Nitrogen Fertilization Boosts Maize Grain Yield, Forage Quality, and Estimated Meat Production in Maize–Forage Intercropping
Abstract
:1. Introduction
2. Materials and Methods
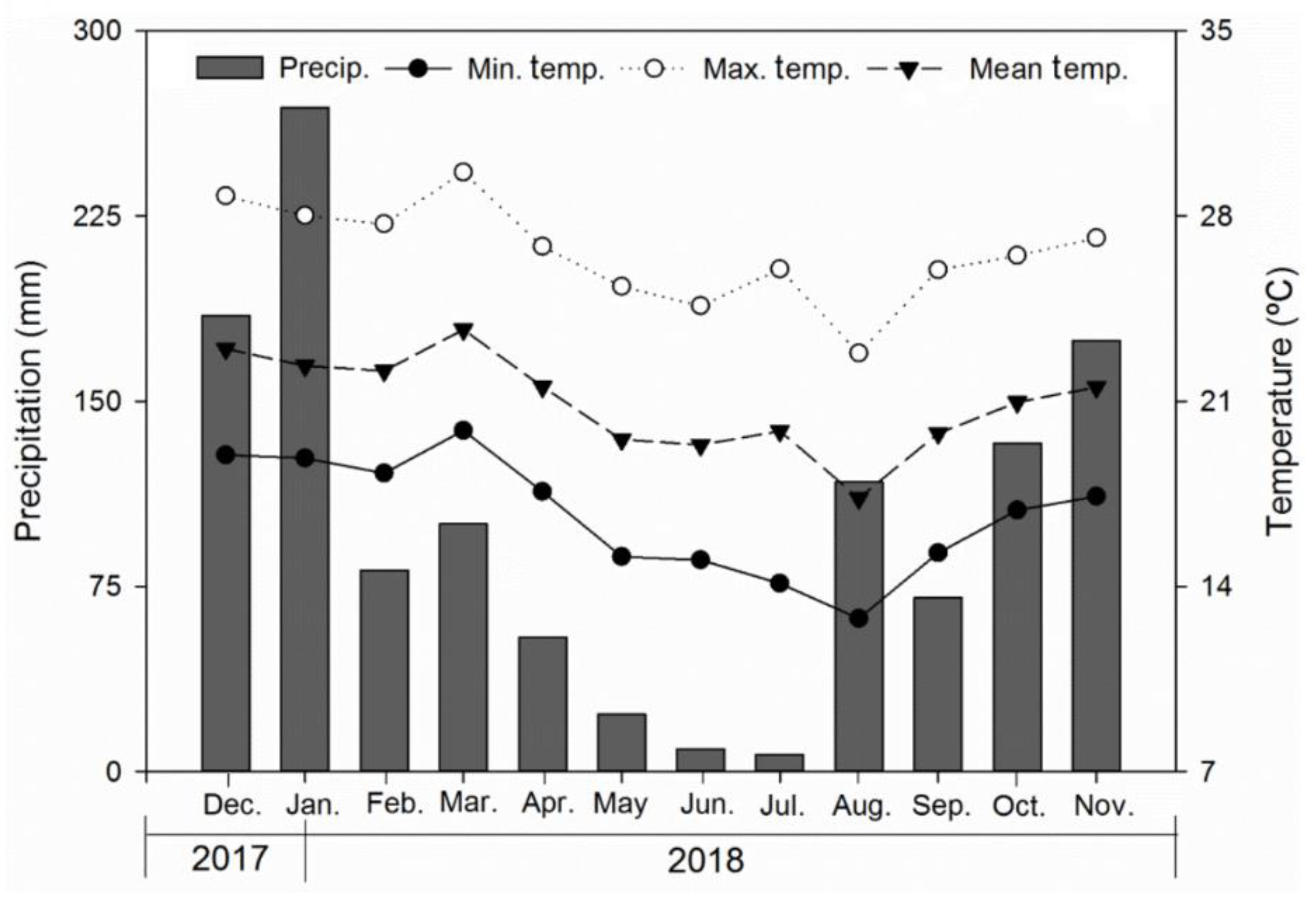
2.1. Study Site
2.2. Treatments and Study Design
2.3. Crop Management
2.4. Bromatological Analysis
2.5. Light Interception, SPAD Index and Height of Grasses
2.6. Estimated Meat Production
2.7. Statistical Analysis
3. Results
3.1. Forage and Maize Yields
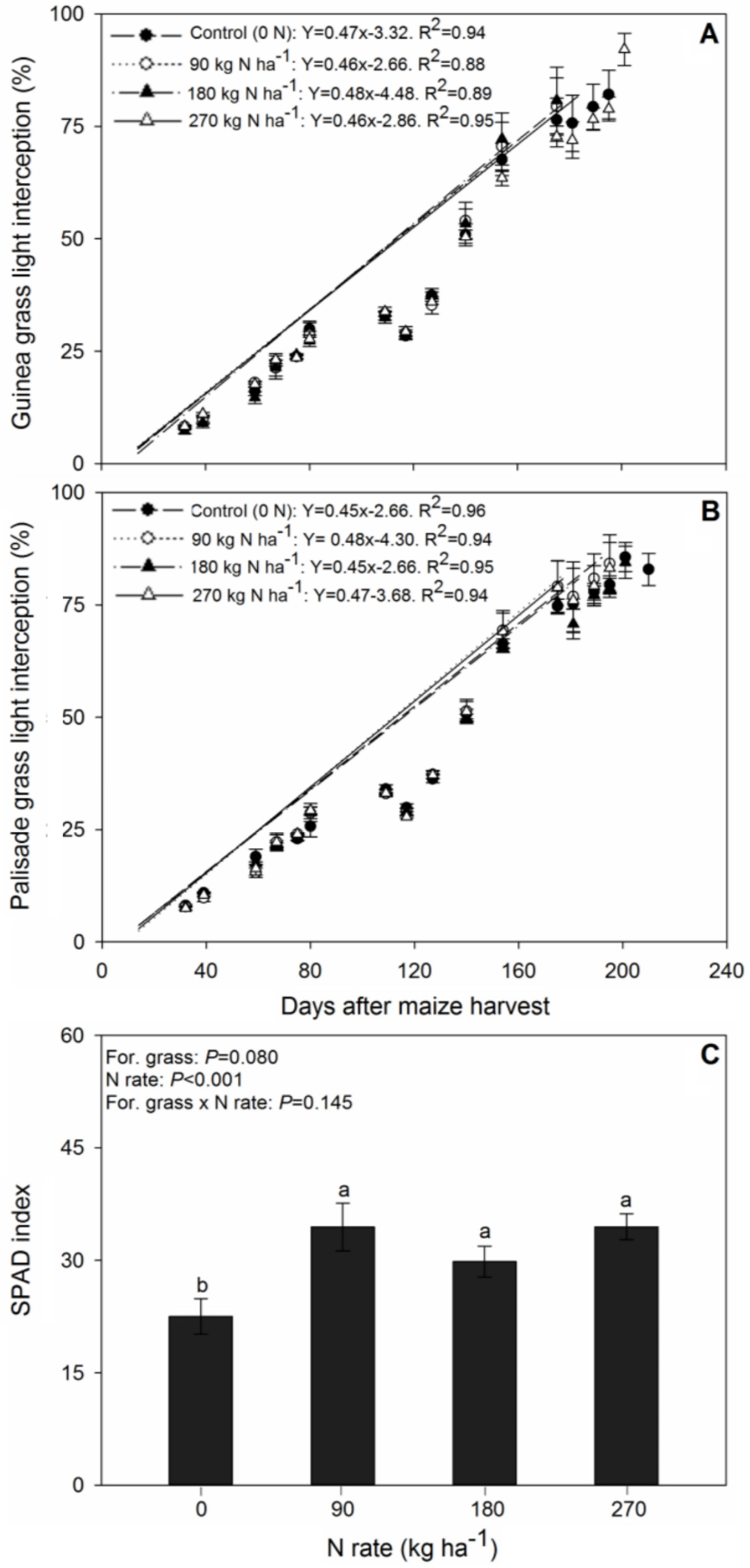
3.2. Forage Light Interception and SPAD Index
3.3. Forage Quality
3.4. Estimated Meat Production
4. Discussion
4.1. Crop Yield and Bromatological Quality at Maize Harvest
4.2. Light Interception and SPAD Index after Maize Harvesting
4.3. Dry Matter, Bromatological Quality, and Estimated Meat Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thierfelder, C.; Cheesman, S.; Rusinamhodzi, L. A comparative analysis of conservation agriculture systems: Benefits and challenges of rotations and intercropping in Zimbabwe. Field Crops Res. 2012, 137, 237–250. [Google Scholar] [CrossRef]
- Angelsen, A. Policies for reduced deforestation and their impact on agricultural production. Proc. Natl. Acad. Sci. USA 2010, 107, 19639–19644. [Google Scholar] [CrossRef] [PubMed]
- Scopel, E.; Triomphe, B.; Affholder, F.; Da Silva, F.A.M.; Corbeels, M.; Xavier, J.H.V.; Lahmar, R.; Recous, S.; Bernoux, M.; Blanchart, E.; et al. Conservation agriculture cropping systems in temperate and tropical conditions, performances and impacts. A review. Agron. Sustain. Dev. 2012, 33, 113–130. [Google Scholar] [CrossRef]
- Calonego, J.C.; Rosolem, C.A. Estabilidade de agregados do solo após manejo com rotações de culturas e escarificação. Rev. Bras. Ciência Solo 2008, 32, 1399–1407. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Crop and cattle production responses to tillage and cover crop management in an integrated crop–livestock system in the southeastern USA. Eur. J. Agron. 2014, 57, 62–70. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Ritz, C.; Cantarella, H.; Galdos, M.V.; Hawkesford, M.J.; Whalley, W.R.; Mooney, S.J. Enhanced Plant Rooting and Crop System Management for Improved N Use Efficiency. Adv. Agron. 2017, 146, 205–239. [Google Scholar] [CrossRef]
- Soratto, R.P.; Rosolem, C.A.; Crusciol, C.A.C. Integração Lavoura-Pecuária-Floresta: Alguns Exemplos No Brasil Centra, 1st ed.; FEPAF: Botucatu, Brazil, 2011; Volume 01, 110p. [Google Scholar]
- Borghi, E.; Crusciol, C.A.C.; Trivelin, P.C.O.; Nascente, A.S.; Costa, C.; Mateus, G.P. Nitrogen fertilization (15NH4NO3) of palisadegrass and residual effect on subsequent no-tillage corn. Rev. Bras. Ciência Solo 2014, 38, 1457–1468. [Google Scholar] [CrossRef]
- Gazola, B.; Mariano, E.; Andrade, M.G.O.; Costa, V.E.; Rosolem, C.A. Fate of fertilizer N applied to maize intercropped with forage grass and recovery of residual N by soybean in a double cropping system. Plant Soil 2023, 492, 1–15. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Soratto, R.P.; Crusciol, C.A.C. Análise da situação geral. In Integração Lavoura Pecuária-Floresta: Alguns Exemplos No Brasil Central; Soratto, R.P., Rosolem, C.A., Crusciol, C.A.C., Eds.; FEPAF: Botucatu, Brazil, 2011; pp. 103–104. [Google Scholar]
- Machado, A.O.; Cecato, U.; Mira, R.T.; Pereira, L.A.F.; Damasceno, J.C. Avaliação da composição química e digestibilidade in vitro da matéria seca de cultivares e acessos de Panicum maximum Jacq. sob duas alturas de corte. Rev. Bras. Zootec. 1998, 27, 1057–1063. [Google Scholar]
- Leonel, F.d.P.; Pereira, J.C.; Costa, M.G.; Marco, P., Jr.; Lara, L.A.; de Queiroz, A.C. Comportamento produtivo e características nutricionais do capim-braquiária cultivado em consórcio com milho. Rev. Bras. Zootec. 2009, 38, 177–189. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis; Klute, A., Ed.; SSSA Book Series, 5.1; SSSA, ASA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Raij, B.V.; Quaggio, J.A.; Cantarella, H.; Abreu, C.A. Os métodos de análise química do sistema IAC de análise de solo no contexto nacional. In Análise Química Para Avaliação da Fertilidade de Solos Tropicais, 2nd ed.; van Raij, B., Andrade, J.C., Cantarella, H., Eds.; IAC: Campinas, Brazil, 2001. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis; Sparks, D.L., Ed.; SSSA Book Series, 5; SSSA, ASA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric oxide. Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Rocha, K.F.; Mariano, E.; Grassmann, C.S.; Trivelin, P.C.; Rosolem, C.A. Fate of 15N fertilizer applied to maize in rotation with tropical forage grasses. Field Crops Res. 2019, 238, 35–44. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J. How a corn plant develops. In Special Report 48; Iowa State University of Science and Technology: Ames, IA, USA, 1986. [Google Scholar]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Symposium: Carbohydrate metodoly, metabolism, and nutritional implications in dairy cattle. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Fox, D.G.; Tedeschi, L.O.; Tylutki, T.P.; Russell, J.B.; Van Amburgh, M.E.; Chase, L.E.; Pell, A.N.; Overton, T.R. The Cornell net carbohydrate and protein system model for evaluating herd nutrition and nutrient excretion. Anim. Feed. Sci. Technol. 2004, 112, 29–78. [Google Scholar] [CrossRef]
- Borghi, E.; Crusciol, C.A.C.; Mateus, G.P.; Nascente, A.S.; Martins, P.O. Intercropping time of corn and palisadegrass or Guinea grass affecting grain yield and forage production. Crop Sci. 2013, 53, 629–636. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E.; Ludkiewicz, M.G.Z. Application of different nitrogen doses to increase nitrogen efficiency in Mombasa Guinegrass (Panicum maximum cv. Mombasa) at dry and rainy seasons. Aust. J. Crop Sci 2017, 11, 1657–1664. [Google Scholar] [CrossRef]
- Weiss, W.P. Predicitng energy values of feeds. J. Dairy Sci. 1993, 76, 1802–1811. [Google Scholar] [CrossRef]
- Macedo, G.L.M., Jr.; Zanine, A.M.; Borges, I.; Pérez, J.R.O. Qualidade da fibra para a dieta de ruminantes. Ciência Anim. 2007, 17, 7–17. [Google Scholar]
- Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: New York, NY, USA, 1994; 476p. [Google Scholar]
- Martuscello, J.A.; Fonseca, D.M.; do Nascimento, D., Jr.; Santos, P.M.; Ribeiro, J.I., Jr.; Cunha, D.N.F.V.; Moreira, L.M. Características morfogênicas e estruturais do capim-xaraés submetido à adubação nitrogenada e desfolhação. Rev. Bras. Zootec. 2005, 34, 1475–1482. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Silva, J.L.O.; Campos, B.A., Jr. Produção de matéria seca e composição mineral da forragem de seis gramíneas tropicais estabelecidas em um sub-bosque de angico vermelho. Rev. Bras. Zootec. 1997, 26, 213–218. [Google Scholar]
- Carnevalli, R.A.; Silva, S.C.; Bueno, A.A.O.; Uebele, M.C.; Bueno, F.O.; Hodgson, J.; Silva, G.N.; Morais, J.P.G. Herbage production and grazing losses in Panicum maximum cv. Mombaça under four grazing managements. Trop. Grassl. 2006, 40, 165–176. [Google Scholar]
- Zanini, G.D.; Santos, G.T.; Sbrissia, A.F. Frequencies and intensities of defoliation in Aruana Guineagrass swards: Morphogenetic and structural characteristics. Rev. Bras. Zootec. 2012, 41, 1848–1857. [Google Scholar] [CrossRef]
- Silva, C.C.F.; Bonomo, P.; Pires, A.J.V.; Maranhão, C.M.A.; Patês, N.M.S.; Santos, L.C. Características morfogênicas e estruturais de duas espécies de braquiária adubadas com diferentes doses de nitrogênio. Rev. Bras. Zootec. 2009, 38, 657–661. [Google Scholar] [CrossRef]
- Almeida, R.E.M.; Favarin, J.L.; Otto, R.; Pierozan, C., Jr.; Oliveira, S.M.; Tezotto, T.; Lago, B.C. Effects of nitrogen fertilization on yield components in a corn-palisadegrass intercropping system. Austr. J. Crop Sci. 2017, 11, 352–359. [Google Scholar] [CrossRef]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 chlorophyll meter for nitrogen management in corn. J. Plant Nutr. 1998, 21, 741–755. [Google Scholar] [CrossRef]
- Lavres, J., Jr.; Santos, J.D.G., Jr.; Monteiro, F.A. Nitrate reductase activity and SPAD readings in leaf tissues of guinea grass submitted to nitrogen and potassium rates. Rev. Bras. Ciência Solo 2010, 34, 801–809. [Google Scholar] [CrossRef]
- Hay, R.K.M.; Walker, A.J. An Introduction to the Physiology of Crop Yield; Longman Scientific and Technical: London, UK, 1989; 292p. [Google Scholar]
- Costa, K.A.P.; Faquin, V.; Oliveira, I.P.; Araújo, J.L.; Rodrigues, R.B. Doses e fontes de nitrogênio em pastagem de capim-marandu: II—Nutrição nitrogenada da planta. Rev. Bras. Ciência Solo 2008, 32, 1601–1607. [Google Scholar] [CrossRef]
- Costa, N.R.; Andreotti, M.; Gameiro, R.A.; Pariz, C.M.; Buzetti, S.; Lopes, K.S.M. Adubação nitrogenada no consórcio de milho com duas espécies de braquiária em sistema plantio direto. Pesqui. Agropecuária Bras. 2012, 47, 1038–1047. [Google Scholar] [CrossRef]
- Almeida, R.E.M.; Oliveira, S.M.; Lago, B.C.; Junior, C.P.; Trivelin, P.C.O.; Favarin, J.L. Palisadegrass effects on N fertilizer dynamic in intercropping systems with corn. An. Acad. Bras. Ciências 2017, 89, 1917–1923. [Google Scholar] [CrossRef]
- Lemaire, G.; Chartier, M. Relationships between growth dynamics and nitrogen uptake for individual sorghum plants growing at different plant densities. In Diagnosis of the Nitrogen Status in Crops; Lemaire, G., Ed.; INRA—Station décophysiologiedes Plantes Fourragères: Paris, France, 1992; pp. 3–43. [Google Scholar]
- Soares, A.B.; Sartor, L.R.; Adami, P.F.; Varella, A.C.; Fonseca, L.; Mezzalira, J.C. Influência da luminosidade no comportamento de onze espécies forrageiras perenes de verão. Rev. Bras. Zootec. 2009, 38, 443–451. [Google Scholar] [CrossRef]
- Kephart, K.D.; Buxton, D.R. Forage Quality Responses of C3 and C4 Perennial Grasses to Shade. Crop Sci. 1993, 33, 831–837. [Google Scholar] [CrossRef]
- Gerdes, L.; Werner, J.C.; Colozza, M.T.; Possenti, R.A.; Schammass, E.A. Avaliação de características de valor nutritivo das gramíneas forrageiras marandu, setária e tanzânia nas estações do ano. R. Bras. Zootecn. 2000, 29, 955–963. [Google Scholar] [CrossRef]
- Mazza, L.D.M.; Pôggere, G.C.; Ferraro, F.P.; Ribeiro, C.B.; Cherobim, V.F.; Motta, A.C.V.; De Moraes, A. Adubação nitrogenada na produtividade e composição química do capim-mombaça no primeiro planalto paranaense. Sci. Agrar. 2009, 10, 257. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Buzetti, S.; Dupas, E.; Teixeira Filho, M.C.M.; Andreotti, M. Sources and rates of nitrogen fertilizer used in Mombasa guineagrass in the Brazilian Cerrado region. Afr. J. Agric. Res. 2015, 10, 2076–2082. [Google Scholar] [CrossRef]


| Forage Grass | N Rate | MM | CP | NDF | ADF | HEM |
|---|---|---|---|---|---|---|
| kg ha−1 | % | |||||
| Guinea grass | - | 9.3 ± 0.2 a | 5.4 ± 0.2 | 76 ± 1 | 45 ± 1 | 31 ± 1 |
| Palisade grass | - | 7.9 ± 0.3 b | 6.2 ± 0.3 | 74 ± 1 | 43 ± 1 | 31 ± 1 |
| p = 0.018 | p = 0.104 | p = 0.083 | p = 0.097 | p = 0.411 | ||
| - | Control | 8.8 ± 0.3 | 5.2 ± 0.2 b | 72 ± 1 b | 39 ± 1 b | 33 ± 1 a |
| - | 90 | 9.0 ± 0.4 | 5.7 ± 0.3 b | 77 ± 1 a | 46 ± 1 a | 31 ± 1 b |
| - | 180 | 8.4 ± 0.5 | 5.4 ± 0.3 b | 76 ± 1 a | 45 ± 1 a | 31 ± 1 ab |
| - | 270 | 8.1 ± 0.4 | 7.1 ± 0.3 a | 76 ± 1 a | 46 ± 1 a | 30 ± 1 b |
| p = 0.056 | p < 0.001 | p = 0.001 | p < 0.001 | p = 0.004 | ||
| Guinea grass | Control | 8.9 ± 0.3 | 5.1 ± 0.3 | 72 ± 2 | 40 ± 1 | 33 ± 1 |
| Guinea grass | 90 | 9.7 ± 0.4 | 5.3 ± 0.4 | 77 ± 1 | 47 ± 1 | 30 ± 1 |
| Guinea grass | 180 | 9.4 ± 0.4 | 5.2 ± 0.4 | 77 ± 1 | 46 ± 2 | 31 ± 1 |
| Guinea grass | 270 | 9.1 ± 0.5 | 6.2 ± 0.4 | 77 ± 1 | 48 ± 2 | 29 ± 1 |
| Palisade grass | Control | 8.6 ± 0.6 | 5.2 ± 0.4 | 72 ± 1 | 39 ± 1 | 33 ± 1 |
| Palisade grass | 90 | 8.3 ± 0.4 | 6.1 ± 0.4 | 76 ± 1 | 45 ± 1 | 31 ± 1 |
| Palisade grass | 180 | 7.4 ± 0.5 | 5.6 ± 0.4 | 75 ± 1 | 44 ± 1 | 31 ± 1 |
| Palisade grass | 270 | 7.1 ± 0.1 | 8.3 ± 0.6 | 75 ± 1 | 44 ± 1 | 30 ± 1 |
| p = 0.080 | p = 0.139 | p = 0.743 | p = 0.791 | p = 0.970 | ||
| Forage Grass | N Rate | MM | CP | NDF | ADF | HEM | CEL | LIG |
|---|---|---|---|---|---|---|---|---|
| kg ha−1 | % | |||||||
| Guinea grass | - | 10.3 ± 0.2 | 10.0 ± 0.7 | 66 ± 1 a | 34 ± 1 a | 31 ± 1 | 30 ± 1 a | 2.2 ± 0.2 |
| Palisade grass | - | 10.4 ± 0.4 | 9.6 ± 0.6 | 62 ± 1 b | 31 ± 1 b | 31 ± 1 | 27 ± 1 b | 1.8 ± 0.2 |
| p = 0.910 | p = 0.809 | p = 0.030 | p = 0.004 | p = 0.631 | p = 0.009 | p = 0.234 | ||
| - | Control | 10.7 ± 0.4 | 7.6 ± 0.5 b | 64 ± 2 | 32 ± 1 | 32 ± 1 | 27 ± 1 | 2.02 ± 0.2 |
| - | 90 | 9.9 ± 0.6 | 11.4 ± 0.9 a | 64 ± 2 | 32 ± 1 | 31 ± 1 | 28 ± 1 | 2.22 ± 0.2 |
| - | 180 | 10.9 ± 0.5 | 10.3 ± 1.1 a | 64 ± 1 | 33 ± 1 | 31 ± 1 | 29 ± 1 | 1.93 ± 0.2 |
| - | 270 | 9.8 ± 0.4 | 9.9 ± 0.5 a | 64 ± 1 | 33 ± 1 | 31 ± 1 | 29 ± 1 | 1.98 ± 0.2 |
| p = 0.151 | p = 0.002 | p = 0.990 | p = 0.529 | p = 0.383 | p = 0.098 | p = 0.728 | ||
| Guinea grass | Control | 10.6 ± 0.5 | 7.8 ± 0.7 | 66 ± 2 | 33 ± 1 | 33 ± 1 | 28 ± 1 | 2.4 ± 0.3 |
| Guinea grass | 90 | 10.4 ± 1.1 | 10.8 ± 1.6 | 65 ± 2 | 34 ± 1 | 31 ± 1 | 29 ± 1 | 2.3 ± 0.3 |
| Guinea grass | 180 | 10.1 ± 0.5 | 11.3 ± 2.0 | 64 ± 1 | 34 ± 1 | 30 ± 1 | 30 ± 1 | 2.1 ± 0.3 |
| Guinea grass | 270 | 10.1 ± 0.5 | 9.9 ± 0.5 | 67 ± 1 | 36 ± 1 | 31 ± 1 | 32 ± 1 | 2.2 ± 0.3 |
| Palisade grass | Control | 10.8 ± 0.8 | 7.4 ± 0.8 | 60 ± 1 | 30 ± 1 | 30 ± 1 | 26 ± 1 | 1.7 ± 0.2 |
| Palisade grass | 90 | 9.4 ± 0.3 | 12.0 ± 1.0 | 62 ± 3 | 31 ± 2 | 31 ± 1 | 27 ± 1 | 2.1 ± 0.3 |
| Palisade grass | 180 | 11.6 ± 0.8 | 9.2 ± 1.0 | 63 ± 1 | 31 ± 1 | 31 ± 1 | 27 ± 1 | 1.8 ± 0.3 |
| Palisade grass | 270 | 9.6 ± 0.7 | 9.9 ± 1.0 | 61 ± 2 | 30 ± 1 | 30 ± 1 | 27 ± 1 | 1.8 ± 0.3 |
| p = 0.148 | p = 0.320 | p = 0.344 | p = 0.489 | p = 0.070 | p = 0.200 | p = 0.826 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilli, B.R.; Grassmann, C.S.; Mariano, E.; Rosolem, C.A. Nitrogen Fertilization Boosts Maize Grain Yield, Forage Quality, and Estimated Meat Production in Maize–Forage Intercropping. Agriculture 2023, 13, 2200. https://doi.org/10.3390/agriculture13122200
Gilli BR, Grassmann CS, Mariano E, Rosolem CA. Nitrogen Fertilization Boosts Maize Grain Yield, Forage Quality, and Estimated Meat Production in Maize–Forage Intercropping. Agriculture. 2023; 13(12):2200. https://doi.org/10.3390/agriculture13122200
Chicago/Turabian StyleGilli, Bruno R., Camila S. Grassmann, Eduardo Mariano, and Ciro A. Rosolem. 2023. "Nitrogen Fertilization Boosts Maize Grain Yield, Forage Quality, and Estimated Meat Production in Maize–Forage Intercropping" Agriculture 13, no. 12: 2200. https://doi.org/10.3390/agriculture13122200
APA StyleGilli, B. R., Grassmann, C. S., Mariano, E., & Rosolem, C. A. (2023). Nitrogen Fertilization Boosts Maize Grain Yield, Forage Quality, and Estimated Meat Production in Maize–Forage Intercropping. Agriculture, 13(12), 2200. https://doi.org/10.3390/agriculture13122200





