocosmus kuriphilus. J. Chem. Ecol. 2011, 37, 49–56. [Google Scholar] [CrossRef]
- Kant, M.; Bleeker, P.M.; Van Wijk, M.; Schhrink, R.C.; Haring, M. Plant Volatiles in Defence. In Advances in Botanical Research; van Loon, L.C., Ed.; Academic Press: London, UK, 2009; Volume 51, pp. 613–666. [Google Scholar] [CrossRef]
- NinKuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpene and Recent Advances in Plant Protection. Int. L Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Mutis, A.; Parra, L.; Monosalva, L.; Palma, R.; Cnadia, O.; Lizama, M.; Pardo, F.; Perich, F.; Quiro, A. Electroantennographic and Behavioral Responses of Adults of Raspberry Weevil Aegorhinus superciliosus (Coleoptera: Curculionidae) to Odors Released from Conspecific Females. Environ. Entomol. 2010, 39, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Mozūraitis, R.; Hall, D.; Trandem, N.; Ralle, B.; Tunstrom, K.; Sigsgaard, L.; Baroffio, C.; Fountain, M.; Cross, J.; Wibe, A.; et al. Composition of Strawberry Floral Volatiles and their Effects on Behavior of Strawberry Blossom Weevil, Anthonomus rubi. J. Chem. Ecol. 2020, 46, 1069–1081. [Google Scholar] [CrossRef]
- Roberts, J.M.; Kundun, J.; Rowley, C.; Hall, D.R.; Douglas, P.; Pope, T.W. Electrophysiological and Behavioral Responses of Vine Weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae), Adults to Host Plant Odors. J. Chem. Ecol. 2019, 45, 858–868. [Google Scholar] [CrossRef]
- Bichão, H.; Borg-Karlson, A.-K.; Araújo, J.; Mustaparta, H. Five Types of Olfactory Receptor Neurons in the Strawberry Blossom Weevil Anthonomus rubi: Selective Responses to Inducible Host-plant Volatiles. Chem. Senses 2005, 30, 153–170. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects-finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
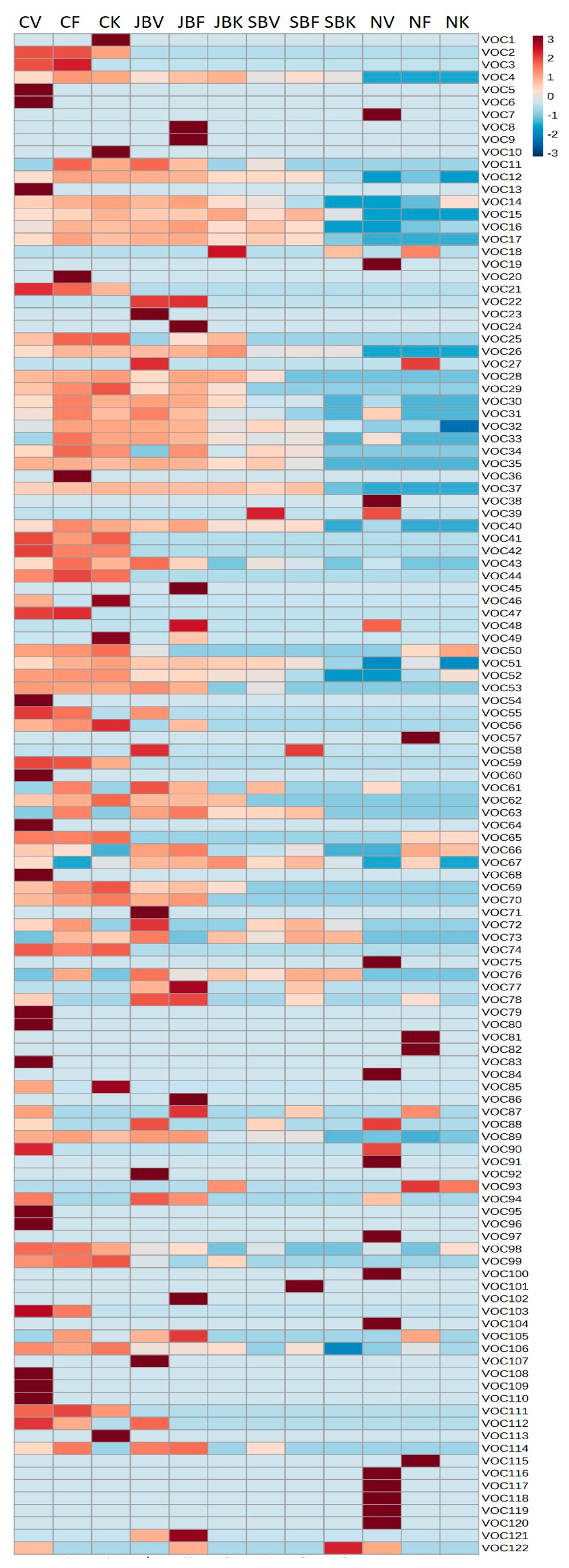








| Catkins | Burs | Nuts | F | M | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | RIl 1 | RIc 2 | Compound | CV | CF | CK | JBV | JBF | JBK | SBV | SBF | SBK | NV | NF | NK | EAD Active | |
| (n = 7) | (n = 5) | ||||||||||||||||
| 1 | 797 | 798 | (Z)-3-Hexenal | ● 3 | |||||||||||||
| 2 | 802 | 798 | Ethyl butanoate | ● | ● | ● | |||||||||||
| 3 | 846 | 841 | Ethyl 2-methylbutanoate | ● | ● | ||||||||||||
| 4 | 850 | 849 | (Z)-3-Hexen-1-ol | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ 4 | √ | |||
| 5 | 854 | 852 | (Z)-2-Hexen-1-ol | ● | |||||||||||||
| 6 | 863 | 860 | n-Hexanol | ● | |||||||||||||
| 7 | 864 | 868 | ο-Xylene | ● | |||||||||||||
| 8 | 894 | 889 | 1-Nonene | ● | |||||||||||||
| 9 | 900 | 900 | n-Nonane | ● | |||||||||||||
| 10 | 911 | 913 | n-Amyl acetate | ● | |||||||||||||
| 11 | 924 | 925 | α-thujene | ● | ● | ● | ● | ● | |||||||||
| 12 | 932 | 929 | α-pinene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | ||
| 13 | 938 | 935 | Ethyl tiglate | ● | |||||||||||||
| 14 | 946 | 947 | Camphene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | |||
| 15 | 969 | 970 | Sabinene | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | |||
| 16 | 974 | 978 | β-pinene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | |||
| 17 | 988 | 988 | β-Myrcene | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | |||
| 18 | 981 | 988 | 6-Methyl-5-hepten-2-one | ● | ● | ● | |||||||||||
| 19 | 995 | 990 | 2,2,4,6,6-Pentamethyl-heptane | ● | |||||||||||||
| 20 | 993 | 993 | Butyl butyrate | ● | |||||||||||||
| 21 | 997 | 998 | Ethyl hexanoate | ● | ● | ● | |||||||||||
| 22 | 1000 | 1000 | n-decane | ● | ● | ||||||||||||
| 23 | 1001 | 1002 | 2-δ-carene | ● | |||||||||||||
| 24 | 1002 | 1004 | α-Phellandrene | ● | |||||||||||||
| 25 | 1001 | 1006 | (Ε)-3-Hexenyl acetate | ● | ● | ● | ● | ● | ● | ||||||||
| 26 | 1004 | 1008 | (Z)-3-Hexenyl acetate | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | |||
| 27 | 1008 | 1010 | 3-δ-carene | ● | ● | ||||||||||||
| 28 | 1010 | 1013 | (E)-2-Hexenyl acetate | ● | ● | ● | ● | ● | ● | ● | |||||||
| 29 | 1007 | 1014 | Hexyl acetate | ● | ● | ● | ● | ● | ● | ||||||||
| 30 | 1014 | 1016 | α-Terpinene | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 31 | 1020 | 1025 | p-cymene | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 32 | 1030 | 1029 | Limonene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| 33 | 1031 | 1030 | β-phelladrene | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 34 | 1026 | 1031 | eucalyptol | ● | ● | ● | ● | ● | ● | ● | |||||||
| 35 | 1032 | 1035 | (Z)-β-Ocimene | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| 36 | 1037 | 1039 | Benzyl alcohol | ● | |||||||||||||
| 37 | 1044 | 1048 | (E)-β-Ocimene | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | |||
| 38 | 1052 | Alkane 1 | ● | ||||||||||||||
| 39 | 1055 | Unknown 1 | ● | ● | |||||||||||||
| 40 | 1059 | 1059 | γ-Terpinene | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | ||||
| 41 | 1061 | 1062 | α-Methyl Benzenemethanol | ● | ● | ● | |||||||||||
| 42 | 1059 | 1069 | Acetophenone | ● | ● | ● | |||||||||||
| 43 | 1086 | 1085 | α-terpinolene | ● | ● | ● | ● | ● | ● | ● | ● | √ | |||||
| 44 | 1084 | 1087 | (E)-Linalool oxide (furanoid) | ● | ● | ● | |||||||||||
| 45 | 1092 | 1092 | 1-Undecene | ● | |||||||||||||
| 46 | 1099 | 1096 | α-Pinene oxide | ● | ● | ||||||||||||
| 47 | 1099 | 1097 | Methyl benzoate | ● | ● | ||||||||||||
| 48 | 1100 | 1100 | Undecane | ● | ● | ||||||||||||
| 49 | 1095 | 1101 | (Ζ)-3-Hexenyl propanoate | ● | ● | ||||||||||||
| 50 | 1101 | 1101 | Linalool | ● | ● | ● | ● | ● | ● | ||||||||
| 51 | 1100 | 1107 | n-Nonanal | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| 52 | 1117 | 1117 | (E)-DMNT | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| 53 | 1128 | 1128 | (Ε)-Allo-ocimene | ● | ● | ● | ● | ● | ● | ||||||||
| 54 | 1130 | 1132 | (E,E)-Cosmene | ● | |||||||||||||
| 55 | 1140 | 1141 | neo-allo-ocimene | ● | ● | ● | |||||||||||
| 56 | 1142 | 1143 | (Z)-3-hexenyl isobutanoate | ● | ● | ● | ● | ||||||||||
| 57 | 1150 | 1152 | Camphor | ● | |||||||||||||
| 58 | 1163 | 1168 | p-Ethylbenzaldehyde | ● | ● | ||||||||||||
| 59 | 1169 | 1173 | Ethyl benzoate | ● | ● | ● | |||||||||||
| 60 | 1184 | 1184 | (Z)-Cinerone | ● | |||||||||||||
| 61 | 1184 | 1184 | 4-Terpineol | ● | ● | ● | ● | ● | |||||||||
| 62 | 1187 | 1187 | (Z)-3-Hexenyl butanoate | ● | ● | ● | ● | ● | ● | √ | |||||||
| 63 | 1187 | 1192 | n-dodecene | ● | ● | ● | ● | ● | ● | ||||||||
| 64 | 1191 | 1193 | Hexyl butanoate | ● | |||||||||||||
| 65 | 1195 | 1195 | Methyl salicylate | ● | ● | ● | ● | ● | ● | ● | ● | √ | √ | ||||
| 66 | 1200 | 1199 | n-Dodecane | ● | ● | ● | ● | ● | ● | ● | ● | ● | √ | ||||
| 67 | 1209 | 1209 | Decanal | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| 68 | 1209 | 1211 | (3E,5E)-2,6-Dimethylocta-3,5,7-trien-2-ol | ● | |||||||||||||
| 69 | 1229 | 1232 | (Z)-3-Hexenyl 2-methyl butanoate | ● | ● | ● | ● | ● | ● | ||||||||
| 70 | 1237 | 1237 | (Ζ)-2-hexenyl isovalerate | ● | ● | ● | ● | ● | |||||||||
| 71 | 1247 | Unknown 2 | ● | ||||||||||||||
| 72 | 1258 | Unknown 3 | ● | ● | ● | ● | ● | ● | |||||||||
| 73 | 1267 | m-Ethylacetophenone | ● | ● | ● | ● | ● | ● | ● | ||||||||
| 74 | 1270 | 1271 | Ethyl salicylate | ● | ● | ● | |||||||||||
| 75 | 1272 | Alkane 2 | ● | ||||||||||||||
| 76 | 1279 | 1288 | p-Ethylacetophenone | ● | ● | ● | ● | ● | ● | ● | |||||||
| 77 | 1290 | 1291 | n-Tridecene | ● | ● | ● | |||||||||||
| 78 | 1300 | 1300 | n-Tridecane | ● | ● | ● | ● | ● | |||||||||
| 79 | 1319 | 1324 | (Z)-Hex-3-enyl (E)-2-methylbut-2-enoate | ● | |||||||||||||
| 80 | 1335 | 1330 | δ-Elemene | ● | |||||||||||||
| 81 | 1348 | alkane 3 | ● | ||||||||||||||
| 82 | 1374 | ester | ● | ||||||||||||||
| 83 | 1374 | 1374 | α-Copaene | ● | |||||||||||||
| 84 | 1374 | 1376 | longicyclene | ● | |||||||||||||
| 85 | 1378 | 1381 | (Z)-3-hexenyl hexanoate | ● | |||||||||||||
| 86 | 1388 | 1393 | 1-Tetradecene | ● | |||||||||||||
| 87 | 1400 | 1400 | n-Tetradecane | ● | ● | ● | ● | ||||||||||
| 88 | 1407 | 1409 | Longifolene | ● | ● | ● | ● | ||||||||||
| 89 | 1419 | 1419 | (E)-β-Caryophyllene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| 90 | 1419 | 1425 | β-Cedrene | ● | ● | ||||||||||||
| 91 | 1429 | 1437 | cis-Thujopsene | ● | |||||||||||||
| 92 | 1444 | 1438 | p-Acetylacetophenone | ● | |||||||||||||
| 93 | 1453 | 1450 | (E)-Geranylacetone | ● | ● | ● | |||||||||||
| 94 | 1452 | 1457 | α-Humulene | ● | ● | ● | ● | ||||||||||
| 95 | 1458 | 1459 | allo-Aromadendrene | ● | |||||||||||||
| 96 | 1462 | 1462 | cis-Muurola-4(15),5-diene | ● | |||||||||||||
| 97 | 1476 | terpene | ● | ||||||||||||||
| 98 | 1484 | 1480 | Germacrene D | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| 99 | 1493 | 1490 | α-Zingiberene | ● | ● | ● | ● | ● | |||||||||
| 100 | 1489 | 1491 | β-Selinene | ● | |||||||||||||
| 101 | 1491 | sesquiterpene | ● | ||||||||||||||
| 102 | 1493 | 1493 | 1-Pentadecene | ● | |||||||||||||
| 103 | 1494 | 1495 | Bicyclogermacrene | ● | ● | ||||||||||||
| 104 | 1498 | 1497 | α-Selinene | ● | |||||||||||||
| 105 | 1500 | 1500 | Pentadecane | ● | ● | ● | ● | ● | |||||||||
| 106 | 1505 | 1504 | (Ε,Ε)-α-Farnesene | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| 107 | 1505 | 1505 | β-bisabolene | ● | |||||||||||||
| 108 | 1513 | 1513 | γ-Cadinene | ● | |||||||||||||
| 109 | 1522 | 1518 | δ-Cadinene | ● | |||||||||||||
| 110 | 1561 | 1562 | (E)-Nerolidol | ● | |||||||||||||
| 111 | 1565 | 1574 | (Z)-3-Hexenyl benzoate | ● | ● | ● | |||||||||||
| 112 | 1577 | 1579 | Spathulenol | ● | ● | ● | |||||||||||
| 113 | 1597 | sesquiterpene | ● | ||||||||||||||
| 114 | 1582 | 1583 | Caryophyllene oxide | ● | ● | ● | ● | ● | |||||||||
| 115 | 1600 | 1600 | n-hexadecane | ● | |||||||||||||
| 116 | 1640 | 1634 | Hinesol | ● | |||||||||||||
| 117 | 1637 | terpene | ● | ||||||||||||||
| 118 | 1652 | 1659 | α-Eudesmol | ● | |||||||||||||
| 119 | 1700 | 1701 | Heptadecane | ● | |||||||||||||
| 120 | 1800 | 1800 | Octadecane | ● | |||||||||||||
| 121 | 1807 | 1807 | 2-Ethylhexyl salicylate | ● | ● | ||||||||||||
| 122 | 1828 | 1830 | Isopropyl myristate | ● | ● | ● | ● | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasaki, E.; Psoma, A.; Toufexi, S.; Partsinevelos, G.; Papachristos, D.; Avtzis, D.; Milonas, P. Electrophysiological Responses of Curculio elephas (Coleoptera: Curculionidae) to Chestnut Plant Volatiles. Agriculture 2023, 13, 1991. https://doi.org/10.3390/agriculture13101991
Anastasaki E, Psoma A, Toufexi S, Partsinevelos G, Papachristos D, Avtzis D, Milonas P. Electrophysiological Responses of Curculio elephas (Coleoptera: Curculionidae) to Chestnut Plant Volatiles. Agriculture. 2023; 13(10):1991. https://doi.org/10.3390/agriculture13101991
Chicago/Turabian StyleAnastasaki, Eirini, Aikaterini Psoma, Savvina Toufexi, Georgios Partsinevelos, Dimitrios Papachristos, Dimitrios Avtzis, and Panagiotis Milonas. 2023. "Electrophysiological Responses of Curculio elephas (Coleoptera: Curculionidae) to Chestnut Plant Volatiles" Agriculture 13, no. 10: 1991. https://doi.org/10.3390/agriculture13101991





