Influence of Farmyard Manure Application on Potential Zinc Solubilizing Microbial Species Abundance in a Ferralsol of Western Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Selection of Treatments
2.4. Soil Sampling and Analysis
2.5. Soil Chemical Analyses
2.6. Determination of Microbial Biomass Carbon
2.7. Deoxyribonucleic Acid (DNA) Extraction from Soil
2.7.1. Soil DNA Sequencing, Bioinformatics Sequence Processing and Taxonomic Identification
2.7.2. Identification of the Potential Zinc Solubilizing Microbial Species
2.8. Statistical Data Analysis
3. Results
3.1. Effects of Management Practices on Soil Chemical and Physical Characteristics in INM3 Site
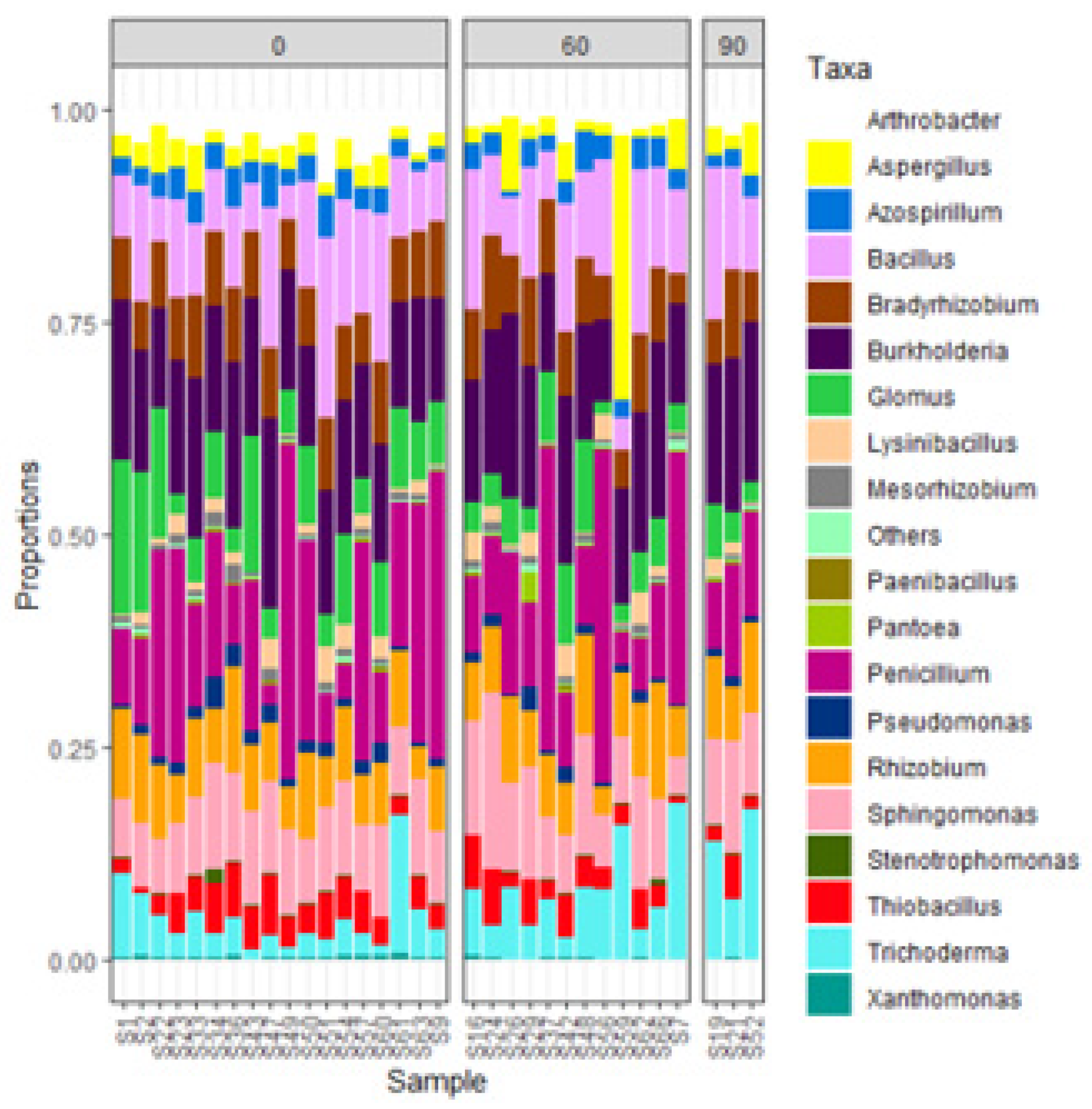
3.2. Overall Microbial Abundance and ZSM Species Abundance in INM3
3.3. Effect of Agronomic Management Practices on ZSM Species
3.4. Relationship between Soil Chemical Characteristics and ZSM Abundance
4. Discussion
4.1. Influence of Soil Chemical Properties on Zinc Solubilizing Microbial Species
4.2. Stimulation of Zinc Solubilizing Microbial Species with FYM Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, V.S.; Madhaiyan, M.; Thangaraju, M. Solubilization of Zinc Compounds by the Diazotrophic, Plant Growth Promoting Bacterium Gluconacetobacter Diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef]
- Cakmak, I.; McLaughlin, M.J.; White, P. Zinc for Better Crop Production and Human Health. Plant Soil 2017, 411, 1–4. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Kihara, J.; Bolo, P.; Kinyua, M.; Rurinda, J.; Piikki, K. Micronutrient Deficiencies in African Soils and the Human Nutritional Nexus: Opportunities with Staple Crops. Environ. Geochem. Health 2020, 42, 3015–3033. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A Review of Zinc Nutrition and Plant Breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cooper, J.M.; Lin, Z.; Li, Y.; Yang, X.; Zhao, B. Soil Microbial Community Structure and Function Are Significantly Affected by Long-Term Organic and Mineral Fertilization Regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Deng, Y.; Chen, R.-Y.; Cui, Z.-L.; Chen, X.-P.; Yost, R.; Zhang, F.-S.; Zou, C.-Q. The Reduction in Zinc Concentration of Wheat Grain upon Increased Phosphorus-Fertilization and Its Mitigation by Foliar Zinc Application. Plant Soil 2012, 361, 143–152. [Google Scholar] [CrossRef]
- Pasley, H.R.; Cairns, J.E.; Camberato, J.J.; Vyn, T.J. Nitrogen Fertilizer Rate Increases Plant Uptake and Soil Availability of Essential Nutrients in Continuous Maize Production in Kenya and Zimbabwe. Nutr. Cycl. Agroecosyst. 2019, 115, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Kashyap, P.L.; Pandiyan, K.; Bhardwaj, A.K. Zinc-Solubilizing Microbes for Sustainable Crop Production: Current Understanding, Opportunities, and Challenges. In Phytobiomes: Current Insights and Future Vistas; Solanki, M.K., Kashyap, P.L., Kumari, B., Eds.; Springer: Singapore, 2020; pp. 281–298. ISBN 9789811531507. [Google Scholar]
- Khanghahi, M.Y.; Ricciuti, P.; Allegretta, I.; Terzano, R.; Crecchio, C. Solubilization of Insoluble Zinc Compounds by Zinc Solubilizing Bacteria (ZSB) and Optimization of Their Growth Conditions. Environ. Sci. Pollut. Res. 2018, 25, 25862–25868. [Google Scholar] [CrossRef] [PubMed]
- Nitu, R.; Rajinder, K.; Sukhminderjit, K. Zinc Solubilizing Bacteria to Augment Soil Fertility—A Comprehensive Review. Int. J. Agricult. Sci. Vet. Med. 2020, 8, 38–44. [Google Scholar]
- Saini, P.; Nagpal, S.; Saini, P.; Kumar, A.; Gani, M. Microbial Mediated Zinc Solubilization in Legumes for Sustainable Agriculture. In Phytomicrobiome Interactions and Sustainable Agriculture; Verma, A., Saini, J.K., Hesham, A.E., Singh, H.B., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 254–276. ISBN 978-1-119-64462-0. [Google Scholar]
- Dhaked, B.S.; Triveni, S.; Reddy, R.S.; Padmaja, G. Isolation and Screening of Potassium and Zinc Solubilizing Bacteria from Different Rhizosphere Soil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1271–1281. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, M.; Jamil, M.; Naveed, M.; Zaman Akhtar, M.F.U. Zinc Solubilizing Bacteria for Zinc Biofortification in Cereals: A Step Toward Sustainable Nutritional Security. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 203–227. ISBN 9789811300431. [Google Scholar]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of Zinc Salts by a Bacterium Isolated from the Air Environment of a Tannery. FEMS Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus Spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Khande, R.; Sharma, S.K.; Ramesh, A.; Sharma, M.P. Zinc Solubilizing Bacillus Strains That Modulate Growth, Yield and Zinc Biofortification of Soybean and Wheat. Rhizosphere 2017, 4, 126–138. [Google Scholar] [CrossRef]
- Vidyashree, D.N.; Muthuraju, R.; Panneerselvam, P. Evaluation of Zinc Solubilizing Bacterial (ZSB) Strains on Growth, Yield and Quality of Tomato (Lycopersicon Esculentum). Int. J. Curr. Microbiol. Appl. Sci 2018, 7, 2018. [Google Scholar] [CrossRef]
- Anuradha, P.; Syed, I.; Swati, M.; Patil, V.D. Solubilization of Insoluble Zinc Compounds by Different Microbial Isolates in Vitro Condition. Int. J. Trop. Agric. 2015, 33, 865–869. [Google Scholar]
- Rawat, N.; Neelam, K.; Tiwari, V.K.; Dhaliwal, H.S. Biofortification of Cereals to Overcome Hidden Hunger. Plant Breed. 2013, 132, 437–445. [Google Scholar] [CrossRef]
- Rengel, Z.; Batten, G.D.; Crowley, D. dy1999 Agronomic Approaches for Improving the Micronutrient Density in Edible Portions of Field Crops. Field Crops Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Klassen-Wigger, P.; Geraets, M.; Messier, M.C.; Detzel, P.; Lenoble, H.P.; Barclay, D.V. Micronutrient Fortification of Bouillon Cubes in Central and West Africa. In Food Fortification in a Globalized World; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–372. [Google Scholar]
- Welch, R.M. The Impact of Mineral Nutrients in Food Crops on Global Human Health. Plant Soil 2002, 247, 83–90. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure Acts as a Better Fertilizer for Increasing Crop Yields than Synthetic Fertilizer Does by Improving Soil Fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Jama, B.; Palm, C.A.; Buresh, R.J.; Niang, A.; Gachengo, C.; Nziguheba, G.; Amadalo, B. Tithonia Diversifolia as a Green Manure for Soil Fertility Improvement in Western Kenya: A Review. Agrofor. Syst. 2000, 49, 201–221. [Google Scholar] [CrossRef]
- Kearney, S.G.; Carwardine, J.; Reside, A.E.; Adams, V.M.; Nelson, R.; Coggan, A.; Spindler, R.; Watson, J.E.M. Saving Species beyond the Protected Area Fence: Threats Must Be Managed across Multiple Land Tenure Types to Secure Australia’s Endangered Species. Conserv. Sci. Prac. 2022, 4, e617. [Google Scholar] [CrossRef]
- Mucheru-Muna, M.; Mugendi, D.; Pypers, P.; Mugwe, J.; JAMES, K.; Vanlauwe, B.; Merckx, R. Enhancing Maize Productivity and Profitability Using Organic Inputs and Mineral Fertilizer in Central Kenya Small-Hold Farms. Exp. Agric. 2014, 50, 250–269. [Google Scholar] [CrossRef]
- Mugwe, J.; Mugendi, D.; Mucheru-Muna, M.; Odee, D.; Mairura, F. Effect of Selected Organic Materials and Inorganic Fertilizer on the Soil Fertility of a Humic Nitisol in the Central Highlands of Kenya. Soil Use Manag. 2009, 25, 434–440. [Google Scholar] [CrossRef]
- Gautam, A.; Sekaran, U.; Guzman, J.; Kovács, P.; Hernandez, J.L.G.; Kumar, S. Responses of Soil Microbial Community Structure and Enzymatic Activities to Long-Term Application of Mineral Fertilizer and Beef Manure. Environ. Sustain. Indic. 2020, 8, 100073. [Google Scholar] [CrossRef]
- Kihanda, F.M.; Warren, G.P.; Micheni, A.N. Effects of Manure Application on Crop Yield and Soil Chemical Properties in a Long-Term Field Trial in Semi-Arid Kenya. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Bationo, A., Waswa, B., Kihara, J., Kimetu, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 471–486. ISBN 978-1-4020-5759-5. [Google Scholar]
- Tang, H.; Li, C.; Xiao, X.; Shi, L.; Cheng, K.; Wen, L.; Li, W. Effects of Short-Term Manure Nitrogen Input on Soil Microbial Community Structure and Diversity in a Double-Cropping Paddy Field of Southern China. Sci. Rep. 2020, 10, 13540. [Google Scholar] [CrossRef]
- Maguta, J.K. Conservation Tillage in Kenya: The Biophysical Processes Affecting Its Effectiveness. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2009. [Google Scholar]
- Bolo, P.; Kihara, J.; Mucheru-Muna, M.; Njeru, E.M.; Kinyua, M.; Sommer, R. Application of Residue, Inorganic Fertilizer and Lime Affect Phosphorus Solubilizing Microorganisms and Microbial Biomass under Different Tillage and Cropping Systems in a Ferralsol. Geoderma 2021, 390, 114962. [Google Scholar] [CrossRef]
- Paul, B.K.; Vanlauwe, B.; Ayuke, F.; Gassner, A.; Hoogmoed, M.; Hurisso, T.T.; Koala, S.; Lelei, D.; Ndabamenye, T.; Six, J. Medium-Term Impact of Tillage and Residue Management on Soil Aggregate Stability, Soil Carbon and Crop Productivity. Agric. Ecosyst. Environ. 2013, 164, 14–22. [Google Scholar] [CrossRef]
- Orwa, P.; Mugambi, G.; Wekesa, V.; Mwirichia, R. Isolation of Haloalkaliphilic Fungi from Lake Magadi in Kenya. Heliyon 2020, 6, e02823. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Educaton and Data Anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Koorem, K.; Gazol, A.; Öpik, M.; Moora, M.; Saks, Ü.; Uibopuu, A.; Sober, V.; Zobel, M. Soil Nutrient Content Influences the Abundance of Soil Microbes but Not Plant Biomass at the Small-Scale. PLoS ONE 2014, 9, e91998. [Google Scholar] [CrossRef]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of Zinc Solubilizing Bacteria in Growth Promotion and Zinc Content of Wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A. Cross Talk between Zinc-Solubilizing Bacteria and Plants: A Short Tale of Bacterial-Assisted Zinc Biofortification. Front. Soil Sci. 2022, 1, 788170. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Rengel, Z.; Marschner, P. Nutrient Availability and Management in the Rhizosphere: Exploiting Genotypic Differences. New Phytol. 2005, 168, 305–312. [Google Scholar] [CrossRef]
- Wallman, M.; Delin, S. Nitrogen Leaching from Tile-drained Fields and Lysimeters Receiving Contrasting Rates and Sources of Nitrogen. Soil Use Manag. 2022, 38, 596–610. [Google Scholar] [CrossRef]
- Kihara, J.; Martius, C.; Bationo, A.; Thuita, M.; Lesueur, D.; Herrmann, L.; Amelung, W.; Vlek, P.L. Soil Aggregation and Total Diversity of Bacteria and Fungi in Various Tillage Systems of Sub-Humid and Semi-Arid Kenya. Appl. Soil Ecol. 2012, 58, 12–20. [Google Scholar] [CrossRef]
- Margenot, A.J.; Sommer, R.; Mukalama, J.; Parikh, S.J. Biological P Cycling Is Influenced by the Form of P Fertilizer in an Oxisol. Biol. Fertil. Soils 2017, 53, 899–909. [Google Scholar] [CrossRef]
- Xue, P.-P.; Carrillo, Y.; Pino, V.; Minasny, B.; McBratney, A.B. Soil Properties Drive Microbial Community Structure in a Large Scale Transect in South Eastern Australia. Sci. Rep. 2018, 8, 11725. [Google Scholar] [CrossRef]
- Niu, H.; Pang, Z.; Fallah, N.; Zhou, Y.; Zhang, C.; Hu, C.; Lin, W.; Yuan, Z. Diversity of Microbial Communities and Soil Nutrients in Sugarcane Rhizosphere Soil under Water Soluble Fertilizer. PLoS ONE 2021, 16, e0245626. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z.; Nian, H. Impact of Intercropping on the Coupling between Soil Microbial Community Structure, Activity, and Nutrient-Use Efficiencies. PeerJ 2019, 7, e6412. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover Crops to Increase Soil Microbial Diversity and Mitigate Decline in Perennial Agriculture. A Review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial Nitrogen Acquisition by Arbuscular Mycorrhizal Fungi from Organic Material Has Implications for N Cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Zhi-Hui, Y.; Stöven, K.; Haneklaus, S.; Singh, B.R.; Schnug, E. Elemental Sulfur Oxidation by Thiobacillus Spp. and Aerobic Heterotrophic Sulfur-Oxidizing Bacteria. Pedosphere 2010, 20, 71–79. [Google Scholar]
- Ashworth, A.J.; DeBruyn, J.M.; Allen, F.L.; Radosevich, M.; Owens, P.R. Microbial Community Structure Is Affected by Cropping Sequences and Poultry Litter under Long-Term No-Tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi Filho, A.; Andrade, D.S.; Dick, R.P. Long-Term Tillage and Crop Rotation Effects on Microbial Biomass and C and N Mineralization in a Brazilian Oxisol. Soil Tillage Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative Agriculture: An Agronomic Perspective. Outlook Agric. 2021, 50, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mucheru-Muna, M.; Mugendi, D.; Kung’u, J.; Mugwe, J.; Bationo, A. Effects of Organic and Mineral Fertilizer Inputs on Maize Yield and Soil Chemical Properties in a Maize Cropping System in Meru South District, Kenya. Agrofor. Syst. 2007, 69, 189–197. [Google Scholar] [CrossRef]
- Ayaga, G.; Todd, A.; Brookes, P.C. Enhanced Biological Cycling of Phosphorus Increases Its Availability to Crops in Low-Input Sub-Saharan Farming Systems. Soil Biol. Biochem. 2006, 38, 81–90. [Google Scholar] [CrossRef]
- Babin, D.; Deubel, A.; Jacquiod, S.; Sørensen, S.J.; Geistlinger, J.; Grosch, R.; Smalla, K. Impact of Long-Term Agricultural Management Practices on Soil Prokaryotic Communities. Soil Biol. Biochem. 2019, 129, 17–28. [Google Scholar] [CrossRef]
- Lladó, S.; Baldrian, P. Community-Level Physiological Profiling Analyses Show Potential to Identify the Copiotrophic Bacteria Present in Soil Environments. PLoS ONE 2017, 12, e0171638. [Google Scholar] [CrossRef] [PubMed]
- Tomsone, L.; Kruma, Z.; Alsina, I.; Lepse, L. The Application of Hierarchical Cluster Analysis for Clasifying Horseradish Genotypes (Armoracia rusticana L.) Roots. Chem. Technol. 2012, 62, 52–56. [Google Scholar]
- Kumar, V.; Ram, S.; Chandra, R. Crop Productivity and Soil Biological Properties Influenced by Long Term Application of Mineral Fertilizers and Manures under Rice-Wheat Sequence on Mollisols of Northern India. Int. J. Curr. Microbiol. App. Sci 2019, 8, 299–312. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Wang, R.; Guo, S. Responses of Nitrification and Denitrification to Nitrogen and Phosphorus Fertilization: Does the Intrinsic Soil Fertility Matter? Plant Soil 2019, 440, 443–456. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
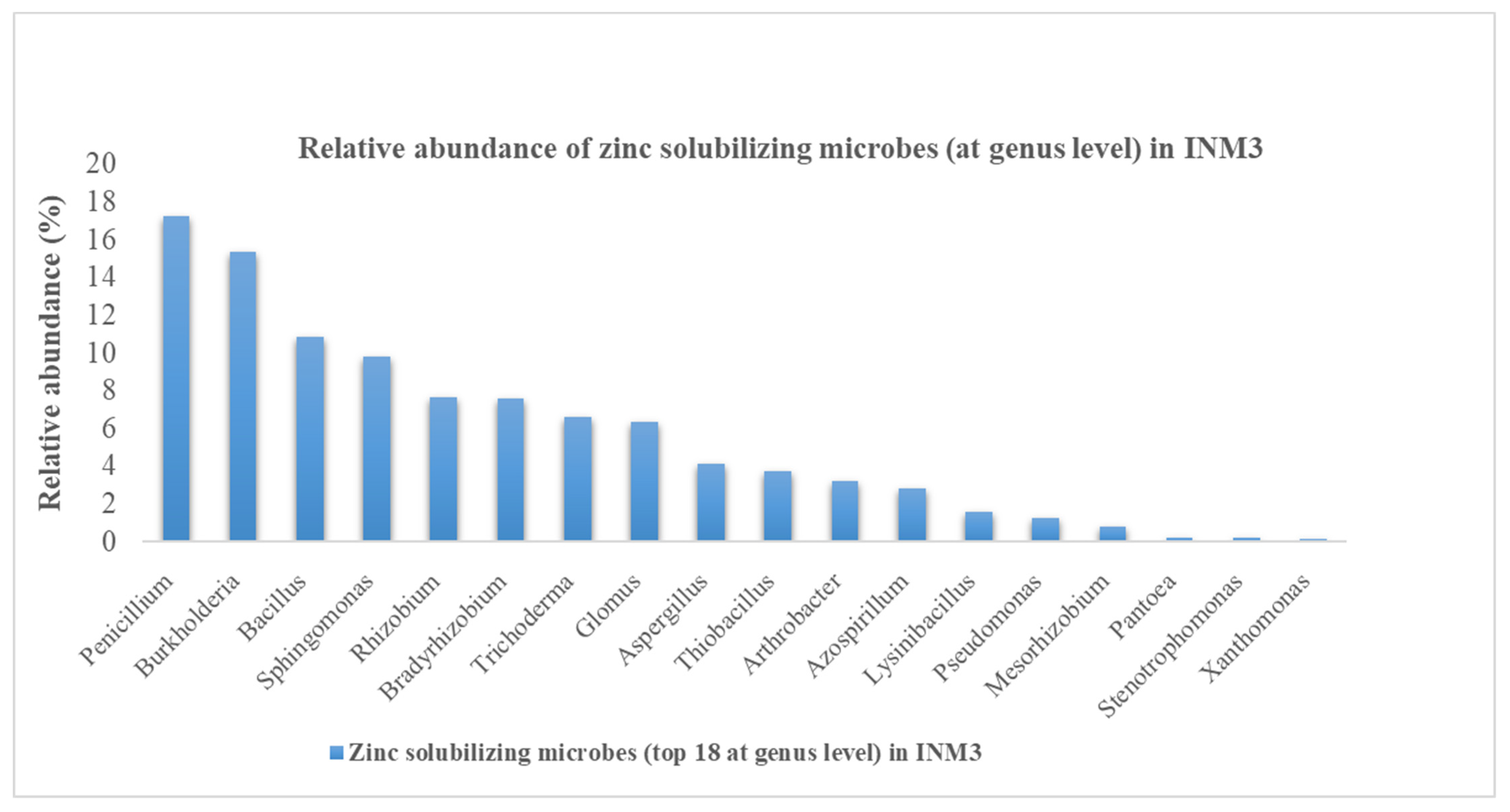

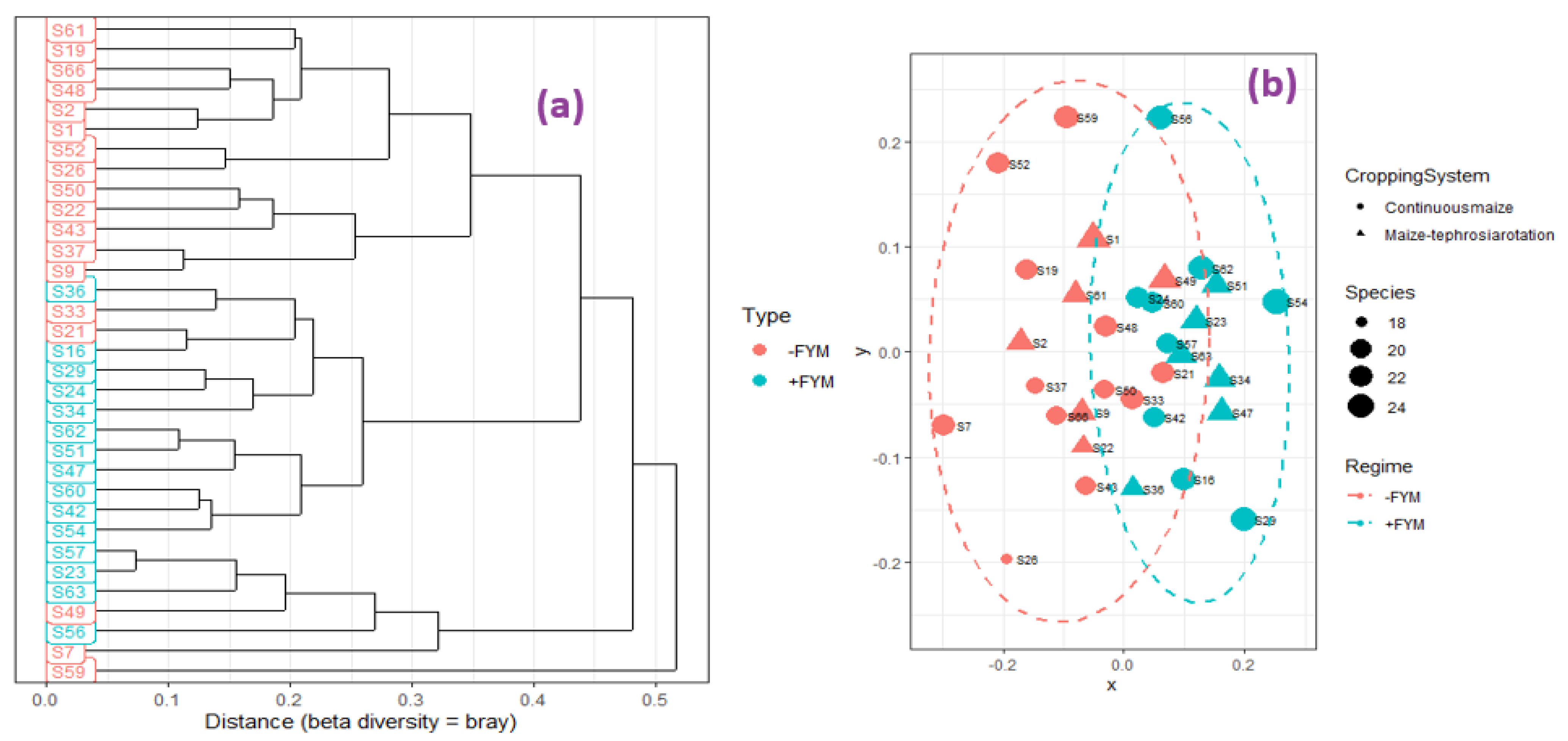
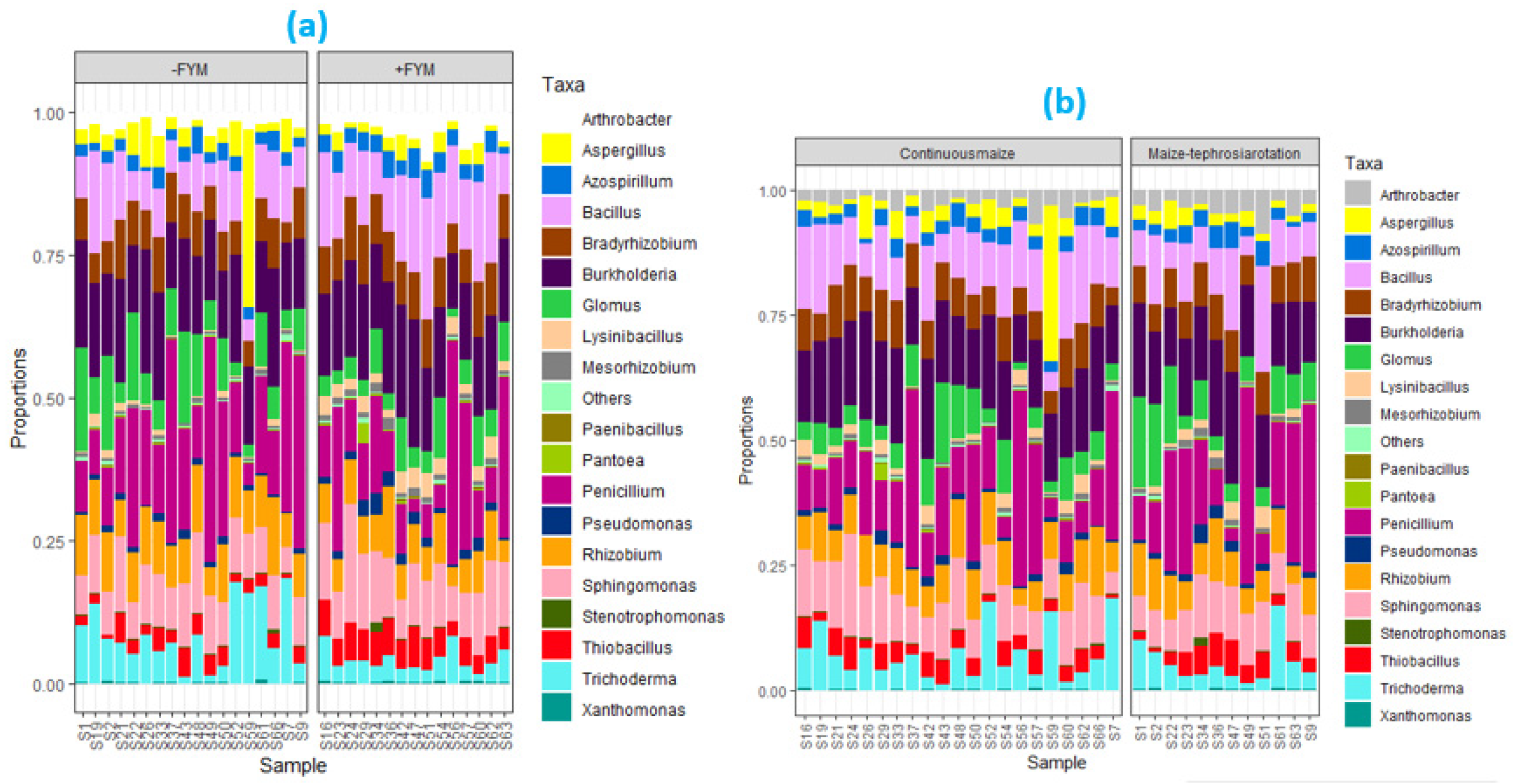
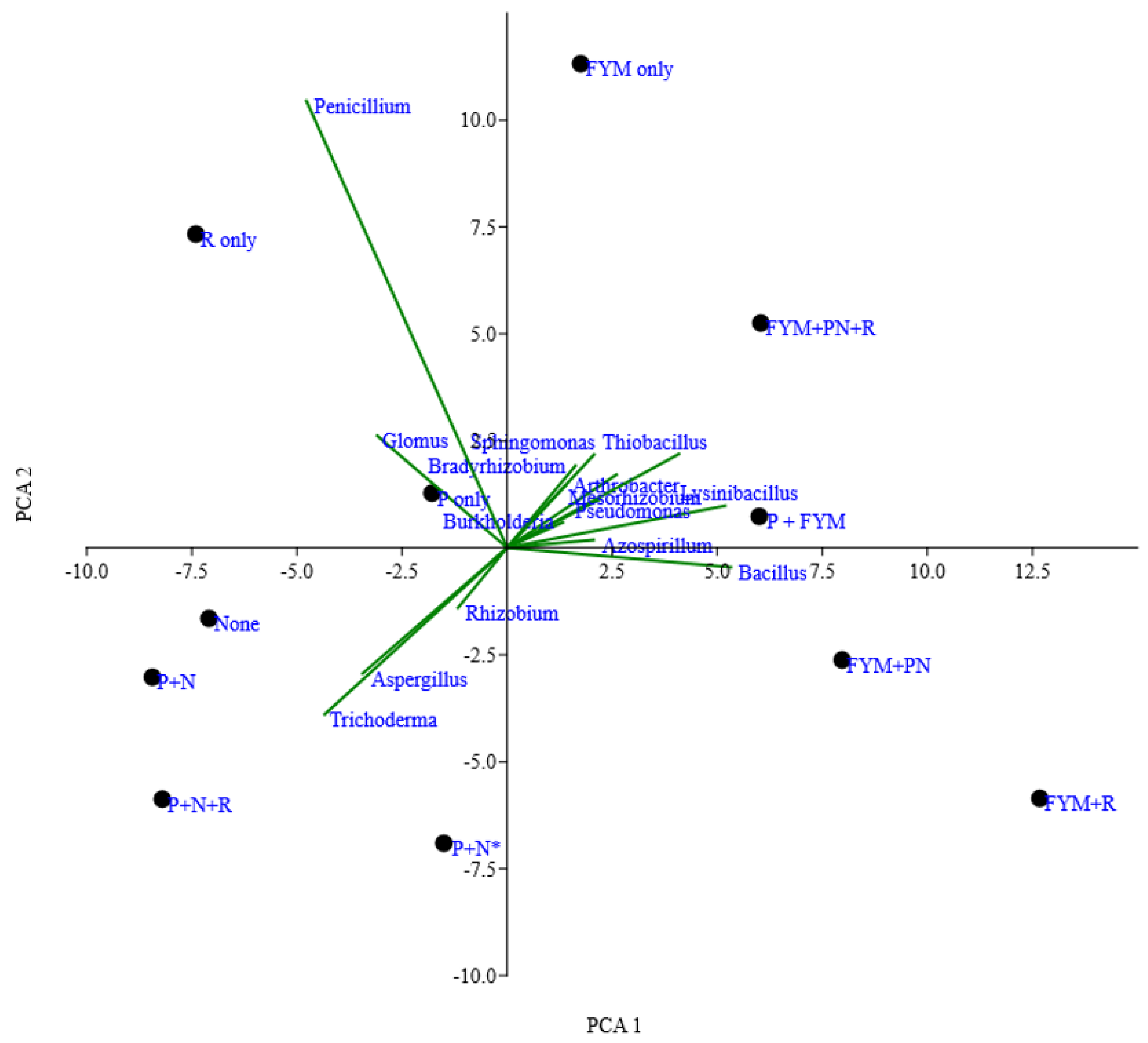


| Code | Treatment Abbreviation | Treatment Description | Cropping System | FYM | Res | N (Kg ha−1) | P (Kg ha−1) |
|---|---|---|---|---|---|---|---|
| Inm1 | None | No input | MT | - | - | 0 | 0 |
| Inm2 | P + N | NP fertilizer | MM | - | - | 60 | 45 |
| Inm3 | R only | Residue | MT | - | + | 0 | 0 |
| Inm4 | FYM only | FYM | MT | + | - | 0 | 0 |
| Inm5 | P + N + R | NP fertilizer + residue only | MM | - | + | 60 | 45 |
| Inm6 | FYM + PN + R | NP fertilizer + residue +FYM | MM | + | + | 60 | 45 |
| Inm7 | FYM + PN | NP fertilizer + FYM only | MT | + | - | 60 | 45 |
| Inm8 | FYM + R | Residue + FYM only | MM | + | + | 0 | 0 |
| Inm9 | P only | P fertilizer only | MM | - | - | 0 | 45 |
| Inm10 | P + FYM | P + FYM only | MM | + | - | 0 | 45 |
| Inm11 | P + N * | 90N + P fertilizer only | MM | - | - | 90 | 45 |
| Treatment | pH | SOC (%) | N (%) | P (mg kg−1) | Mg (mg kg−1) | Mn (mg kg−1) | S (mg kg−1) | Cu (mg kg−1) | B (mg kg−1) | Zn (mg kg−1) | Fe (mg kg−1) | EC * | CEC # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inm1 | 4.84 de | 1.70 cd | 0.13 ef | 18.1 e | 37.5 d | 394 ab | 17.1 d | 5.26 c | 0.13 cd | 1.00 c | 118 c | 31.7 b | 6.54 d |
| Inm2 | 4.51 f | 1.64 d | 0.12 f | 79.0 a | 37.5 d | 431 ab | 22.1 bc | 5.51 bc | 0.17 bcd | 1.30 c | 128 bc | 43.6 ab | 6.84 d |
| Inm3 | 4.88 de | 1.83 bcd | 0.14 def | 13.0 e | 45.3 d | 400 ab | 19.0 cd | 5.62 abc | 0.18 abcd | 1.23 c | 117 c | 32.0 b | 6.64 d |
| Inm4 | 5.16 abc | 2.05 a | 0.17 ab | 23.8 de | 122.3 ab | 396 ab | 19.8 cd | 5.92 abc | 0.23 abc | 2.61 ab | 143 b | 42.0 ab | 11.8 ab |
| Inm5 | 4.66 ef | 1.68 cd | 0.13 f | 71.2 ab | 36.7 d | 441 a | 23.3 ab | 5.65 abc | 0.12 cd | 1.28 c | 133 bc | 44.0 ab | 6.71 d |
| Inm6 | 5.06 bcd | 1.96 ab | 0.16 bcd | 65.6 ab | 138.7 a | 397 ab | 21.1 bc | 6.29 ab | 0.19 abcd | 3.57 a | 169 a | 52.2 a | 12.9 a |
| Inm7 | 4.95 cd | 1.86 abc | 0.15 cde | 55.9 bc | 126.7 ab | 366 b | 23.7 ab | 6.12 abc | 0.17 bcd | 3.29 a | 174 a | 50.4 a | 12.6 a |
| Inm8 | 5.43 a | 2.07 a | 0.18 a | 20.5 e | 152.3 a | 420 ab | 21.8 bc | 6.37 a | 0.29 a | 3.46 a | 126 bc | 42.5 ab | 12.1 ab |
| Inm9 | 4.98 cd | 1.70 cd | 0.13 ef | 56.7 bc | 89.8 bc | 389 ab | 24.0 ab | 5.85 abc | 0.17 bcd | 1.84 bc | 126 bc | 42.7 ab | 10.1 bc |
| Inm10 | 5.32 ab | 1.83 bcd | 0.16 bc | 59.2 abc | 161.5 a | 405 ab | 26.2 a | 6.23 ab | 0.27 ab | 2.58 ab | 139 bc | 47.8 a | 13.0 a |
| Inm11 | 4.54 f | 1.74 cd | 0.14 ef | 42.5 cd | 55.4 cd | 425 ab | 24.3 ab | 5.77 abc | 0.12 d | 1.61 bc | 129 bc | 48.2 a | 8.62 cd |
| P † | ||||||||||||||||
| P † | 1 | N (%) | ||||||||||||||
| N (%) | −0.43 * | 1 | SOC (%) | |||||||||||||
| SOC (%) | −0.43 * | 0.85 ** | 1 | pH | ||||||||||||
| pH (1:2) ₩ | −0.34 | 0.76 ** | 0.70 ** | 1 | K † | |||||||||||
| K † | −0.40 * | 0.76 ** | 0.72 ** | 0.80 ** | 1 | Ca † | ||||||||||
| Ca † | −0.10 | 0.71 ** | 0.54 ** | 0.82 ** | 0.58 ** | 1 | Mg † | |||||||||
| Mg † | −0.10 | 0.70 ** | 0.73 ** | 0.85 ** | 0.70 ** | 0.87 ** | 1 | Mn † | ||||||||
| Mn † | 0.07 | 0.15 | 0.07 | −0.02 | −0.11 | −0.04 | −0.07 | 1 | S † | |||||||
| S † | 0.45 * | −0.02 | −0.18 | −0.01 | −0.16 | 0.32 | 0.25 | −0.11 | 1 | Cu † | ||||||
| Cu † | −0.07 | 0.44 * | 0.54 ** | 0.49 ** | 0.46 ** | 0.56 ** | 0.65 ** | 0.01 | 0.26 | 1 | B † | |||||
| B † | −0.30 | 0.73 ** | 0.60 ** | 0.72 ** | 0.54 ** | 0.69 ** | 0.63 ** | 0.23 | 0.11 | 0.43 * | 1 | Zn † | ||||
| Zn † | −0.02 | 0.73 ** | 0.70 ** | 0.65 ** | 0.62 ** | 0.67 ** | 0.84 ** | −0.04 | 0.23 | 0.54 ** | 0.42 * | 1 | Fe † | |||
| Fe † | 0.36 * | 0.19 | 0.30 | 0.14 | 0.21 | 0.29 | 0.41 * | −0.14 | 0.10 | 0.28 | −0.09 | 0.56 ** | 1 | Na † | ||
| Na † | 0.13 | −0.01 | 0.24 | 0.21 | −0.05 | 0.38 * | 0.41 * | −0.04 | 0.18 | 0.38 * | 0.08 | 0.18 | 0.26 | 1 | EC | |
| EC ¥ | 0.43 * | 0.18 | −0.05 | −0.10 | −0.02 | 0.23 | 0.19 | −0.22 | 0.47 ** | 0.28 | −0.04 | 0.40 * | 0.47 ** | 0.09 | 1 | CEC |
| CEC # | 0.06 | 0.67 ** | 0.60 ** | 0.67 ** | 0.45 ** | 0.85 ** | 0.82 ** | 0.08 | 0.28 | 0.60 ** | 0.53 ** | 0.74 ** | 0.55 ** | 0.45 * | 0.41 * | 1 |
| Inputs | Diversity (Shannon) | Richness | pH | SOC (%) | N (%) | Olsen P (mg kg−1) | K (mg kg−1) | Zn (mg kg−1) | Fe (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Nitrogen (kg ha−1) 0 | 2.33 ± 0.15 a | 21.2 ± 1.44 a | 5.10 ± 0.26 a | 1.85 ± 0.17 | 0.15 ± 0.02 a | 31.9 ± 22.0 b | 343 ± 123 a | 2.12 ± 1.02 a | 128 ± 15.5 a |
| 60 | 2.27 ± 0.19 a | 21.3 ± 2.10 a | 4.80 ± 0.27 a | 1.79 ± 0.18 | 0.14 ± 0.02 a | 66.6 ± 15.8 a | 251 ± 94 a | 2.36 ± 1.27 a | 151 ± 25.2 a |
| 90 | 2.32 ± 0.07 a | 21.0 ± 1.00 a | 4.54 ± 0.13 a | 1.75 ± 0.10 | 0.14 ± 0.01 a | 42.5 ± 16.7 ab | 162 ± 39 a | 1.61 ± 0.50 a | 129 ± 7.8 a |
| Phosphorus (kg ha−1) 0 | 2.30 ± 0.16 a | 21.1 ± 1.24 a | 5.08 ± 0.26 a | 1.90 ± 0.19 | 0.16 ± 0.02 a | 18.9 ± 5.65 b | 381 ± 124 a | 2.07 ± 1.18 a | 126 ± 14.6 a |
| 45 | 2.31 ± 0.16 a | 21.3 ± 1.85 a | 4.86 ± 0.32 a | 1.77 ± 0.15 | 0.14 ± 0.02 a | 60.7 ± 18.0 a | 243 ± 89.3 a | 2.21 ± 1.04 a | 143± 23.1 a |
| FYM (t ha−1) 0 | 2.25 ± 0.15 b | 20.44 ± 1.42 b | 4.74 ± 0.21 b | 1.72 ± 0.11 | 0.13 ± 0.01 b | 46.8 ± 27.0 a | 212 ± 60.5 b | 1.38 ± 0.45 b | 125 ± 9.8 b |
| 4 | 2.38 ± 0.15 a | 22.2 ± 1.37 a | 5.19 ± 0.24 a | 1.94 ± 0.15 | 0.16 ± 0.02 a | 43.9 ± 23.5 a | 390 ± 104 a | 3.10 ± 0.82 a | 150 ± 24.3 a |
| Residue (t ha−1) 0 | 2.35 ± 0.12 a | 21.2 ± 1.61 a | 4.9 ± 0.32 a | 1.79 ± 0.15 | 0.14 ± 0.02 a | 47.1 ± 23.1 a | 257 ± 103 a | 2.03 ± 0.84 a | 137 ± 21.1 a |
| 2 | 2.23 ± 0.20 a | 21.3 ± 1.76 a | 5.01 ± 0.31 a | 1.87 ± 0.20 | 0.15 ± 0.02 a | 42.6 ± 29.1 a | 357 ± 130 a | 2.38 ± 1.42 a | 136 ± 23.8 a |
| Microbial Indices | Olsen P (mg kg−1) | N (%) | SOC (%) | pH | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | Zn (mg kg−1) | Fe (mg kg−1) | CEC (meq 100 g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Diversity (Shannon) | −0.137 | 0.286 | 0.167 | 0.375 * | 0.312 | 0.498 ** | 0.480 ** | 0.468 ** | 0.283 | 0.350 * |
| Species richness | 0.105 | 0.252 | 0.390 * | 0.272 | 0.300 | 0.382 * | 0.422 * | 0.348 * | 0.642 ** | 0.514 ** |
| Variables | Burkholderia spp. | Bacillus spp. | Trichoderma spp. | Rhizobium spp. | Sphingomonas spp. | Bradyrhizobium spp. | Glomus spp. | Aspergillus spp. | Arthrobacter spp. | Thiobacillus spp. | Pseudomonas spp. | Azospirillum spp. | Lysinibacillus spp. | Mesorhizobium spp. | Paenibacillus spp. | Pantoea spp. | Xanthomonas spp. | Staphylococcus spp. | Mucor spp. | Serratia spp. | Agrobacterium spp. | Micrococcus spp. | Sporosarcina spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P §§ | 0.01 | 0.17 | 0.37* | 0.24 | 0.02 | −0.06 | −0.23 | 0.26 | −0.33 | −0.19 | −0.24 | 0.08 | 0.09 | −0.24 | 0.19 | 0.08 | −0.33 | 0.25 | 0.01 | 0.25 | −0.08 | −0.02 | 0.10 |
| N (%) | 0.41 * | 0.37 * | −0.35 * | −0.21 | 0.41 * | 0.47 ** | −0.30 | −0.19 | 0.64 ** | 0.77 ** | 0.57 ** | 0.52 ** | 0.49 ** | 0.78 ** | 0.17 | −0.02 | 0.46 ** | 0.12 | −0.30 | −0.17 | 0.35 * | 0.16 | 0.07 |
| SOC (%) | 0.38 * | 0.37 * | −0.31 | −0.44* | 0.60 ** | 0.57 ** | −0.18 | −0.12 | 0.47 ** | 0.74 ** | 0.54 ** | 0.45* | 0.52 ** | 0.71 ** | 0.05 | −0.03 | 0.21 | 0.18 | −0.25 | −0.12 | 0.37 * | 0.30 | 0.20 |
| pH | 0.11 | 0.30 | −0.53 ** | −0.35* | 0.24 | 0.48 ** | −0.04 | −0.15 | 0.65 ** | 0.65 ** | 0.60 ** | 0.51 ** | 0.47 ** | 0.65 ** | 0.06 | 0.03 | 0.42 * | 0.14 | −0.42 * | −0.09 | 0.37 * | 0.19 | −0.08 |
| K §§ | 0.17 | 0.33 | −0.48 ** | −0.30 | 0.23 | 0.44 * | −0.08 | −0.27 | 0.46 ** | 0.57 ** | 0.55 ** | 0.49 ** | 0.54 ** | 0.69 ** | −0.09 | 0.09 | 0.22 | −0.01 | −0.29 | −0.24 | 0.48 ** | 0.22 | −0.04 |
| Ca §§ | 0.20 | 0.52 ** | −0.42 * | −0.33 | 0.24 | 0.39 * | −0.27 | −0.14 | 0.60 ** | 0.71 ** | 0.66 ** | 0.57 ** | 0.61 ** | 0.58 ** | 0.29 | 0.12 | 0.50 ** | 0.18 | −0.45 ** | 0.07 | 0.32 | 0.16 | 0.19 |
| Mg §§ | 0.24 | 0.50 ** | −0.45 ** | −0.37* | 0.41 * | 0.55 ** | −0.30 | −0.16 | 0.42* | 0.71 ** | 0.60 ** | 0.53 ** | 0.66 ** | 0.60 ** | 0.14 | 0.11 | 0.30 | 0.21 | −0.41 * | 0.06 | 0.28 | 0.23 | 0.19 |
| Mn §§ | 0.34 * | −0.19 | 0.40 * | 0.62 ** | 0.18 | −0.10 | −0.22 | 0.49 ** | 0.17 | −0.03 | 0.06 | 0.23 | −0.34 | 0.22 | −0.07 | −0.13 | −0.11 | 0.26 | 0.28 | −0.21 | −0.11 | 0.21 | −0.36 * |
| S §§ | −0.04 | 0.30 | −0.12 | 0.07 | −0.12 | −0.08 | −0.40 * | −0.05 | −0.08 | 0.05 | 0.04 | 0.06 | 0.17 | −0.19 | 0.40 * | 0.03 | 0.05 | 0.08 | −0.26 | 0.37 * | −0.09 | −0.15 | 0.06 |
| Cu §§ | 0.09 | 0.37 * | −0.30 | −0.09 | 0.41 * | 0.21 | −0.21 | −0.01 | 0.26 | 0.45 ** | 0.51 ** | 0.31 | 0.45 ** | 0.30 | 0.23 | −0.02 | 0.05 | 0.16 | −0.15 | −0.09 | 0.18 | 0.46 ** | 0.21 |
| B §§ | 0.15 | 0.28 | −0.26 | −0.31 | 0.11 | 0.14 | −0.2 | −0.10 | 0.62 ** | 0.47 ** | 0.47 ** | 0.43 * | 0.26 | 0.60 ** | 0.27 | −0.12 | 0.38* | 0.14 | −0.31 | −0.12 | 0.14 | 0.11 | −0.03 |
| Zn §§ | 0.29 | 0.48 ** | −0.32 | −0.20 | 0.52 ** | 0.59 ** | −0.43 * | −0.21 | 0.28 | 0.71 ** | 0.48 ** | 0.46 ** | 0.65 ** | 0.59 ** | 0.11 | 0.21 | 0.30 | 0.13 | −0.34 | 0.11 | 0.21 | 0.14 | 0.23 |
| Fe §§ | 0.29 | 0.37 * | −0.05 | −0.16 | 0.61 ** | 0.55 ** | −0.21 | −0.01 | −0.08 | 0.52 ** | 0.33 | 0.25 | 0.54 ** | 0.33 | 0.19 | 0.50 ** | 0.05 | 0.14 | −0.26 | 0.02 | 0.34 | 0.10 | 0.36 * |
| Na §§ | 0.25 | −0.02 | −0.23 | −0.25 | 0.45 ** | 0.27 | −0.10 | 0.11 | 0.02 | 0.37* | 0.33 | 0.01 | 0.10 | 0.04 | 0.05 | 0.09 | −0.03 | 0.05 | −0.30 | 0.16 | 0.11 | 0.15 | 0.22 |
| EC ¥ | 0.21 | 0.32 | 0.10 | 0.01 | 0.23 | 0.03 | −0.33 | 0.01 | −0.02 | 0.23 | −0.05 | −0.03 | 0.36 * | 0.02 | 0.48 ** | −0.08 | 0.14 | 0.03 | −0.02 | 0.21 | 0.07 | −0.05 | 0.41 * |
| CEC # | 0.41 * | 0.51 ** | −0.18 | −0.19 | 0.54 ** | 0.51 ** | −0.42 * | 0.18 | 0.50 ** | 0.82 ** | 0.64 ** | 0.57 ** | 0.63 ** | 0.62 ** | 0.30 | 0.15 | 0.37 * | 0.37 * | −0.34 | 0.01 | 0.25 | 0.23 | 0.32 |
| CCA1 | CCA2 | R2 | p-Value | |
|---|---|---|---|---|
| Zinc solubilizing microbial genera Agrobacterium | 0.91 | 0.42 | 0.27 | 0.02 |
| Arthrobacter | 0.64 | 0.76 | 0.66 | 0.001 |
| Aspergillus | 0.98 | −0.21 | 0.51 | 0.001 |
| Azospirillum | 0.96 | 0.26 | 0.39 | 0.001 |
| Bacillus | 1.00 | 0.03 | 0.57 | 0.001 |
| Bradyrhizobium | 0.90 | −0.43 | 0.42 | 0.001 |
| Burkholderia | 1.00 | −0.01 | 0.03 | 0.67 |
| Enterobacter | 1.00 | 0.10 | 0.02 | 0.73 |
| Glomus | 0.23 | −0.97 | 0.19 | 0.05 |
| Gluconacetobacter | 0.62 | 0.79 | 0.02 | 0.71 |
| Klebsiella | 0.99 | 0.12 | 0.02 | 0.68 |
| Lysinibacillus | 1.00 | −0.05 | 0.76 | 0.001 |
| Mesorhizobium | 0.92 | 0.40 | 0.33 | 0.01 |
| Paenibacillus | 0.81 | 0.58 | 0.07 | 0.38 |
| Pantoea | 0.33 | −0.94 | 0.69 | 0.004 |
| Penicillium | 0.24 | 0.97 | 0.84 | 0.001 |
| Pseudomonas | 0.98 | −0.22 | 0.32 | 0.03 |
| Rhizobium | −1.00 | −0.04 | 0.38 | 0.002 |
| Serratia | −0.09 | −1.00 | 0.02 | 0.64 |
| Sphingomonas | 0.75 | −0.66 | 0.26 | 0.03 |
| Staphylococcus | 0.67 | 0.74 | 0.06 | 0.47 |
| Stenotrophomonas | −0.31 | 0.95 | 0.01 | 0.84 |
| Thiobacillus | 1.00 | 0.04 | 0.59 | 0.001 |
| Trichoderma | 1.00 | −0.01 | 0.67 | 0.001 |
| Xanthomonas | 0.71 | 0.70 | 0.22 | 0.05 |
| Soil chemical properties Olsen P | −0.11 | −0.41 | 0.04 | |
| N | 0.71 | 0.45 | 0.001 | |
| Zn | 0.76 | −0.17 | 0.02 | |
| pH | 0.77 | 0.44 | 0.13 | |
| SOC | 0.38 | −0.06 | 0.05 | |
| S | 0.4 | 0.03 | 0.22 | |
| B | 0.55 | 0.62 | 0.69 | |
| Fe | 0.57 | −0.76 | 0.03 | |
| Cu | 0.31 | 0.09 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolo, P.; Mucheru-Muna, M.W.; Mwirichia, R.K.; Kinyua, M.; Ayaga, G.; Kihara, J. Influence of Farmyard Manure Application on Potential Zinc Solubilizing Microbial Species Abundance in a Ferralsol of Western Kenya. Agriculture 2023, 13, 2217. https://doi.org/10.3390/agriculture13122217
Bolo P, Mucheru-Muna MW, Mwirichia RK, Kinyua M, Ayaga G, Kihara J. Influence of Farmyard Manure Application on Potential Zinc Solubilizing Microbial Species Abundance in a Ferralsol of Western Kenya. Agriculture. 2023; 13(12):2217. https://doi.org/10.3390/agriculture13122217
Chicago/Turabian StyleBolo, Peter, Monicah Wanjiku Mucheru-Muna, Romano Kachiuru Mwirichia, Michael Kinyua, George Ayaga, and Job Kihara. 2023. "Influence of Farmyard Manure Application on Potential Zinc Solubilizing Microbial Species Abundance in a Ferralsol of Western Kenya" Agriculture 13, no. 12: 2217. https://doi.org/10.3390/agriculture13122217




