Journey of Trichoderma from Pilot Scale to Mass Production: A Review
Abstract
:1. Introduction
2. Trichoderma Serves as a Biocontrol Agent
| Name of Trichoderma Species | Name of Plant Pathogens | Crop Name | Inhibition/Efficacy (%) | Experiment Condition | References |
|---|---|---|---|---|---|
| T. harzianum | Sclerotium rolfsii and Rhizoctonia solani | Ryegrass | 42–47 | Greenhouse and field condition | [51] |
| T. harzianum | Phytophthora cinnamon | Pine | 28.5–37.5 | Greenhouse | [52] |
| T. hamatum and T. harzianum | P. cinnamomi | Avocado | Greenhouse | [53] | |
| T. viride, T. virens T. harzianum, T. pseudokoningii T. koningii | Aspergillus niger, Rhizoctonia solani and Geotrichum candidum | Sapodilla (Manilkara zapota L.) | 54–74 | Laboratory | [54] |
| T. harzianum strain Ths97 | F. solani | Olive trees | 25–50 | - | [55] |
| Trichoderma viride 1433 Mutant strains | Pythium aphanidermatum | Mustard | 85 | Lab and field | [56] |
| Trichoderma spp. | Pythium aphanidermatum | Chilli | 88.00 | Lab and field | [57] |
| Trichoderma harzianumRifai, Trichoderma viride | Pythium aphanidermatum | Tobacco | 100 | Lab and greenhouse | [58] |
| Trichoderma virens Trichoderma harzianum, Trichoderma viride | Phomopsis vexans | Brinjal | 38–49 | Lab | [59] |
| Trichoderma harzianum T22 | Alternaria alternata | Sunflower | 75 | Lab | [60] |
| T. atrobrunneum | Armillaria mellea | Strawberry | 91 | Field | [61] |
| Trichoderma harzianum | Fusarium moniliforme | Maize | 73.33 | Lab | [62] |
| T. viride, Gliocladium virens | Rhizoctonia solani | Rice | 67.94% and 68.62% respectively | Lab | [63] |
| S.No. | Identified Genes | Trichoderma Species | Function | References |
|---|---|---|---|---|
| 1 | Tvsp1 | T. virens | This gene protects cotton seedlings against Rhizoctonia solani. | [64,65] |
| 2 | Tag3 | T. asperellum | Responsible for glucanase production for cell wall degradation. | [66] |
| 3 | TgaA and TgaB | T. virens | Bicontrol efficacy for management of R. solani and Sclerotium rolfsii. | [67] |
| 4 | ThPG1 | T. harzianum | This gene is required for beneficial interaction between Trichoderma harzianum and the host. | [68] |
| 5 | ThPRT2 | T. harzianum | Mycoparasitism activity for Botrytis cinerea. | [69] |
| 6 | tri5 | T. brevicompactum IBT40841 | Production of antifungal activity and trichodermin against fungus causing infection in the human body (Candida spp. and Aspergillus fumigatus). | [70] |
| 7 | erg1 | T. harzianum CECT 2413 | Ergosterol biosynthetic pathway (EBP). | [71] |
| 8 | TvGST | T. virens | This gene provides enhanced tolerance against cadmium stress. | [72] |
| 9 | TrCCD1 | T. reesei | This gene facilitates pigment production and hyphal growth. | [73] |
| 10 | egl1 | T. longibrachiatum | Exhibits antagonistic activity against Pythium ultimum. | [74] |
| 11 | qid74 | T. harzianum | Plant biofertilization and root architecture. | [75] |
| 12 | Taabc2 | T. atroviride | ATP binding cassette transporter plays a major role in cell membranes. | [76] |
| 13 | tac1 | T. virens IMI 304061 | This gene shows mycoparasitism against S. rolfsii and R. solani. | [77] |
| 14 | TrCCD1 | T. reesei | This gene promotes condia formation and elongation of fungal hyphae. | [73] |
| 15 | gluc78 w | T. atroviride | Cell wall disintegartion of Pythium and Rhizoctonia spp. | [78] |
| 16 | SL41 | T. harzinum | Showed mycoparasitic action. | [79] |
| 17 | Taabc2 | T. atroviride | This gene is important for biological control of necrotrophs (B. cinerea and R. solani). | [76] |
| 18 | Monooxygenase | T. hamatum | This gene shows antagonistic activity against Sclerotinia sclerotiourum and S. cepivorum. | [80] |
| 19 | Xl1 | Trichoderma strain Y | This gene is helpful in hemicellulose breakdown. | [81] |
| 20 | eg1, β-1,4 glucanase | T longibrachiatum | This gene shows antagonistic activity against Pythium ultimum in cucumber. | [82] |
| 21 | pacC | T. virens | This gene acts as myctorphy. | [83] |
| 22 | tvhydii1 | T. reesei | Important in mycoparasitism and plant-fungus interaction. | [84] |
| 23 | hmgR | T. koningii | These genes inhibit the pathogen Rhizoctonia solani. | [85] |
| 24 | Tasx1 | T. asperellum | This gene plays a key role in morphological development, mycoparasitism, and antibiosis. | [86] |
| 25 | gpr1 | T. atroviride | This gene is required for the stability of cell walls and hyphal growth. | [87] |
| 26 | TvCyt2 | T. virens | Trichoderma–Arabidopsis interaction | [88] |
| 27 | gluc31 | T. harzianum | Mycoparasitism ability and influence cell wall organization. | [89] |
| 28 | ipa-1 | T. virens | Antibiosis of R. solani. | [90] |
| 29 | TasXyn24.2, TasXyn29.4 | T. asperellum | Induced resistance and promoted growth in seedlings. | [91] |
| 30 | agl1 | T. atroviride | Biological control of plant pathogen. | [92] |
3. Biocontrol Properties of Trichoderma against Phytopathogens
3.1. Mycoparasitism
3.2. Antibiosis
3.3. Competition
3.4. Production of Antibiotics and Other Antifungal Compounds
3.5. Induced Systemic Resistance
4. Trichoderma Selective Medium
5. Methods for Isolation of Trichoderma
6. Identification
7. Preservation of Trichoderma Cultures
8. Estimation of Colony Forming Units (cfu)
9. How to Find a New Bio Control Agent?
10. Use of Carriers
11. Pilot Experiments
12. Types of Formulations
12.1. Liquid Formulations
12.2. Solid Formulations
12.3. Talc Based Formulation
12.4. Vermiculite–Wheat Bran-Based Formulation
12.5. Oil-Based Formulations
12.6. Sodium Alginate Encapsulation of Trichoderma
12.7. Press Mud-Based Formulation
12.8. Coffee Husk-Based Formulation
12.9. Banana Waste-Based Formulations
12.10. Dry Flowable Formulation
12.11. Sawdust-Based Formulation
| S.No. | Substrate | Composition/Components | Efficacy | References |
|---|---|---|---|---|
| 1 | Cereals (wheat, moong, maize) | Grains (200 g + sugar (1%) + Trichoderma harzianum | 12.96% | [216] |
| 2 | Vermicompost fortified with Trichoderma | Vermicompost + cereals + pulses | Reduction of 10.01%incidence of wilt in chili | [216] |
| 3 | Wheat seeds-based formulation | Grinded grain + sugar solution (1%) + Trichoderma harzianum | 38 × 107 cfu/g | [217] |
| 4 | Cow dung enriched formulation | Decomposed cow dung + Trichoderma formulation | 37.5 × 107 cfu/g | [217] |
| 5 | Talc-based formulation | Talcum powder + CMC + Trichoderma culture | 37 × 107 cfu/g | [217] |
| 6 | Sorghum grain | Partial crushed grain + sugar (1%) solution + distilled water | 6.1 × 104 cfu/g | [218] |
| 7 | Wood pellets-based formulation | Beech, fir, and chestnut + conidial suspensions of T. atroviride + distilled water + soy flour | Growth increase by ten-fold | [219] |
| 8 | Vermiculite–wheat bran-based formulation | Vermiculite (100 g) + wheat bran (33 g) + fermented Trichoderma biomass (20 g) + 0.05N HCl (175 mL) | - | [202] |
| 9 | Vermiculite bentonite-based formulation | Oat (20 g) + bentonite (50 mL) + vermiculite + T. harzianum + water (60 mL) | Maintained cfu after 8 weeks | [220] |
| 10 | Coffee husk-based formulation | Coffee fruit skin decomposed with cow dung + poultry manure + T. harzianum suspension | 9 × 1011 to 3 × 1012 cfu/g substrate | [178] |
| 11 | Oil-based formulation | Glycerol (1%), PVP (1%), Tween 20 (1%) as an emulsifying agent, ZnSO4 (0.5%) to increase the shelf-life, coconut oil, and distilled water | More than 180 days | [221] |
| 12 | Pesta granules-based formulation | Wheat flour (100 g) + fermenter biomass + sterile water (52 mL) | Viable for a long time | [222] |
| 13 | Banana waste-based | Banana waste (chopped 5–6 cm length) + rock phosphate | Six months | [207,223] |
| 14 | Trichoderma sodium alginate encapsulation | Trichoderma suspension + sodium alginate (0.6%) solution + CaCl2 (1.5%) | Viable for more than six years at room temperature | [163] |
| 15 | Wheat flour- kaolin | Wheat flour (80 gm) + kalolin (20 gm) + fermenter biomass (52 mL) | Few months | [224] |
| 16 | T2- liquid formulation (NIPHM medium) | Liquid formulation (NIPHM medium) Trichoderma filtrate (250 mL) + water (750 mL) + glycerol (3%) | - | [225] |
| 17 | Trichoderma-based compost activator | Fifty grams of soil + rice straw (5 g) + Trichoderma biomass (500 mg) | 5.3 × 1010 cfu/g | [226] |
| 18 | Graphite and silica-based formulation | B. subtilis and T. harzianum + 5% graphite 80 mesh + 1% silica NPs | 35–54% efficacy (in vitro) | [227] |
| 19 | Chitosan-PEG based formulation | Chitosan-PEG + T. harzianum spores + glacial acetic acid (0.1%) | More than six months | [228] |
| 20 | Rice powder-based formulation | Sterilized rice powder + dextrose + talc powder + Trichoderma viride | cfu/g 10 × 109 up to six months at room temperature | [229] |
| 21 | Dextrin-based formulation | T. harzianum filtrate (500 g) + Paraffin oil (500 mL) + CMC (0.2%) + chitosan (0.1%) | Efficacy 26.10%; 4.33 × 107 cfu/g) for six months | [230] |
| 22 | Oil-based liquid formulation | T. asperellum + paraffin oil | 28.67 × 108 cfu/mL for 30 days | [231] |
| 23 | Glycerol based formulation | Molasses yeast extract (MYE) medium+ glycerol (3%) (V/V) +T. harzianum + Talc powder | Extended the shelf-life for 7 to 12 months | [232] |
| 24 | Paste formulation of T. harzianum | Starch (10%) + copper sulphate (20 ppm) + T. harzianum | 11.6 × 1010 cfu/g for 120 days | [233] |
13. Exemplary Properties of Trichoderma Formulation
14. Quality Control Parameters
15. Methods of Application of Trichoderma
15.1. Seed Treatment
15.2. Seed Biopriming
15.3. Root Dipping
15.4. Foliar Spraying/Wound Dressing
15.5. Seed Material Treatment
15.6. Soil Application
15.7. Cutting/Seedling Root Dip Application
15.8. Nursery Bed Treatment
15.9. Soil Drenching
16. Other Applications of Trichoderma
17. Major Challenges and Future Strategies on Biopesticides
18. Constraints in the Development of Trichoderma-Based Biopesticide
19. Future Prospects of Trichoderma Application
20. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Larralde-Corona, C.P.; Santiago-Mena, M.R.; Sifuentes-Rincón, A.M.; Rodríguez-Luna, I.C.; Rodríguez-Pérez, M.A.; Shirai, K.; Narváez-Zapata, J.A. Biocontrol potential and polyphasic characterization of novel native Trichoderma strains against Macrophomina phaseolina isolated from sorghum and common bean. Appl. Microbiol. Biotechnol. 2008, 80, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.H.P.; Parra, J.R.P.; Bettiol, W.; Lenteren, J.v. Biological control in Brazil. In Biological Control in Latin America and the Caribbean: Its Rich History and Bright Future; CABI: Wallingford, UK, 2020; pp. 78–107. [Google Scholar]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. The Role of Cell Wall Degrading Enzymes in Antagonistic Traits of Trichoderma virens Against Rhizoctonia solani. Iran. J. Biotechnol. 2020, 18, e2333. [Google Scholar]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Monte, E.; Bettiol, W.; Hermosa, R. Trichoderma e seus mecanismos de ação para o controle de doenças de plantas. In Trichoderma: Usos na Agricultura; Meyer, M.C., Mazaro, S.M., da Silva, J.C., Eds.; Embrapa Soja: Brasília, Brazil, 2019; pp. 181–200. [Google Scholar]
- De Rezende, L.C.; de Andrade Carvalho, A.L.; Costa, L.B.; de Almeida Halfeld-Vieira, B.; Silva, L.G.; Pinto, Z.V.; Morandi, M.A.B.; de Medeiros, F.H.V.; Mascarin, G.M.; Bettiol, W. Optimizing mass production of Trichoderma asperelloides by submerged liquid fermentation and its antagonism against Sclerotinia sclerotiorum. World J. Microbiol. Biotechnol. 2020, 36, 113. [Google Scholar] [CrossRef]
- Harman, G.; Jin, X.; Stasz, T.; Peruzzotti, G.; Leopold, A.; Taylor, A. Production of conidial biomass of Trichoderma harzianum for biological control. Biol. Control 1991, 1, 23–28. [Google Scholar] [CrossRef]
- Mawar, R.; Manjunatha, B.; Kumar, S. Commercialization, diffusion and adoption of bioformulations for sustainable disease management in indian arid agriculture: Prospects and challenges. Circ. Econ. Sust. 2021, 1, 1367–1385. [Google Scholar] [CrossRef] [PubMed]
- Howell, C. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Papavizas, G. Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol. Annu. Rev. Phytopathol. 1985, 23, 23–54. [Google Scholar] [CrossRef]
- Persoon, C.H. Disposita methodical fungorum. Romers. Neues. Mag. Bot. 1794, 1, 81–128. [Google Scholar]
- Tulasne, L.-R.; Tulasne, C. Selecta Fungorum Carpologia, 2; Imperial: Paris, France, 1863. [Google Scholar]
- Weindling, R. Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Weindling, R. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 1934, 24, 1153–1179. [Google Scholar]
- Gutter, Y. Effect of light in sporulation of Trichoderma viride. Bull. Res. Council Israel Sect. D 1957, 5, 273–286. [Google Scholar]
- Wells, H.D. Efficacy of Trichoderma harzianum as a biocontrol for Sclerotium rolfsii. Phytopathology 1972, 62, 442. [Google Scholar] [CrossRef]
- Montenecourt, B.S.; Eveleigh, D.E. Selective screening methods for the isolation of high yielding cellulase mutants of Trichoderma reesei. Adv. Chem. 1979, 181, 289–301. [Google Scholar]
- Shoemaker, S.; Schweickart, V.; Ladner, M.; Gelfand, D.; Kwok, S.; Myambo, K.; Innis, M. Molecular cloning of exo–cellobiohydrolase I derived from Trichoderma reesei strain L27. Biotechnology 1983, 1, 691–696. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, Y.-C.; Baker, R. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986, 70, 145–148. [Google Scholar] [CrossRef]
- Penttilä, M.; Nevalainen, H.; Rättö, M.; Salminen, E.; Knowles, J. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 1987, 61, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Harkki, A.; Uusitalo, J.; Bailey, M.; Penttilä, M.; Knowles, J.K. A novel fungal expression system: Secretion of active calf chymosin from the filamentous fungus Trichoderma reesei. Biotechnology 1989, 7, 596–603. [Google Scholar] [CrossRef]
- Inbar, J.; Chet, I. Biomimics of fungal cell-cell recognition by use of lectin-coated nylon fibers. J. Bacteriol. 1992, 174, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Geremia, R.A.; Goldman, G.H.; Jacobs, D.; Ardrtes, W.; Vila, S.B.; Van Montagu, M.; Herrera-Estrella, A. Molecular characterization of the proteinase-encoding gene, prb1, related to mycoparasitism by Trichoderma harzianum. Mol. Microbiol. 1993, 8, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Bigirimana, J.; De Meyer, G.; Poppe, J.; Elad, Y.; Höfte, M. Induction of systemic resistance on bean (Phaseolus vulgaris) by Trichoderma harzianum. Meded. Fac. Landbouwkd. Toegepaste Biol. Wet. Univ. Gent. 1997, 62, 1001–1007. [Google Scholar]
- Cortes, C.; Gutierrez, A.; Olmedo, V.; Inbar, J.; Chet, I.; Herrera-Estrella, A. The expression of genes involved in parasitism by Trichoderma harzianum is triggered by a diffusible factor. Mol. Gen. Genet. 1998, 260, 218–225. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Rocha-Ramírez, V.; Omero, C.; Chet, I.; Horwitz, B.A.; Herrera-Estrella, A. Trichoderma atroviride G-protein α-subunit gene tga1 is involved in mycoparasitic coiling and conidiation. Eukaryot. Cell 2002, 1, 594–605. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Pozo, M.J.; Grzegorski, D.; Martínez, P.; García, J.M.; Olmedo-Monfil, V.; Cortés, C.; Kenerley, C.; Herrera-Estrella, A. Enhanced biocontrol activity of Trichoderma through inactivation of a mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 15965–15970. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Latha, J.; Hadar, R.; Horwitz, B.A. TmkA, a mitogen-activated protein kinase of Trichoderma virens, is involved in biocontrol properties and repression of conidiation in the dark. Eukaryot. Cell 2003, 2, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Casas-Flores, S.; Rios-Momberg, M.; Bibbins, M.; Ponce-Noyola, P.; Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 2004, 150, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Harel, M.; Horwitz, B.A.; Chet, I.; Mukherjee, P.K. Trichoderma mitogen-activated protein kinase signaling is involved in induction of plant systemic resistance. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Djonović, S.; Pozo, M.J.; Dangott, L.J.; Howell, C.R.; Kenerley, C.M. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant Microbe Interact. 2006, 19, 838–853. [Google Scholar] [CrossRef] [PubMed]
- Stricker, A.R.; Grosstessner-Hain, K.; Würleitner, E.; Mach, R.L. Xyr1 (xylanase regulator 1) regulates both the hydrolytic enzyme system and D-xylose metabolism in Hypocrea jecorina. Eukaryot. Cell 2006, 5, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Berka, R.M.; Henrissat, B.; Saloheimo, M.; Arvas, M.; Baker, S.E.; Chapman, J.; Chertkov, O.; Coutinho, P.M.; Cullen, D.; et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 2008, 26, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kenerley, C.M. Regulation of morphogenesis and biocontrol properties in Trichoderma virens by a VELVET protein, Vel1. Appl. Environ. Microbiol. 2010, 76, 2345–2352. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Schuster, A.; Bruno, K.S.; Collett, J.R.; Baker, S.E.; Seiboth, B.; Kubicek, C.P.; Schmoll, M. A versatile toolkit for high throughput functional genomics with Trichoderma reesei. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Yang, H.-T.; Zhang, X.-J.; Zhou, F.-Y.; Wu, X.-Q.; Xie, X.-Y.; Zhao, X.-Y.; Zhou, H.-Z. Five new species of Trichoderma from moist soils in China. MycoKeys 2022, 87, 133. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Sarrocco, S. Biological Disease Control by Beneficial (Micro)Organisms: Selected Breakthroughs in the Past 50 Years. Phytopathology 2023, 113, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Omann, M. Trichoderma biocontrol: Signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul. Syst. Bio. 2007, 1, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.J.; Bruckart, W.L.; Coulson, J.R.; Goettel, M.S.; Humber, R.A.; Lumsden, R.D.; Maddox, J.V.; McManus, M.L.; Moore, L.; Meyer, S.F. Safety of microorganisms intended for pest and plant disease control: A framework for scientific evaluation. Biol. Control 1996, 7, 333–351. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Boyle, P.; Henis, Y. Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology 1983, 73, 85–88. [Google Scholar] [CrossRef]
- Kelley, W.D. Interactions of Phytophthora cinnamomi and Trichoderma spp. in relation to propagule production in soil cultures at 26 °C. Can. J. Microbiol. 1977, 23, 288–294. [Google Scholar] [CrossRef]
- McLeod, A.; Labuschagne, N.; Kotzé, J. Evaluation of Trichoderma for biological control of avocado root rot in bark medium artificially infested with Phytophthora cinnamomi. S. Afr. Avocado Grow. Assoc. Yearb. 1995, 18, 32–37. [Google Scholar]
- Bhale, U.; Wagh, P.; Rajkonda, J. Antagonistic confrontation of Trichoderma spp. against fruit rot pathogens on Sapodilla (Manilkara zapota L.). J. Yeast Fungal Res. 2013, 4, 5–11. [Google Scholar]
- Ben Amira, M.; Lopez, D.; Triki Mohamed, A.; Khouaja, A.; Chaar, H.; Fumanal, B.; Gousset-Dupont, A.; Bonhomme, L.; Label, P.; Goupil, P.; et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol. Control 2017, 110, 70–78. [Google Scholar] [CrossRef]
- Khare, A.; Singh, B.; Upadhyay, R. Biological control of Pythium aphanidermatum causing damping-off of mustard by mutants of Trichoderma viride 1433. J. Agric. Technol. 2010, 6, 231–243. [Google Scholar]
- Muthukumar, A.; Eswaran, A.; Sanjeevkumas, K. Exploitation of Trichoderma species on the growth of Pythium aphanidermatum in chilli. Braz. J. Microbiol. 2011, 42, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Fajola, A.; Alasoadura, S. Antagonistic effects of Trichoderma harzianum on Pythium aphanidermatum causing the damping-off disease of tobacco in Nigeria. Mycopathologia 1975, 57, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Thesiya, M.; Rakholiya, K.; Lokesh, R.; Shekhda, M. Comparative Efficacy of Fungicides and Biocontrol Agents against Stem Blight and Fruit Rot Disease of Brinjal under Pot Culture Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2020. [Google Scholar]
- Arzanlou, M.; Khodaei, S.; Narmani, A.; Babai-Ahari, A.; Azar, A.M. Inhibitory effect of Trichoderma isolates on growth of Alternaria alternata, the causal agent of leaf spot disease on sunflower, under laboratory conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 1592–1599. [Google Scholar] [CrossRef]
- Naher, L.; Yusuf, U.K.; Ismail, A.; Hossain, K. Trichoderma spp.: A biocontrol agent for sustainable management of plant diseases. Pak. J. Bot. 2014, 46, 1489–1493. [Google Scholar]
- Bhandari, C.; Karuna, V. Screening of different isolates of Trichoderma harzianum and Pseudomonas fluorescens against Fusarium moniliforme infecting maize. Pantnagar J. Res. 2013, 11, 243–247. [Google Scholar]
- Lenka, S.; Mishra, S.; Mohanty, S.; Das, K.; Medhi, B. Bio-control of rice sheath blight through antagonists. ORYZA-Int. J. Rice 2012, 49, 68–69. [Google Scholar]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spp. Genes Involved in the Biocontrol Activity Against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef] [PubMed]
- Emani, C.; Garcia, J.M.; Lopata-Finch, E.; Pozo, M.J.; Uribe, P.; Kim, D.-J.; Sunilkumar, G.; Cook, D.R.; Kenerley, C.M.; Rathore, K.S. Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens. Plant Biotechnol. J. 2003, 1, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Marcello, C.M.; Steindorff, A.S.; da Silva, S.P.; do Nascimento Silva, R.; Bataus, L.A.M.; Ulhoa, C.J. Expression analysis of the exo-β-1, 3-glucanase from the mycoparasitic fungus Trichoderma asperellum. Microbiol. Res. 2010, 165, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Latha, J.; Hadar, R.; Horwitz, B.A. Role of two G-protein alpha subunits, TgaA and TgaB, in the antagonism of plant pathogens by Trichoderma virens. Appl. Environ. Microbiol. 2004, 70, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol. Plant Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, J.; Cardoza, R.; Hauser, M.; Hermosa, R.; Rey, M.; Llobell, A.; Becker, J.; Gutiérrez, S.; Monte, E. ThPTR2, a di/tri-peptide transporter gene from Trichoderma harzianum. Fungal Genet. Biol. 2006, 43, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Tijerino, A.; Cardoza, R.E.; Moraga, J.; Malmierca, M.G.; Vicente, F.; Aleu, J.; Collado, I.G.; Gutiérrez, S.; Monte, E.; Hermosa, R. Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet. Biol. 2011, 48, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.E.; Malmierca, M.G.; Gutiérrez, S. Overexpression of erg1 gene in Trichoderma harzianum CECT 2413: Effect on the induction of tomato defence-related genes. J. Appl. Microbiol. 2014, 117, 812–823. [Google Scholar] [CrossRef]
- Dixit, P.; Mukherjee, P.K.; Ramachandran, V.; Eapen, S. Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS ONE 2011, 6, e16360. [Google Scholar] [CrossRef]
- Zhong, Y.H.; Wang, T.H.; Wang, X.L.; Zhang, G.T.; Yu, H.N. Identification and characterization of a novel gene, TrCCD1, and its possible function in hyphal growth and conidiospore development of Trichoderma reesei. Fungal Genet. Biol. 2009, 46, 255–263. [Google Scholar] [CrossRef]
- Migheli, Q. Biodiversity of the Genus Trichoderma and Identification of Marker Genes Involved in the Antagonism between Trichoderma spp. and Plant Pathogenic Fungi. Ph.D. Thesis, University of Sassari, Sassari, Italy, 2008. [Google Scholar]
- Samolski, I.; Rincon, A.M.; Pinzon, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a new biocontrol gene in Trichoderma atroviride: The role of an ABC transporter membrane pump in the interaction with different plant-pathogenic fungi. Mol. Plant Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Mukherjee, P.K.; Kale, S.P. cAMP signalling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology 2007, 153, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, B.G.G.; Lorito, M.; Scala, F.; Harman, G. Cloning, sequence and structure of a gene encoding an antifungal glucan 1, 3-β-glucosidase from Trichoderma atroviride (T. harzianum). Gene 2001, 277, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Song, J. A new serine protease gene from Trichoderma harzianum is expressed in Saccharomyces cerevisiae. Appl. Biochem. Microbiol. 2009, 45, 22–26. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Ridgway, H.J.; Stringer, A.M.; Hay, A.J.; Stewart, A. Characterisation of a Trichoderma hamatum monooxygenase gene involved in antagonistic activity against fungal plant pathogens. Curr. Genet. 2008, 53, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Kim, B.-G.; Lee, C.; Hur, H.-G.; Ahn, J.-H. Purification, characterization, and cDNA cloning of xylanase from fungus Trichoderma strain SY. J. Microbiol. Biotechnol. 2002, 12, 890–894. [Google Scholar]
- Migheli, Q.; González-Candelas, L.; Dealessi, L.; Camponogara, A.; Ramón-Vidal, D. Transformants of Trichoderma longibrachiatum overexpressing the β-1, 4-endoglucanase gene egl1 show enhanced biocontrol of Pythium ultimum on cucumber. Phytopathology 1998, 88, 673–677. [Google Scholar] [CrossRef]
- Trushina, N.; Levin, M.; Mukherjee, P.K.; Horwitz, B.A. PacC and pH–dependent transcriptome of the mycotrophic fungus Trichoderma virens. BMC Genom. 2013, 14, 138. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef]
- Gajera, H.; Hirpara, D.G.; Katakpara, Z.A.; Patel, S.; Golakiya, B. Molecular evolution and phylogenetic analysis of biocontrol genes acquired from SCoT polymorphism of mycoparasitic Trichoderma koningii inhibiting phytopathogen Rhizoctonia solani Kuhn. Infect. Genet. Evol. 2016, 45, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, P. The gene task1 is involved in morphological development, mycoparasitism and antibiosis of Trichoderma asperellum. Biocontrol. Sci. Technol. 2017, 27, 620–635. [Google Scholar] [CrossRef]
- Atanasova, L.; Gruber, S.; Lichius, A.; Radebner, T.; Abendstein, L.; Münsterkötter, M.; Stralis-Pavese, N.; Łabaj, P.P.; Kreil, D.P.; Zeilinger, S. The Gpr1-regulated Sur7 family protein Sfp2 is required for hyphal growth and cell wall stability in the mycoparasite Trichoderma atroviride. Sci. Rep. 2018, 8, 12064. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valdespino, C.A.; Porras-Troncoso, M.D.; Corrales-Escobosa, A.R.; Wrobel, K.; Martínez-Hernández, P.; Olmedo-Monfil, V. Functional characterization of TvCyt2, a member of the p450 monooxygenases from Trichoderma virens relevant during the association with plants and mycoparasitism. Mol. Plant Microbe Interact. 2018, 31, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Suriani Ribeiro, M.; Graciano de Paula, R.; Raquel Voltan, A.; de Castro, R.G.; Carraro, C.B.; José de Assis, L.; Stecca Steindorff, A.; Goldman, G.H.; Silva, R.N.; Ulhoa, C.J. Endo-β-1, 3-glucanase (GH16 family) from Trichoderma harzianum participates in cell wall biogenesis but is not essential for antagonism against plant pathogens. Biomolecules 2019, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Rivera, M.; Hernández-Oñate, M.Á.; Dautt-Castro, M.; Gallardo-Negrete, J.d.J.; Rebolledo-Prudencio, O.G.; Uresti-Rivera, E.E.; Arenas-Huertero, C.; Herrera-Estrella, A.; Casas-Flores, S. IPA-1 a putative chromatin remodeler/helicase-related protein of Trichoderma virens plays important roles in antibiosis against Rhizoctonia solani and induction of Arabidopsis systemic disease resistance. Mol. Plant Microbe Interact. 2020, 33, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Ji, S.; Wang, Z.; Zhang, H.; Wang, Y.; Liu, Z. Trichoderma asperellum xylanases promote growth and induce resistance in poplar. Microbiol. Res. 2021, 248, 126767. [Google Scholar] [CrossRef]
- Dubey, M.; Jensen, D.F.; Karlsson, M. Functional characterization of the AGL1 aegerolysin in the mycoparasitic fungus Trichoderma atroviride reveals a role in conidiation and antagonism. Mol. Genet. Genom. 2021, 296, 131–140. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of versatile biocontrol agent, its secrets and insights into mechanism of biocontrol potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Chet, I.; Inbar, J.; Hadar, I. Fungal antagonists and mycoparasites. In The Mycota IV: Environmental and Microbial Relationships; Springer: Berlin/Heidelberg, Germany, 1997; pp. 165–184. [Google Scholar]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Chet, I.; Benhamou, N.; Haran, S. Trichoderma and Gliocladium. In Mycoparasitism and Lytic Enzymes; Taylor and Francis: London, UK, 1998; pp. 153–172. [Google Scholar]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Barak, R.; Chet, I. Determination, by fluorescein diacetate staining, of fungal viability during mycoparasitism. Soil Biol. Biochem. 1986, 18, 315–319. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I. Possible role of competition for nutrients in biocontrol of Pythium damping-off by bacteria. Phytopathology 1987, 77, 190–195. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 25–39. [Google Scholar] [CrossRef]
- Lu, Z.; Tombolini, R.; Woo, S.; Zeilinger, S.; Lorito, M.; Jansson, J.K. In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter systems. Appl. Environ. Microbiol. 2004, 70, 3073–3081. [Google Scholar] [CrossRef]
- Hjeljord, L.; Tronsmo, A.; Harman, G.; Kubicek, C. Trichoderma and gliocladium. Biological control: An overview. In Trichoderma & Gliocladium: Enzymes, Biological Control and Commercial Applications; Harman, G.E., Kubice, C.P., Eds.; CRC Press: Boca Raton, FL, USA, 1998; Volume 2, pp. 131–151. [Google Scholar]
- Brunner, K.; Peterbauer, C.K.; Mach, R.L.; Lorito, M.; Zeilinger, S.; Kubicek, C.P. The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr. Genet. 2003, 43, 289–295. [Google Scholar]
- Zeilinger, S.; Galhaup, C.; Payer, K.; Woo, S.L.; Mach, R.L.; Fekete, C.; Lorito, M.; Kubicek, C.P. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet. Biol. 1999, 26, 131–140. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Buensanteai, N.; Moran-Diez, M.E.; Druzhinina, I.S.; Kenerley, C.M. Functional analysis of non-ribosomal peptide synthetases (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NRPS hybrid enzyme involved in the induced systemic resistance response in maize. Microbiology 2012, 158, 155–165. [Google Scholar] [CrossRef]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system–a review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal metabolites antagonists towards plant pests and human pathogens: Structure-activity relationship studies. Molecules 2018, 23, 834. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Hu, M.; Li, Q.-L.; Yang, Y.-B.; Liu, K.; Miao, C.-P.; Zhao, L.-X.; Ding, Z.-T. Koninginins RS from the endophytic fungus Trichoderma koningiopsis. Nat. Prod. Res. 2017, 31, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Ramachander Turaga, V. Peptaibols: Antimicrobial peptides from fungi. In Bioactive Natural Products in Drug Discovery; Springer: Singapore, 2020; pp. 713–730. [Google Scholar]
- Dunlop, R.W.; Simon, A.; Sivasithamparam, K.; Ghisalberti, E.L. An Antibiotic from Trichoderma Koningii Active Against Soilborne Plant Pathogens. J. Nat. Prod. 1989, 52, 67–74. [Google Scholar] [CrossRef]
- Singh, S.; Dureja, P.; Tanwar, R.; Singh, A. Production and antifungal activity of secondary metabolites of Trichoderma virens. Pestic. Res. J. 2005, 17, 26–29. [Google Scholar]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.M.; Abada, K.; Abd-El-Moneim, M.L.; Abo-Shosha, Y.Z. Effect of different mixtures of some bioagents and Rhizobium phaseoli on bean damping-off under field condition. Int. J. Sci. Eng. Res. 2015, 6, 1009–1106. [Google Scholar]
- Chen, J.-L.; Sun, S.-Z.; Miao, C.-P.; Wu, K.; Chen, Y.-W.; Xu, L.-H.; Guan, H.-L.; Zhao, L.-X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef]
- De Sousa Rocha, J.d.R.; de Oliveira, N.T. In vitro antagonistic potential of Trichoderma spp. against Colletotrichum gloeosporoides agent of anthracnose in the passion fruit (passiflora). Boletín Micológico 1998, 13, 103–110. [Google Scholar] [CrossRef]
- Mahmood, A.; Kataoka, R. Potential of biopriming in enhancing crop productivity and stress tolerance. In Advances in Seed Priming; Springer: Berlin/Heidelberg, Germany, 2018; pp. 127–145. [Google Scholar]
- Da Costa, A.C.; de Miranda, R.F.; Costa, F.A.; Ulhoa, C.J. Potential of Trichoderma piluliferum as a biocontrol agent of Colletotrichum musae in banana fruits. Biocatal. Agric. Biotechnol. 2021, 34, 102028. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Oskiera, M.; Szczech, M.; Bartoszewski, G. Molecular identification of Trichoderma strains collected to develop plant growth-promoting and biocontrol agents. J. Hortic. Res. 2015, 23, 75–86. [Google Scholar] [CrossRef]
- Saber, W.I.; Ghoneem, K.M.; Rashad, Y.M.; Al-Askar, A.A. Trichoderma harzianum WKY1: An indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol Sci. Technol. 2017, 27, 654–676. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-Ściseł, J. Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the Biolog GEN III MicroPlateTM Test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef]
- Srinivasan, U.; Highley, T.; Bruce, A. In The role of siderophore production in the biological control of wood decay fungi by Trichoderma spp. In Proceedings of the 9th International Biodeterioration and Biodegradation Symposium; Institution of Chemical Engineers: Rugby, UK, 1995; pp. 226–231. [Google Scholar]
- Mokhtar, H.; Aid, D. Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. Agric. Biol. J. N. Am. 2013, 4, 145–154. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, K.; Reddy, C.; Arora, D.; Bharat, R.; Mukerji, K.; Knudsen, G. Handbook of Applied Mycology: Fungal Biotechnology 4; Marcel Dekker, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jedryczka, M. Trichoderma spp.—Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Keswani, C.; Singh, S.P.; Sansinenea, E.; Hoat, T.X. Trichoderma spp. mediated induction of systemic defense response in brinjal against Sclerotinia sclerotiorum. Curr. Res. Microb. Sci. 2021, 2, 100051. [Google Scholar]
- Gomes, E.V.; Costa, M.d.N.; de Paula, R.G.; Ricci de Azevedo, R.; da Silva, F.L.; Noronha, E.F.; José Ulhoa, C.; Neves Monteiro, V.; Elena Cardoza, R.; Gutiérrez, S. The Cerato-Platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci. Rep. 2015, 5, 17998. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Sun, J.; Li, Y.; Chen, J. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 1981, 9, 59–67. [Google Scholar] [CrossRef]
- Gil, S.V.; Pastor, S.; March, G. Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and actinomycetes from soil with culture media. Microbiol. Res. 2009, 164, 196–205. [Google Scholar]
- Liu, B.; Ji, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Guo, R.; Ji, S.; Fan, H.; Wang, J.; Wang, Y.; Liu, Z. Isolation of Trichoderma from forestry model base and the antifungal properties of isolate TpsT17 toward Fusarium oxysporum. Microbiol. Res. 2020, 231, 126371. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Gao, J.; Zhang, C.; Yang, H.; Jiang, X.; Li, J.; Li, Y.; Wang, W.; Xian, H.; Li, S. Trichoderma biodiversity in major ecological systems of China. J. Microbiol. 2019, 57, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Z.; Zhang, Y.; Wang, Y.; Liu, Z. Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 2021, 242, 126596. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Ismaiel, A.; Bon, M.-C.; De Respinis, S.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef] [PubMed]
- Gezgin, Y.; Güralp, G.; Barlas, A.B.; Eltem, R. Morphological and molecular identification of trichoderma isolates used as biocontrol agents by DNA barcoding. Eur. J. Biol. 2023, 82, 59–69. [Google Scholar] [CrossRef]
- Kullnig-Gradinger, C.M.; Szakacs, G.; Kubicek, C.P. Phylogeny and evolution of the genus Trichoderma: A multigene approach. Mycol. Res. 2002, 106, 757–767. [Google Scholar] [CrossRef]
- Kopchinskiy, A.; Komoń, M.; Kubicek, C.P.; Druzhinina, I.S. TrichoBLAST: A multilocus database for Trichoderma and Hypocrea identifications. Mycol. Res. 2005, 109, 658–660. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kopchinskiy, A.G.; Komoń, M.; Bissett, J.; Szakacs, G.; Kubicek, C.P. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef]
- Bochner, B.R.; Gadzinski, P.; Panomitros, E. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001, 11, 1246–1255. [Google Scholar] [CrossRef]
- Chaverri, P.; Samuels, G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 2013, 67, 2823–2837. [Google Scholar] [CrossRef] [PubMed]
- Marik, T.; Urbán, P.; Tyagi, C.; Szekeres, A.; Leitgeb, B.; Vágvölgyi, M.; Manczinger, L.; Druzhinina, I.S.; Vágvölgyi, C.; Kredics, L. Diversity profile and dynamics of peptaibols produced by green mould Trichoderma species in interactions with their hosts Agaricus bisporus and Pleurotus ostreatus. Chem. Biodivers. 2017, 14, e1700033. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A.; Mulaw, T.B.; Szakacs, G.; Druzhinina, I.S.; Kubicek, C.P.; Jaklitsch, W.M. The Longibrachiatum Clade of Trichoderma: A revision with new species. Fungal Divers. 2012, 55, 77–108. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ashfaq, M.; Malik, A.; Khan, K.; Mathew, P. Isolation, preservation and revival of Trichoderma viride in culture media. J. Entomol. Zool. Stud. 2017, 5, 1640–1646. [Google Scholar]
- Elad, Y.; Chet, I.; Katan, J. Trichoderma harzianum: A biocontrol agent effective against Sclerotium rolfsii and Rhizoctonia solani. Phytopathology 1980, 70, 119–121. [Google Scholar] [CrossRef]
- Sikandar, S.; Ujor, V.C.; Ezeji, T.C.; Rossington, J.L.; Michel, F.C.; McMahan, C.M.; Ali, N.; Cornish, K. Thermomyces lanuginosus STm: A source of thermostable hydrolytic enzymes for novel application in extraction of high-quality natural rubber from Taraxacum kok-saghyz (Rubber dandelion). Ind. Crops Prod. 2017, 103, 161–168. [Google Scholar] [CrossRef]
- López Lastra, C.C.; Hajek, A.E.; Humber, R.A. Comparing methods of preservation for cultures of entomopathogenic fungi. Can. J. Bot. 2002, 80, 1126–1130. [Google Scholar] [CrossRef]
- Bakerspigel, A. Soil as a storage medium for fungi. Mycologia 1953, 45, 596–604. [Google Scholar] [CrossRef]
- Buell, C.B.; Weston, W.H. Application of the mineral oil conservation method to maintaining collections of fungous cultures. Am. J. Bot. 1947, 34, 555–561. [Google Scholar] [CrossRef]
- Elliott, M.L. Survival, Growth and Pathogenicity of Gaeumannomyces graminis var. graminis with Different Methods of Long-Term Storage. Mycologia 2005, 97, 901–907. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Yassin, M.A.; Moslem, M.A.; Bahkali, A.H.; de Wit, P.J.; McKenzie, E.H.; Stephenson, S.L.; Cai, L.; Hyde, K.D. Culture collections, the new herbaria for fungal pathogens. Fungal Divers. 2010, 45, 21–32. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Stoccho, M.; Mónaco, C.; Cordo, C. A comparison of preservation methods for Trichoderma harzianum cultures. Rev. Iberoam. Micol. 2010, 27, 213. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.W.; Rice, E.; Johnson, C. Total viable counts. Spread plate technique. Encycl. Food Microbiol. 2000, 3, 2159–2160. [Google Scholar]
- Hoben, H.; Somasegaran, P. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl. Environ. Microbiol. 1982, 44, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sekhar, A.C.; Upreti, R.; Mujawar, M.M.; Pasha, S.S. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. 2015, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M.; Blum, B. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol. Control 2011, 57, 1–12. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jørgensen, H.J.; Latz, M.A.; Manzotti, A.; Ntana, F.; Rojas, E.C.; Jensen, B. Searching for novel fungal biological control agents for plant disease control among endophytes. Endophytes Grow. World 2019, 31, 25. [Google Scholar]
- Vafadar, F.; Amooaghaie, R.; Otroshy, M. Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J. Plant Interact. 2014, 9, 128–136. [Google Scholar] [CrossRef]
- Pohjanen, J.; Koskimäki, J.J.; Sutela, S.; Ardanov, P.; Suorsa, M.; Niemi, K.; Sarjala, T.; Häggman, H.; Pirttilä, A.M. Interaction with ectomycorrhizal fungi and endophytic Methylobacterium affects nutrient uptake and growth of pine seedlings in vitro. Tree Physiol. 2014, 34, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.L.; Baruah, K.P.A.; Talukdar, R.B.K.; Kataky, L.; Phukan, A. Review on bacterial blight of rice caused by Xanthomonas oryzae pv. oryzae: Different management approaches and role of Pseudomonas fluorescens as a potential biocontrol agent. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 982–1005. [Google Scholar] [CrossRef]
- Gupta, R.; Saxena, R.; Goel, S. Photoinduced sporulation in Trichoderma harzianum–an experimental approach to primary events. World J. Microbiol. Biotechnol. 1997, 13, 249–250. [Google Scholar] [CrossRef]
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Rukmani, S.; Mariappan, V. Influence of organic amendments with Trichoderma viride on the control of root rot of blackgram. Plant Dis. Res. 1990, 5, 244. [Google Scholar]
- Sawant, I.S.; Sawant, S. A simple method for achieving high cfu of Trichoderma harzianum on organic wastes for field applications. Indian Phytopathol. 1996, 49, 185–187. [Google Scholar]
- Saju, K.; Anandaraj, M.; Sarma, Y. On farm production of Trichoderma harzianum using organic matter. Indian Phytopathol. 2002, 55, 277–281. [Google Scholar]
- Sangle, U.; Bambawale, O. Evaluation of substrates for mass multiplication of Trichoderma spp. Indian J. Plant Prot. 2005, 33, 298. [Google Scholar]
- Simon, S. Agro-based waste products as a substrate for mass production of Trichoderma spp. J. Agric. Sci. 2011, 3, 168. [Google Scholar]
- Babu, K.N.; Pallavi, P. Isolation, identification and mass multiplication of Trichoderma an important bio-control agent. Int. J. Pharm. Life Sci. 2013, 4, 2320–2323. [Google Scholar]
- Mustafa, A.; Khan, M.A.; Inam-ul-Haq, M.; Khan, S.; Pervez, M.A. Mass multiplication of Trichoderma spp. on organic substrate and their effect in management of seed borne fungi. Pak. J. Phytopathol. 2009, 21, 108–114. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Jeyarajan, R. Prospects of Indigenous Mass Production and Formulation of Trichoderma, Current status of biological control of plant diseases using antagonistic organisms in India. In Proceedings of the Group Meeting on Antagonistic Organisms in Plant Disease Management Held at Project Directorate of Biological Control, Bangalore, India, 10–11 July 2003; Project Directorate of Biological Control, Indian Council of Agricultural Research: Bangalore, India, 2006; pp. 74–80. [Google Scholar]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Srinivas, V. Formulations of plant growth-promoting microbes for field applications. Microb. Inoculants Sustain. Agric. Product. Funct. Appl. 2016, 2, 239–251. [Google Scholar]
- Nakkeeran, S.; Fernando, W.D.; Siddiqui, Z.A. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2006; pp. 257–296. [Google Scholar]
- Keswani, C.; Bisen, K.; Singh, V.; Sarma, B.K.; Singh, H.B. Formulation technology of biocontrol agents: Present status and future prospects. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 35–52. [Google Scholar]
- Bejarano, A.; Puopolo, G. Bioformulation of microbial biocontrol agents for a sustainable agriculture. In How Research Can Stimulate the Development of Commercial Biological Control against Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 275–293. [Google Scholar]
- Anith, K.; Vaishakhi, A.; Viswanathan, A.; Varkey, S.; Aswini, S. Population dynamics and efficiency of coconut water based liquid formulation of Pseudomonas fluorescens AMB-8. J. Trop. Agric. 2017, 54, 184. [Google Scholar]
- Mbarga, J.B.; Begoude, B.; Ambang, Z.; Meboma, M.; Kuaté, J.; Schiffers, B.; Ewbank, W.; Dedieu, L.; Ten Hoopen, G.M. A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora megakarya. Biol. Control 2014, 77, 15–22. [Google Scholar] [CrossRef]
- Peeran, M.F.; Krishnan, N.; Thangamani, P.R.; Gandhi, K.; Thiruvengadam, R.; Kuppusamy, P. Development and evaluation of water-in-oil formulation of Pseudomonas fluorescens (FP7) against Colletotrichum musae incitant of anthracnose disease in banana. Eur. J. Plant Pathol. 2014, 138, 167–180. [Google Scholar] [CrossRef]
- Herrera, W.; Valbuena, O.; Pavone-Maniscalco, D. Formulation of Trichoderma asperellum TV190 for biological control of Rhizoctonia solani on corn seedlings. Egypt J. Biol. Pest Control 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Patil, S.S.; Guldegar, D.D.; Potdukhe, S.R.; Khobragade, H.M. Investigation of Liquid Formulation of Trichoderma asperellum against Fusarium Wilt of Chickpea. Biol. Forum Int. J. 2021, 13, 571–576. [Google Scholar]
- Whipps, J.M. Developments in the biological control of soil-borne plant pathogens. Adv. Bot. Res. 1997, 26, 1–134. [Google Scholar]
- Jin, X.; Hayes, C.K.; Harman, G.E. Principles in the development of biological control systems employing Trichoderma species against soil-borne plant pathogenic fungi. In Frontiers in Industrial Mycology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 174–195. [Google Scholar]
- Morgan, C.A.; Herman, N.; White, P.; Vesey, G. Preservation of micro-organisms by drying; a review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef]
- Cumagun, C.J.R. Advances in formulation of Trichoderma for biocontrol. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–531. [Google Scholar]
- Jeyarajan, R.; Ramakrishnan, G.; Dinakaran, D.; Sridar, R. Development of products Trichoderma viride and Bacillus subtilis for biocontrol of root rot diseases. In Biotechnology in India; Dwivedi, B.K., Ed.; Bioved Research Society: Allahabad, India, 1994; pp. 25–36. [Google Scholar]
- Pradhan, P.C.; Mukhopadhyay, A.; Kumar, R.; Kundu, A.; Patanjali, N.; Dutta, A.; Kamil, D.; Bag, T.K.; Aggarwal, R.; Bharadwaj, C. Performance appraisal of Trichoderma viride based novel tablet and powder formulations for management of Fusarium wilt disease in chickpea. Front. Plant Sci. 2022, 13, 990392. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, S.; Balabaskar, P. Biocontrol efficacy of Trichoderma spp. against wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. J. Appl. Biol. Biotechnol. 2013, 1, 036–040. [Google Scholar]
- Lewis, J. Formulation and delivery systems of biocontrol agents with emphasis on fungi. In The Rhizosphere and Plant Growth, Proceedings of the Beltsville Symposia in Agricultural Research, Beltsville, MD, USA, 8–11 May 1989; Springer: Berlin/Heidelberg, Germany, 1991; pp. 279–287. [Google Scholar]
- Batta, Y. Control of postharvest diseases of fruit with an invert emulsion formulation of Trichoderma harzianum Rifai. Postharvest Biol. Technol. 2007, 43, 143–150. [Google Scholar] [CrossRef]
- Bhai, R. Preservation and long-term storage of Trichoderma spp. by sodium alginate encapsulation. J. Plant Crops 2020, 48, 36–44. [Google Scholar]
- Byakodi, A.; Babu, B.S. Experimental studies on effects of selected species of fungi on aerobic composting of sugar pressmud. Environ. Res. Eng. Manag. 2021, 77, 74–85. [Google Scholar] [CrossRef]
- Cavalcante, R.S.; Lima, H.L.S.; Pinto, G.A.S.; Gava, C.A.T.; Rodrigues, S. Effect of Moisture on Trichoderma Conidia Production on Corn and Wheat Bran by Solid State Fermentation. Food Bioproc. Tech. 2008, 1, 100–104. [Google Scholar] [CrossRef]
- Balasubramanian, C.; Udaysoorian, P.; Prabhu, C.; Kumar, G. Enriched compost for yield and quality enhancement in sugarcane. J. Ecobiol. 2008, 22, 173–176. [Google Scholar]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Jin, X.; Custis, D. Microencapsulating aerial conidia of Trichoderma harzianum through spray drying at elevated temperatures. Biol. Control 2011, 56, 202–208. [Google Scholar] [CrossRef]
- Dos Santos, G.; Locatelli, G.; Coêlho, D.; Botelho, P.; De Amorim, M.; De Vasconcelos, T.; Bueno, L. Factorial design, preparation and characterization of new beads formed from alginate, polyphosphate and glycerol gelling solution for microorganism microencapsulation. J. Solgel Sci. Technol. 2015, 75, 345–352. [Google Scholar] [CrossRef]
- Oancea, F.; Raut, I.; Şesan, T.E.; Cornea, P.C. Dry Flowable Formulation of Biostimulants Trichoderma Strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef]
- Arora, N.K.; Kumar, V.; Maheshwari, D.K. Constraints, development and future of the inoculants with special reference to rhizobial inoculants. In Innovative Approaches in Microbiology; Maheshwari, D.K., Dubey, R.C., Eds.; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2001; pp. 241–245. [Google Scholar]
- Arora, N.; Khare, E.; Naraian, R.; Maheshwari, D. Sawdust as a superior carrier for production of multipurpose bioinoculant using plant growth promoting rhizobial and pseudomonad strains and their impact on productivity of Trifolium repense. Curr. Sci. 2008, 95, 90–94. [Google Scholar]
- Moses, K. Assessment of sawdust as carrier material for fungal inoculum intended for faster composting. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 608–613. [Google Scholar]
- Khan, M.R.; Majid, S.; Mohidin, F.A.; Khan, N. A new bioprocess to produce low cost powder formulations of biocontrol bacteria and fungi to control fusarial wilt and root-knot nematode of pulses. Biol. Control 2011, 59, 130–140. [Google Scholar] [CrossRef]
- Nabi, S.U.; Raja, W.H.; Sharma, A.; Malik, G. Evaluation of Different Substrates for Development of Trichoderma harzianum Based Stock Cultures and their Utilization in Management of Chilli Wilt Disease. Chem. Sci. Rev. Lett. 2017, 6, 2229–2235. [Google Scholar]
- Mohiddin, F.; Bashir, I.; Padder, S.A.; Hamid, B. Evaluation of different substrates for mass multiplication of Trichoderma species. J. Pharmacogn. Phytochem. 2017, 6, 563–569. [Google Scholar]
- Ananda, N.; Vadlani, P.V.; Prasad, P.V.V. Evaluation of drought and heat stressed grain sorghum (Sorghum bicolor) for ethanol production. Ind. Crops Prod. 2011, 33, 779–782. [Google Scholar] [CrossRef]
- Chammem, H.; Nesler, A.; Pertot, I. Wood pellets as carriers of conidia of Trichoderma atroviride SC1 for soil application. Fungal Biol. 2021, 125, 989–998. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Pascual, J.A. Performance of a Trichoderma harzianum bentonite–vermiculite formulation against Fusarium wilt in seedling nursery melon plants. HortScience 2009, 44, 2025–2027. [Google Scholar] [CrossRef]
- Sreenayana, B.; Nakkeeran, S. Effect of Oil Based Formulation of Trichoderma spp. on Growth Parameters of Cucumber Seedlings. Int. J. Curr. Microbiol. App. Sci 2019, 8, 200–209. [Google Scholar] [CrossRef]
- Connick, W.J.; Boyette, C.D.; McAlpine, J.R. Formulation of mycoherbicides using a pasta-like process. Biol. Control 1991, 1, 281–287. [Google Scholar] [CrossRef]
- Thangavelu, R.; Palaniswami, A.; Velazhahan, R. Mass production of Trichoderma harzianum for managing fusarium wilt of banana. Agric. Ecosyst. Environ. 2004, 103, 259–263. [Google Scholar] [CrossRef]
- Ramanujam, B.; Prasad, R.; Sriram, S.; Rangeswaran, R. Mass production, formulation, quality control and delivery of Trichoderma for plant disease management. J. Plant Prot. Sci. 2010, 2, 1–8. [Google Scholar]
- Komala, G.; Madhavi, G.B.; Nath, R. Shelf life studies of different formulations of Trichoderma harzianum. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 1100–1105. [Google Scholar]
- Organo, N.D.; Granada, S.M.J.M.; Pineda, H.G.S.; Sandro, J.M.; Nguyen, V.H.; Gummert, M. Assessing the potential of a Trichoderma-based compost activator to hasten the decomposition of incorporated rice straw. Sci. Rep. 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Prasad, R.D.; Poorna Chandrika, K.S.; Desai, S.; Greeshma, K.; Vijaykumar, S. Development, Production, and Storage of Trichoderma Formulations for Agricultural Applications. In Advances in Trichoderma Biology for Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 371–385. [Google Scholar]
- Rini, C.; Ramya, J.; Jayakumar, G.; Shajan, V. Low cost carrier material for mass production of Trichoderma inoculants. J. Trop. Agric. 2018, 56, 63–68. [Google Scholar]
- Rai, D.; Tewari, A. Shelf life studies of different formulations based on Trichoderma harzianum (Th14). Ann. Biol. Res. 2016, 7, 1–5. [Google Scholar]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Sriram, S.; Roopa, K.P.; Savitha, M.J. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot. 2011, 30, 1334–1339. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Parthasarathy, S.; Kamalakannan, A.; Kuppusamy, K.; Gopalakrishnan, C. A novel method to develop paste formulation of Trichoderma harzianum. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3286–3293. [Google Scholar]
- Jeyarajan, R.; Nakkeeran, S. Exploitation of microorganisms and viruses as biocontrol agents for crop disease management. In Biocontrol Potential and its Exploitation in Sustainable Agriculture: Crop Diseases, Weeds, and Nematodes; Springer: Berlin/Heidelberg, Germany, 2000; pp. 95–116. [Google Scholar]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and nutrition of cowpea (Vigna unguiculata) under water deficit as influenced by microbial inoculation via seed coating. J. Agron. Crop Sci. 2019, 205, 447–459. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.N.; Shrestha, S.M.; Mukherjee, P.K. Biological seed treatment for control of soil-borne plant pathogens. FAO Plant Prot. Bull. 1992, 40, 21–30. [Google Scholar]
- Jogani, V.; John, P. Evaluation of different application methods of Trichoderma harzianum (Rifai) against Fusarium wilt of tomato. Crop Res. 2014, 48, 76–79. [Google Scholar]
- Tewari, A.; Mukhopadhyay, A. Testing of different formulations of Gliocladium virens against chickpea wilt-complex. Indian Phytopathol. 2001, 54, 67–71. [Google Scholar]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Corrales, D.C.; Soltani, E. Biological seed treatments promote crop establishment and yield: A global meta-analysis. Agron. Sustain. Dev. 2022, 42, 45. [Google Scholar] [CrossRef]
- Couto, A.P.S.; Pereira, A.E.; Abati, J.; Fontanela, M.L.C.; Dias-Arieira, C.R.; Krohn, N.G. Seed treatment with Trichoderma and chemicals to improve physiological and sanitary quality of wheat cultivars. Rev. Caatinga 2021, 34, 813–823. [Google Scholar] [CrossRef]
- Sudana, I.M.; Raka, I.G.N. Seeds Treatment Using Trichoderma spp. Formulated in Biosluryy and Vermicompost to Induct the Resistance of the Peanut (Arachis hypogaea L.) Diseases. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1253–1265. [Google Scholar] [CrossRef]
- Hossain, S.; Akter, F. Effects of Trichoderma-enriched biofertilizer and farmyard manure on the growth and yield of brinjal (Solanum melongena L.). Dhaka Univ. J. Biol. Sci. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Kim, K.; Heo, Y.M.; Jang, S.; Lee, H.; Kwon, S.-L.; Park, M.S.; Lim, Y.W.; Kim, J.-J. Diversity of Trichoderma spp. in marine environments and their biological potential for sustainable industrial applications. Sustainability 2020, 12, 4327. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, S.; Parihar, M.; Rakshit, A. Seed bio-priming with microbial inoculants: A tailored approach towards improved crop performance, nutritional security, and agricultural sustainability for smallholder farmers. Curr. Res. Environ. Sustain. 2021, 3, 100093. [Google Scholar] [CrossRef]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Mukherjee, P.K.; Bhattacharyya, P.; Behera, S.; Bagchi, T.B.; Patro, R.; Khandual, A.; Bag, M. Novel Trichoderma strains isolated from tree barks as potential biocontrol agents and biofertilizers for direct seeded rice. Microbiol. Res. 2018, 214, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.S.; Singh, P.; Singh, J.; Vaishnav, A.; Ray, S.; Singh, H. Trichoderma mediated seed biopriming augments antioxidant and phenylpropanoid activities in tomato plant against Sclerotium rolfsii. J. Pharmacogn. Phytochem. 2019, 8, 2641–2647. [Google Scholar]
- Singh, V.; Upadhyay, R.S.; Sarma, B.; Singh, H. Seed bio-priming with Trichoderma asperellum effectively modulate plant growth promotion in pea. Int. J. Agric. Environ. Biotechnol. 2016, 9, 361–365. [Google Scholar] [CrossRef]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradation capacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S. Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Raja, M.; Singh, B.; Katoch, S.; Kumar, S.; Sharma, P. Potential native Trichoderma strains against Fusarium verticillioides causing post flowering stalk rot in winter maize. Crop Prot. 2022, 152, 105838. [Google Scholar] [CrossRef]
- Singh, U.S.; Zaidi, N.W. Current status of formulation and delivery of fungal and bacterial antagonists for disease management in India. In Microbial Biopesticide Formulations and Application; Project Directorate of Biological Control: Bangalore, India, 2002; p. 269. [Google Scholar]
- Yang, Z.; Zhi, P.; Chang, C. Priming seeds for the future: Plant immune memory and application in crop protection. Front. Plant Sci. 2022, 13, 961840. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Panwar, V.; Aggarwal, A.; Singh, G.; Verma, A.; Sharma, I.; Saharan, M. Efficacy of foliar spray of Trichoderma isolates against Fusarium graminearum causing head blight of wheat. J. Wheat Res. 2014, 6, 59–63. [Google Scholar]
- Karthikeyan, M.; Radhika, K.; Bhaskaran, R.; Mathiyazhagan, S.; Sandosskumar, R.; Velazhahan, R.; Alice, D. Biological control of onion leaf blight disease by bulb and foliar application of powder formulation of antagonist mixture. Arch. Phytopathol. Plant Prot. 2008, 41, 407–417. [Google Scholar] [CrossRef]
- Gade, R.; Wardhe, S.; Armarker, S. Shelf life study of Trichoderma spp. in different carrier materials. J. Maharashtra Agric. Univ. 2009, 34, 181–182. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]
- Uddin, A.; Hussain, M.; Rahman, S.; Ahmad, H.; Roni, M. Effect of Trichoderma concentrations on growth and yield of tomato. Bangladesh Res. Publ. J. 2015, 11, 228–232. [Google Scholar]
- Dursun, A.; Ekinci, M.; Dönmez, M.F. Effects of foliar application of plant growth promoting bacterium on chemical contents, yield and growth of tomato (Lycopersicon esculentum L.) and cucumber (Cucumis sativus L.). Pak. J. Bot. 2010, 42, 3349–3356. [Google Scholar]
- Syam, N.; Hidrawati; Sabahannur, S.; Nurdin, A. Effects of Trichoderma and Foliar Fertilizer on the Vegetative Growth of Black Pepper (Piper nigrum L.) Seedlings. Int. J. Agron. 2021, 2021, 9953239. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Sehim, A.E.; Hewedy, O.A.; Altammar, K.A.; Alhumaidi, M.S.; Abd Elghaffar, R.Y. Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Front. Microbiol. 2023, 14, 1140378. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.; Kay, W.; Kilaru, S.; Schuster, M.; Gurr, S.J.; Steinberg, G. Multi-site fungicides suppress banana Panama disease, caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. PLoS Pathog. 2022, 18, e1010860. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato dry rot disease: Current status, pathogenomics and management. 3 Biotech 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liu, Q.; Yang, Z. Pathogenicity, Mycotoxin Production, and Control of Potato Dry Rot Caused by Fusarium spp.: A Review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Jeyalakshmi, C.; Rettinassababady, C.; Nema, S. Integrated management of sesame diseases. J. Biopestic. 2013, 6, 68. [Google Scholar]
- El-Mohamedy, R.S.; Ziedan, E.; Abdalla, A. Biological soil treatment with Trichoderma harzianum to control root rot disease of grapevine (Vitis vinifera L.) in newly reclaimed lands in Nobaria province. Arch. Phytopathol. Plant Prot. 2010, 43, 73–87. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Cao, H.; Fan, Y.; Du, K.; Bu, X.; Gao, D. Effects of Trichoderma harzianum biofertilizer on growth, yield, and quality of Bupleurum chinense. Plant Direct 2022, 6, e461. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, Q.; Li, W.; Gao, L.; Liu, G. Strain improvement of Trichoderma harzianum for enhanced biocontrol capacity: Strategies and prospects. Front. Microbiol. 2023, 14, 1146210. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C.; Jiang, X.; Tan, Z.; Li, H.; Xu, S.; Zhang, S.; Shang, Q.; Deising, H.B.; Behrens, S.E.; et al. The Newly Identified Trichoderma harzianum Partitivirus (ThPV2) Does Not Diminish Spore Production and Biocontrol Activity of Its Host. Viruses 2022, 14, 1532. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kalia, A.; Lore, J.S.; Kaur, A.; Yadav, I.; Sharma, P.; Sandhu, J.S. Trichoderma sp. endochitinase and β-1,3-glucanase impede Rhizoctonia solani growth independently, and their combined use does not enhance impediment. Plant Pathol. 2021, 70, 1388–1396. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Kumhar, K.C.; Kumar, K.; Arora, I.; Bhatia, A.K.; Batra, V.K.; Raj, H. Management of tomato damping-off disease in the nursery using of Trichoderma asperellum. Int. J. Econ. Plants 2022, 9, 145–151. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, X.; Xu, H.; Ding, G. Trichoderma longibrachiatum Inoculation Improves Drought Resistance and Growth of Pinus massoniana Seedlings through Regulating Physiological Responses and Soil Microbial Community. J. Fungi 2023, 9, 694. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 1–23. [Google Scholar] [CrossRef]
- Van Jaarsveld, W.J.; Halleen, F.; Bester, M.C.; Pierron, R.J.; Stempien, E.; Mostert, L. Investigation of Trichoderma species colonization of nursery grapevines for improved management of black foot disease. Pest Manag. Sci. 2021, 77, 397–405. [Google Scholar] [CrossRef]
- Lanzuise, S.; Manganiello, G.; Guastaferro, V.M.; Vincenzo, C.; Vitaglione, P.; Ferracane, R.; Vecchi, A.; Vinale, F.; Kamau, S.; Lorito, M.; et al. Combined Biostimulant Applications of Trichoderma spp. with Fatty Acid Mixtures Improve Biocontrol Activity, Horticultural Crop Yield and Nutritional Quality. Agronomy 2022, 12, 275. [Google Scholar] [CrossRef]
- Galante, Y.; De Conti, A.; Monteverdi, R. Trichoderma and Gliocladium. In Trichoderma & Gliocladium: Enzymes, Biological Control and Commercial Applications; Taylor & Francis: Abingdon, UK, 1998. [Google Scholar]
- Kaczmarek, J.; Jedryczka, M. Characterization of two coexisting pathogen populations of Leptosphaeria spp., the cause of stem canker of brassicas. Acta Agrobot. 2011, 64, 2. [Google Scholar] [CrossRef]
- Zafra, G.; Moreno-Montaño, A.; Absalón, Á.E.; Cortés-Espinosa, D.V. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 2015, 22, 1034–1042. [Google Scholar] [CrossRef]
- Vázquez, M.B.; Barrera, V.; Bianchinotti, V. Molecular identification of three isolates of Trichoderma harzianum isolated from agricultural soils in Argentina, and their abilities to detoxify in vitro metsulfuron methyl. Botany 2015, 93, 793–800. [Google Scholar] [CrossRef]
- Amira, R.D.; Roshanida, A.; Rosli, M.; Zahrah, M.S.F.; Anuar, J.M.; Adha, C.N. Bioconversion of empty fruit bunches (EFB) and palm oil mill effluent (POME) into compost using Trichoderma virens. Afr. J. Biotechnol. 2011, 10, 18775–18780. [Google Scholar]
- Haddadin, M.S.; Haddadin, J.; Arabiyat, O.I.; Hattar, B. Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Bioresour. Technol. 2009, 100, 4773–4782. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Singh, R.P. Current challenges, constraints and future strategies for development of successful market for biopesticides. Clim. Chang. Environ. Sustain. 2016, 4, 129–136. [Google Scholar] [CrossRef]
- Mishra, J.; Tewari, S.; Singh, S. Biopesticides: Where we stand? In Plant Microbes Symbiosis; Arora, N.K., Ed.; Springer: New Delhi, India, 2015. [Google Scholar]
- Chakraborty, N.; Mitra, R.; Pal, S.; Ganguly, R.; Acharya, K.; Minkina, T.; Sarkar, A.; Keswani, C. Biopesticide Consumption in India: Insights into the Current Trends. Agriculture 2023, 13, 557. [Google Scholar] [CrossRef]
- Topolovec-Pintarić, S. Trichoderma: Invisible partner for visible impact on agriculture. In Trichoderma—The Most Widely used Fungicide; IntechOpen: London, UK, 2019; p. 15. [Google Scholar]
- Kumar, S.; Thakur, M.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]
- Köberl, M.; Ramadan, E.M.; Roßmann, B.; Staver, C.; Fürnkranz, M.; Lukesch, B.; Grube, M.; Berg, G. Using ecological knowledge and molecular tools to develop effective and safe biocontrol strategies. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; IntechOpen: London, UK, 2011; pp. 3–26. [Google Scholar]
- Oros, G.; Naár, Z.; Cserháti, T. Growth response of Trichoderma species to organic solvents. Mol. Inform. 2011, 30, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hajieghrari, B. Effect of some metal-containing compounds and fertilizers on mycoparasite Trichoderma species mycelia growth response. Afr. J. Biotechnol. 2010, 9, 4025–4033. [Google Scholar]
- Niti, C.; Sunita, S.; Kamlesh, K.; Rakesh, K. Bioremediation: An emerging technology for remediation of pesticides. Res. J. Chem. Environ. 2013, 17, 4. [Google Scholar]
- Argumedo-Delira, R.; Alarcón, A.; Ferrera-Cerrato, R.; Almaraz, J.J.; Peña-Cabriales, J.J. Tolerance and growth of 11 Trichoderma strains to crude oil, naphthalene, phenanthrene and benzo [a] pyrene. J. Environ. Manage. 2012, 95, S291–S299. [Google Scholar] [CrossRef]
- Matsubara, M.; Lynch, J.; De Leij, F. A simple screening procedure for selecting fungi with potential for use in the bioremediation of contaminated land. Enzyme Microb. Technol. 2006, 39, 1365–1372. [Google Scholar] [CrossRef]
- Ezzi, M.I.; Lynch, J.M. Biodegradation of cyanide by Trichoderma spp. and Fusarium spp. Enzyme Microb. Technol. 2005, 36, 849–854. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, M.; Zeng, Z.; Du, A.; Tan, H.; Liu, Y. Trichoderma atroviride F6 improves phytoextraction efficiency of mustard (Brassica juncea (L.) Coss. var. foliosa Bailey) in Cd, Ni contaminated soils. Chemosphere 2008, 71, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.; De-Leij, F.A.; Lynch, J. Trichoderma harzianum Rifai 1295-22 mediates growth promotion of crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil. Microb. Ecol. 2007, 54, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Moffat, A.J. Bioremediation–prospects for the future application of innovative applied biological research. Ann. Appl. Biol. 2005, 146, 217–221. [Google Scholar] [CrossRef]
- Huang, J.; Pang, Y.; Zhang, F.; Huang, Q.; Zhang, M.; Tang, S.; Fu, H.; Li, P. Suppression of Fusarium wilt of banana by combining acid soil ameliorant with biofertilizer made from Bacillus velezensis H-6. Eur. J. Plant Pathol. 2019, 154, 585–596. [Google Scholar] [CrossRef]
- Smily, J.R.M.B.; Sumithra, P.A. Optimization of chromium biosorption by fungal adsorbent, Trichoderma sp. BSCR02 and its desorption studies. HAYATI J. Biosci. 2017, 24, 65–71. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turrà, D.; Di Pietro, A.; Zeilinger, S. Chemotropism assays for plant symbiosis and mycoparasitism related compound screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 601251. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Banga, S.S. Combating Climate Change: An Agricultural Perspective; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
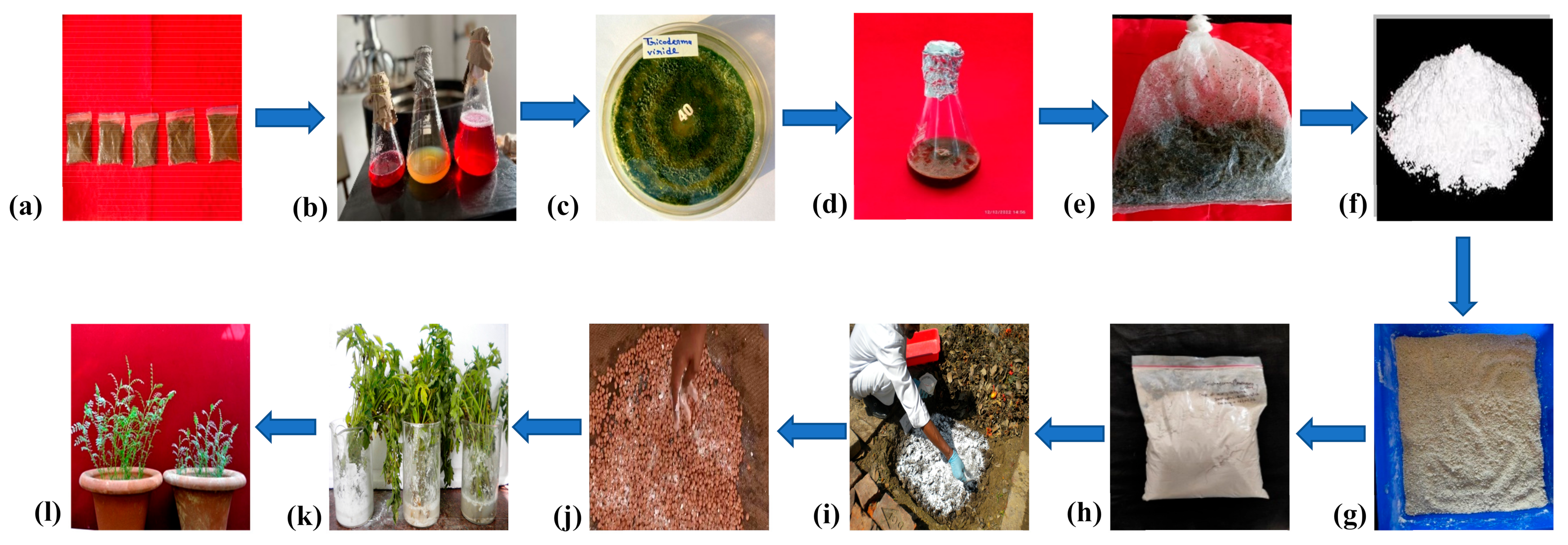
| Decades | Year | Landmarks in Trichoderma Research | References |
|---|---|---|---|
| 1700s | 1794 | In this year, the name Trichoderma was introduced. | [16] |
| 1800s | 1865 | The sexual stage of Trichoderma viride (Hypocrea rufa) was reported. | [17] |
| 1900s | 1932 | First evidence that Trichoderma lignorum (Hypocrea virens) has mycoparasitic and biocontrol abilities. | [18] |
| 1934 | The invention of the first anti-microbial compound (e.g., gliotoxin). | [19] | |
| 1957 | Discovery of the effect of light on T. viride; syn. T. reesei. | [20] | |
| 1972 | Demonstration of biocontrol activity of T. harzianum against Sclerotium rolfsii in field conditions. | [21] | |
| 1979 | RUT C30, a carbon catabolite mutant of T. reesei, was isolated using ultraviolet (UV) mutagenesis. | [22] | |
| 1983 | Cloning of the first Trichoderma species (e.g., T. reesei). | [23] | |
| 1984 | The first international workshop was held on Trichoderma. | - | |
| 1985 | Papavizas wrote the first review on biology, ecology, and potential for biocontrol by Trichoderma. | [15] | |
| 1986 | Report on the expression of growth promotion in the root. | [24] | |
| 1987 | Successful transformation of T. reesei. | [25] | |
| 1989 | First registration of commercial formulation (e.g., Binab T). | [26] | |
| 1992 | Evidence of cloning of lectin-coated fibers by Trichoderma species. | [27] | |
| 1993 | prb1 gene cloning related to mycoparasitism and induced by cell wall. | [28] | |
| 1997 | Expression of enhanced plant immunity (ISR) by application of Trichoderma spp. | [29] | |
| 1998 | Identification of factors that induce genes for mycoparasitism. | [30] | |
| 1999 | Trichoderma internal colonization of plant roots demonstrated. | [31] | |
| 2000s | 2002 | Role of signaling pathways and G protein in mechanism of biocontrol and conidia formation. | [32] |
| 2003 | Role of mitogen-activated protein kinase (MAPK), which negatively regulates conidiation in T. virens. | [33,34] | |
| 2004 | Identification of photoreceptors (Brl1 and Brl2) in T. atroviride. | [35] | |
| 2005 | Function of Trichoderma MAPK (mitogen-activated protein kinase) in Induce systemic resistance (ISR). | [36] | |
| 2006 | Purification of the first true elicitor protein (Sm1/EplI) of Hypocreaatro viridis on glucose. | [37] | |
| 2006 | Cellulases and xylanases regulators identified in T. reesei (XYR1). | [38] | |
| 2008 | First genome sequenced of Trichoderma species (e.g., T. reesei). | [39] | |
| 2009 | Endophytic Trichoderma for stress tolerance in plants. | [40] | |
| 2010 | Discovery of VELVET protein (Vel1) as a key regulator in biocontrol agents (e.g., T. virens) and Trichoderma spp. peptide pheromone precursor genes have been identified. | [41] | |
| 2011 | Genome comparison of three species of Trichoderma. | [42] | |
| 2012 | Next-generation sequencing and high-throughput procedures were used for the sequencing of Trichoderma spp. (T. asperellum, T. harzianum, T. citrinoviride, T. longibrachiatum) genome. | [43] | |
| 2022 | Five new Trichoderma species were reported. | [44] | |
| 2014–2022 | 144 Trichoderma strains were registered in 40 countries. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Koul, B.; Taak, P.; Yadav, D.; Song, M. Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture 2023, 13, 2022. https://doi.org/10.3390/agriculture13102022
Kumar V, Koul B, Taak P, Yadav D, Song M. Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture. 2023; 13(10):2022. https://doi.org/10.3390/agriculture13102022
Chicago/Turabian StyleKumar, Vipul, Bhupendra Koul, Pooja Taak, Dhananjay Yadav, and Minseok Song. 2023. "Journey of Trichoderma from Pilot Scale to Mass Production: A Review" Agriculture 13, no. 10: 2022. https://doi.org/10.3390/agriculture13102022
APA StyleKumar, V., Koul, B., Taak, P., Yadav, D., & Song, M. (2023). Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture, 13(10), 2022. https://doi.org/10.3390/agriculture13102022








