Comparison of Physiological, Anatomical, and Morphological Traits between Sugarcane Hybrids and Their Parents with Different Stalk Dry Weights in the Early Growth Stage under Hydroponic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Culturing Procedure
2.2. Meteorological Conditions
2.3. Data Collection
2.3.1. Growth Characteristics
2.3.2. Root Characteristics
2.3.3. Physiological Traits
2.3.4. Anatomical Traits
2.4. Statistical Analysis
3. Results
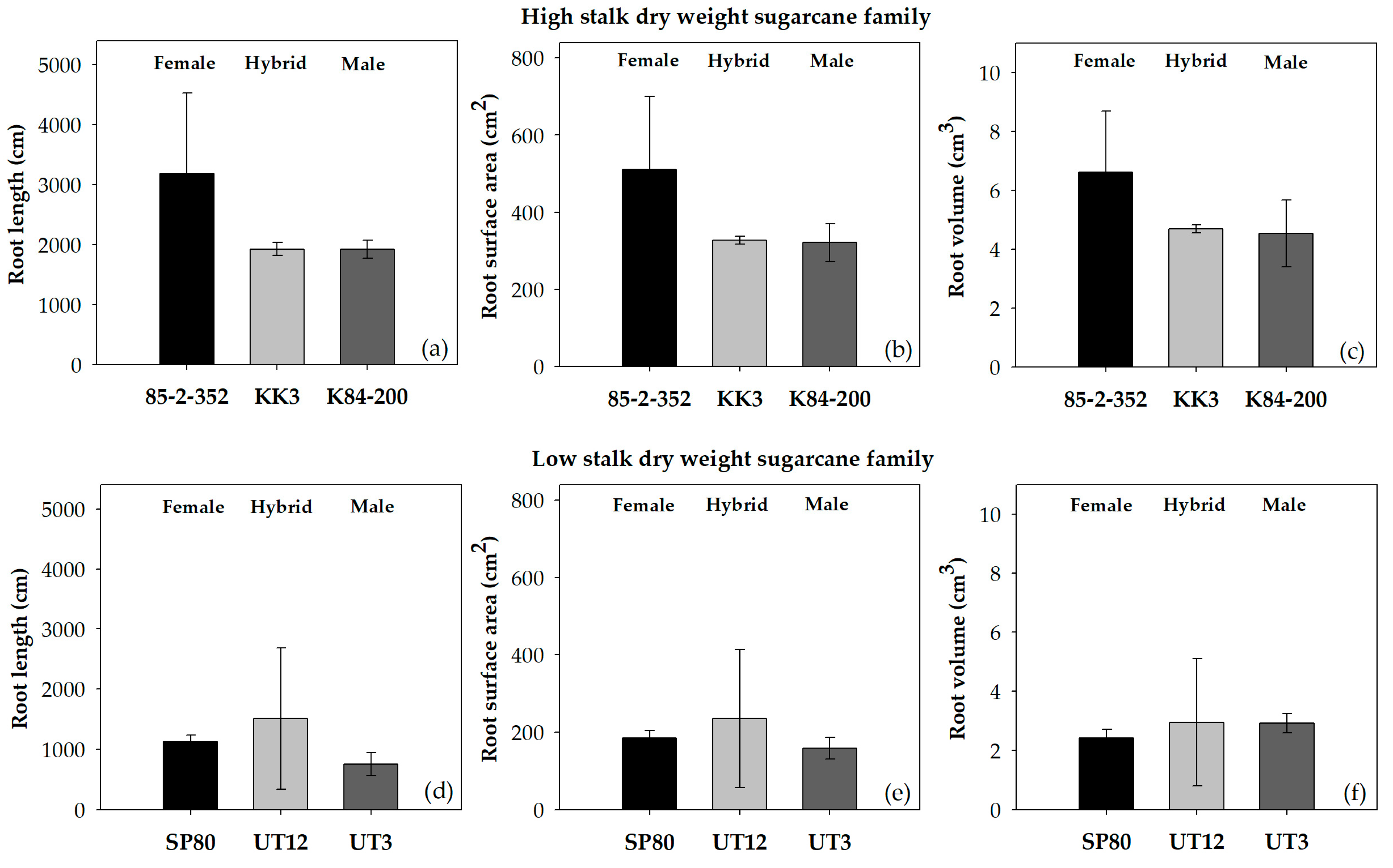
3.1. Belowground Traits
3.2. Aboveground Traits
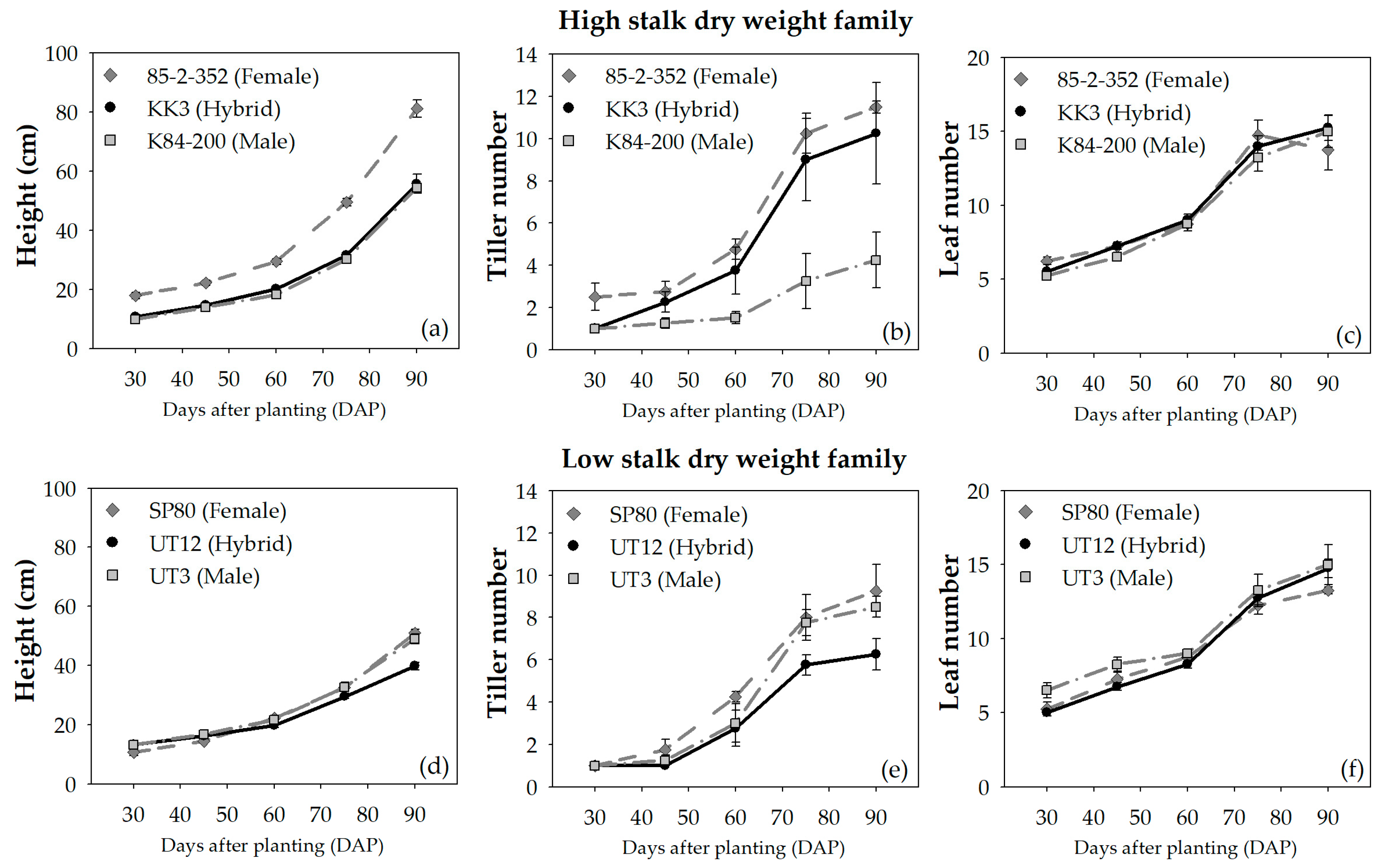
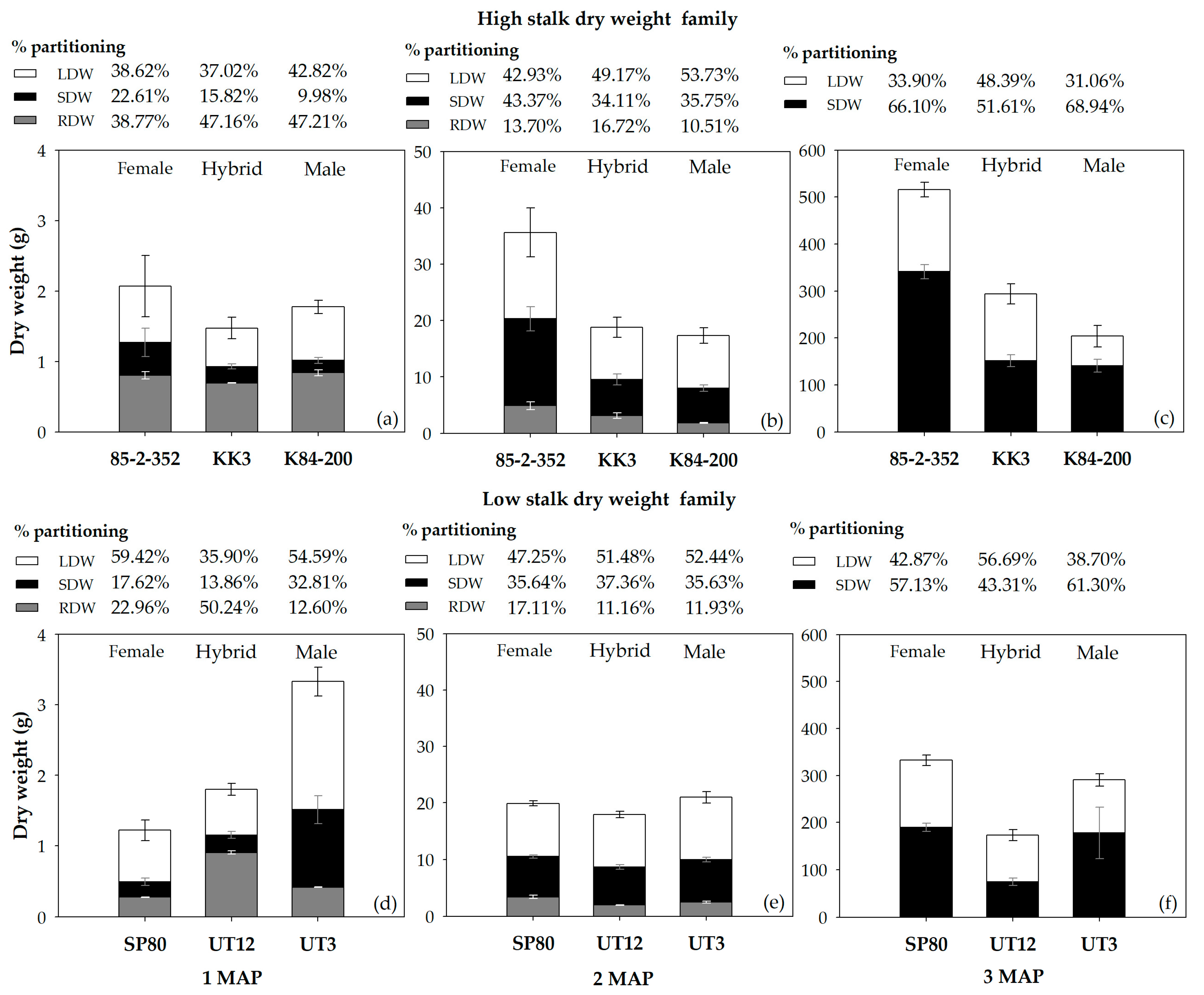
3.3. Growth Performances
4. Discussion
4.1. Root Anatomical and Morphological Traits
4.2. Leaf Anatomical, Morphological, and Physiological Traits
4.3. Growth and Biomass Performance between Different Biomass Potential Cultivars
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formann, S.; Hahn, A.; Janke, L.; Stinner, W.; Strauber, H.; Logrono, W.; Nikolausz, M. Beyond sugar and ethanol production: Value generation opportunities through sugarcane residues. Front. Energy Res. 2020, 8, 579577. [Google Scholar] [CrossRef]
- Zingaretti, S.M.; Rodrigues, F.A.; Graca, J.P.; Pereira, L.M.; Lourenco, M.V. Sugarcane responses at water deficit conditions. In Water Stress, 1st ed.; Rahman, I.M.M., Ed.; Intech Open: Shanghai, China, 2012; pp. 255–276. [Google Scholar]
- Leanasawat, N.; Kosittrakun, M.; Lontom, W.; Songsri, P. Physiological and agronomic traits of certain sugarcane genotypes grown under field conditions as influenced by early drought stress. Agronomy 2021, 11, 2319. [Google Scholar] [CrossRef]
- Wongkhunkaew, P.; Konyai, S.; Sriboonlue, V. Climate variability and rainfed sugarcane production: Thailand a case study. In Proceedings of the 11th International Conference, Chulabhorn International Convention Center (Wora Wana Hua Hin Hotel & Convention) Hua Hin, APRIL, Prachuap Khiri Khan, Thailand, 26–27 April 2018. [Google Scholar]
- Set-Tow, S.; Songsri, P.; Jongrungklang, N. Variations in root distribution patterns and cane yield of 16 elite sugarcane clones grown under varied soil conditions. Sugar Tech. 2020, 22, 1018–1031. [Google Scholar] [CrossRef]
- Tippayawat, A.; Jogloy, S.; Vorasoot, N.; Songsri, P.; Kimbeng, C.A.; Jifon, J.L.; Janket, A.; Thangthong, N.; Jongrungklang, N. Differential physiological responses to different drought durations among a diverse set of sugarcane genotypes. Agronomy 2023, 13, 2594. [Google Scholar] [CrossRef]
- da Silva, P.P.; Soares, L.; da Costa, J.G.; da Silva Viana, L.; de Andrade, J.C.F.; Gonçalves, E.R.; dos Santos, J.M.; de Souza Barbosa, C.V.; Nascimento, V.X.; Todaro, A.R.; et al. Path analysis for selection of drought tolerant sugarcane genotypes through physiological components. Ind. Crops Prod. 2012, 37, 11–19. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Understanding growth rate patterns among different drought resistant sugarcane cultivars during plant and ratoon crops encountered water deficit at early growth stage under natural field conditions. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Toomsa, B.; Jongrungklang, N. Rooting and physiological trait responses to early drought stress of sugarcane cultivars. Sugar Tech. 2018, 20, 396–406. [Google Scholar] [CrossRef]
- Riajaya, D.P.; Hariyono, B.; Cholid, M.; Kadarwati, F.T.; Santoso, B.; Djumali; Subiyakto. Growth and yield potential of new sugarcane varieties during plant and first ratoon Crops. Sustainability 2022, 14, 14396. [Google Scholar] [CrossRef]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef]
- Ramesh, P.; Mahadevaswamy, M. Effect of formative phase drought on different classes of shoots, shoot mortality, cane attributes, yield and quality of four sugarcane cultivars. J. Agron. Crop Sci. 2008, 185, 249–258. [Google Scholar] [CrossRef]
- Silva, M.A.; Silva, J.A.G.; Enciso, J.; Sharma, V.; Jifon, J. Yield components as indicators of drought tolerance of sugarcane. Sci. Agric. 2008, 65, 620–627. [Google Scholar] [CrossRef]
- Begum, M.K.; Islam, M.S. Effect of drought stress on yield and yield components of sugarcane. Agrofor. Environ. 2012, 6, 105–109. [Google Scholar]
- Endres, L.; Silva, J.V.; Ferreira, V.M.; De Souza Barbosa, G.V. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. 2010, 4, 31–37. [Google Scholar] [CrossRef]
- Silva, M.A.; Jifon, J.L.; dos Santos, C.M.; Jadoski, C.J.; da Silva, J.A.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev. Biol. Trop. 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Jongrungklang, N. Hydroponics: An Alternative Method for Root and Shoot Classification on Sugarcane Genotypes. Agrivita 2019, 41, 350–362. [Google Scholar] [CrossRef]
- Smith, J.P.; Lawam, R.J.; Nable, R.O. Investigation into the root:shoot relationship of sugarcane and some implications for crop productivity in presence of sub-optimal conditions. In Proceedings of the Australian Society of Sugar Cane Technologists, Townsville, QL, Australia, 27–30 April 1999; Volume 21, pp. 108–113. [Google Scholar]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Root characters of individual tillers and the relationships with above-ground growth and dry matter accumulation in sugarcane. Pak. J. Bot. 2020, 52, 101–109. [Google Scholar] [CrossRef]
- Chang, Y.S.; Milligan, S.B. Estimating the potential of sugarcane families to produce elite genotypes using bivariate methods. Theor. Appl. Genet. 1992, 84, 633–639. [Google Scholar] [CrossRef]
- Cox, M.C.; Stringer, J.K. Efficacy of early generation selection in a sugarcane improvement program. In Proceedings of the Australian Society of Sugar Cane Technologists, Ballina, Australia, 28 April–1 May 1998; Volume 20, pp. 148–153. [Google Scholar]
- Kimbeng, C.A. Early generation selection of sugarcane families and clones in Australia: A review. J. Am. Soc. Sugar Cane Technol. 2003, 23, 20–39. [Google Scholar]
- Ranjan, R.; Kumar, B. Study of genetic variability for cane yield and its component traits in early maturing sugarcane. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1739–1748. [Google Scholar] [CrossRef]
- Ranjan, S.; Gautam, A. Heritability Estimate. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springe: Cham, Switzerland, 2018; pp. 3088–3091. [Google Scholar]
- Zhang, D.; Li, A.; Lam, S.K.; Li, P.; Zong, Y.; Gao, Z.; Hao, X. Increased carbon uptake under elevated CO2 concentration enhances water-use efficiency of C4 broomcorn millet under drought. Agric. Water Manag. 2021, 245, 106631. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characters of leaf stomata and their relationship with photosynthesis in saccharum officinarum under drought and silicon application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Riddech, N.; Ma, N.Y.; Ho, P.N.; Sarin, P. A plant growth promoting of rhizobacteria and endophytic bacteria in vegetable rhizosphere and root samples. J. Pure. Appl. Microbiol. 2022, 16, 1909–1921. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; pp. 241–423. [Google Scholar]
- Piriyapongsa, J.; Kaewprommal, P.; Vaiwsri, S.; Anuntakarun, S.; Wirojsirasak, W.; Punpee, P.; Klomsa-ard, P.; Shaw, P.J.; Pootakham, W.; Yoocha, T.; et al. Uncovering full-length transcript isoforms of sugarcane cultivar Khon Kaen 3 using single-molecule long-read sequencing. PeerJ 2018, 6, e5818. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Gonkhamdee, S.; Jongrungklang, N. Understanding drought responses of sugarcane cultivars controlled under low water potential conditions. Chil. J. Agric. Res. 2020, 80, 370–380. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-Are they compatible, dissonant, or mutually exclusive. Crop Pasture Sci. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef]
- Inman-Bamber, N.G.; Smith, D. Water relations in sugarcane and response to water deficits. Field Crops Res. 2005, 92, 185–202. [Google Scholar] [CrossRef]
- Smit, M.; Singels, A. The response of sugarcane canopy development to water stress. Field Crops Res. 2006, 98, 91–97. [Google Scholar] [CrossRef]
- Cominelli, E.; Conti, L.; Tonelli, C.; Galbiati, M. Challenges and perspectives to improve crop drought and salinity tolerance. N. Biotechnol. 2013, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jifon, J.L.; de Silva, J.A.G.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef]
- da Graca, J.P.; Rodrigues, F.A.; Farias, J.R.B.; de Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S.M. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef]
- Medeiros, D.B.; da Silva, E.C.; Nogueira, R.J.M.C.; Teixeira, M.M.; Buckeridge, M.S. Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theor. Exp. Plant Physiol. 2013, 25, 213–222. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, R.S.; Machado, E.C.; Machado, D.F.S.P.; Magalhães Filho, J.R.; Landell, M.G.A. Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp. Agric. 2013, 49, 212–224. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. J. Exp. Bot. 2015, 66, 3945–3958. [Google Scholar] [CrossRef]
- De Silva, A.; De Costa, W. Varietal variation in stomatal conductance, transpiration and photosynthesis of commercial sugarcane varieties under two contrasting water regimes. Trop. Agr. Res. Ext. 2011, 12, 97–102. [Google Scholar] [CrossRef]
- Saliendra, N.Z.; Meinzer, F.C. Genotypic, developmental and drought-induced differences in root hydraulic conductance of contrasting sugarcane cultivars. J. Exp. Bot. 1992, 43, 1209–1217. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Richards, R.A.; Passioura, J.B. Seminal root morphology and water-use of wheat. 1. Environmental effects. Crop Sci. 1981, 21, 249–252. [Google Scholar] [CrossRef]
- Smith, D.M.; Inman-Bamber, N.G.; Thorburn, P.J. Growth and function of the sugarcane root system. Field Crops Res. 2005, 92, 169–183. [Google Scholar] [CrossRef]
- Thangthong, N.; Jogloy, S.; Punjansing, T.; Kvien, C.K.; Kesmala, T.; Vorasoot, N. Changes in root anatomy of peanut (Arachis hypogaea L.) under different durations of early season drought. Agronomy 2019, 9, 215. [Google Scholar] [CrossRef]
- Wachsman, G.; Sparks, E.E.; Benfey, P.N. Genes and networks regulating root anatomy and architecture. New Phytol. 2015, 208, 26–38. [Google Scholar] [CrossRef]
- Uga, Y.; Okuno, K.; Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 2011, 62, 2485–2494. [Google Scholar] [CrossRef]
- Inman-Bamber, N.G.; Lakshmanan, P.; Park, S. Sugarcane for water-limited environments: Theoretical assessment of suitable traits. Field Crops Res. 2012, 134, 95–104. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araujo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Inman-Bamber, N.G.; Baillie, C.; Willcox, J.; Attard, S.; Spillman, M.F. Increased Profitability and Water Use Efficiency through Best Use of Limited Water under Supplementary Irrigation in Sugarcane. Final Report for Rural Water Use Efficiency Initiative; Queensland Department of Natural Resources and Mines, CSIRO Sustainable Ecosystems: Townsville, Australia, 31 May 2003; Available online: https://www.academia.edu/22407748/Increased_profitability_and_water_use_efficiency_through_best_use_of_limited_water_under_supplementary_irrigation_in_sugarcane._Final_Report_for_Rural_Water_Use_Efficiency_Initiative_Queensland_Department_of_Natural_Reso (accessed on 21 June 2023).
- Olsen, J.T.; Caudle, K.L.; Johnson, L.C.; Baer, S.G.; Maricle, B.R. Environmental and genetic variation in leaf anatomy among populations of Andropogon gerardii (Poaceae) along a precipitation gradient. Am. J. Bot. 2013, 100, 1957–1968. [Google Scholar] [CrossRef]
- Stace, C.A. Plant Taxonomy and Biosystematics; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Raso, S.; Maneerattanarungroj, P. leaf anatomical responses to drought stress condition in hybrid sugarcane leaf (Saccharum officinarum ‘KK3’). Malays. Appl. Biol. 2019, 48, 181–188. [Google Scholar]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech. 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, A. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Gailing, O.; Langenfeld-Heyser, R.; Polle, A.; Finkeldey, R. Quantitative trait loci affecting stomatal density and growth in a Quercus robur progeny: Implications for the adaptation to changing environments. Glob. Chang. Biol. 2008, 14, 1934–1946. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, H.; Wang, S.; Zhu, X.; Luo, C.; Li, Y.; Zhao, X. Gene or environment? Species-specific control of stomatal density and length. Ecol. Evol. 2012, 2, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Sober, A.; Rahi, M. Leaf anatomical characters associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust. J. Plant Physiol. 2001, 28, 765–774. [Google Scholar]
- Beaulieu, J.M.; Leitch, I.J.; Patel, S.; Knight, C.A.; Pendharkar, A. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 2008, 179, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Zhou, G.S. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L., from the salt range, Pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Silva, M.A.; Pincelli, R.P.; Barbosa, A.D.M. Water stress effects on chlorophyll fluorescence and chlorophyll content in sugarcane cultivars with contrasting tolerance. Bioscience 2018, 34, 75–87. [Google Scholar] [CrossRef]
- Natarajan, S.; Basnayake, J.; Lakshmanan, P.; Fukai, S. Genotypic variation in intrinsic transpiration efficiency correlates with sugarcane yield under rainfed and irrigated field conditions. Physiol. Plant. 2020, 172, 976–989. [Google Scholar] [CrossRef]
- Halpin, C.; Barakate, A.; Askari, B.M.; Abbot, J.C.; Ryan, M.D. Enabling technologies for manipulating multiple genes on complex pathways. Plant Mol. Biol. 2001, 47, 295–310. [Google Scholar] [CrossRef]
- Luguang, W.; Birch, R.G. Doubling sugar content in sugarcane plants modified to produce an isomer. Plant Biotechnol. J. 2007, 5, 109–117. [Google Scholar]
- McCormick, A.J.; Cramer, M.D.; Watt, D.A. Changes in Photosynthetic Rates and Gene Expression of Leaves during a source–sink perturbation in sugarcane. Ann. Bot. 2008, 101, 89–102. [Google Scholar] [CrossRef]
- Khan, Q.; Qin, Y.; Guo, D.J.; Zeng, X.P.; Chen, J.Y.; Huang, Y.Y.; Ta, Q.K.; Yang, L.T.; Liang, Q.; Song, X.P.; et al. Morphological, agronomical, physiological and molecular characterization of a high sugar mutant of sugarcane in comparison to mother variety. PLoS ONE 2022, 17, e0264990. [Google Scholar] [CrossRef]
- Khan, I.A.; Dahot, M.U.; Khatri, A. Study of genetic variability in sugarcane induced through mutation breeding. Pak. J. Bot. 2007, 39, 1489–1501. [Google Scholar]
- Akhtar, M.; Elahi, N.; Ashraf, M. Morphological characters of some exotic sugarcane varieties. Pak. J. Biol. Sci. 2001, 4, 471–476. [Google Scholar]
- Edmeadesa, G.O.; McMasterb, G.S.; Whitec, J.W.; Campos, H. Genomics and the physiologist: Bridging the gap between genes and crop response. Field Crops Res. 2004, 90, 5–18. [Google Scholar] [CrossRef]
- Tena, E.; Mekbib, F.; Ayana, A. Heritability and correlation among sugarcane (Saccharum spp.) yield and some agronomic and sugar quality traits in Ethiopia. Am. J. Plant Sci. 2016, 7, 1453–1477. [Google Scholar] [CrossRef]
- Pandey, D.; Singh, S.P.; Jeena, A.S.; Khan, K.A.; Tabassum, N.A.; Koujalagi, D. Study of genetic variability, heritability and genetic advance for various yield and quality traits in sugarcane genotypes (Saccharum officinarum). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1464–1472. [Google Scholar] [CrossRef]
- Zhao, D.; Barry, G.; Comstock, J.C. Sugarcane response to water-deficit stress during early growth on organic and sand soils. Am. J. Agric. Biol. Sci. 2010, 5, 403–414. [Google Scholar] [CrossRef]
- Jones, M.R.; Singels, A.; Chinorumba, S.; Patton, A.; Poser, C.; Singh, M.; Martiné, J.F.; Christina, M.; Shine, J.; Annandale, J.; et al. Exploring process-level genotypic and environmental effects on sugarcane yield using an international experi-mental dataset. Field Crops Res. 2019, 244, 107622. [Google Scholar] [CrossRef]
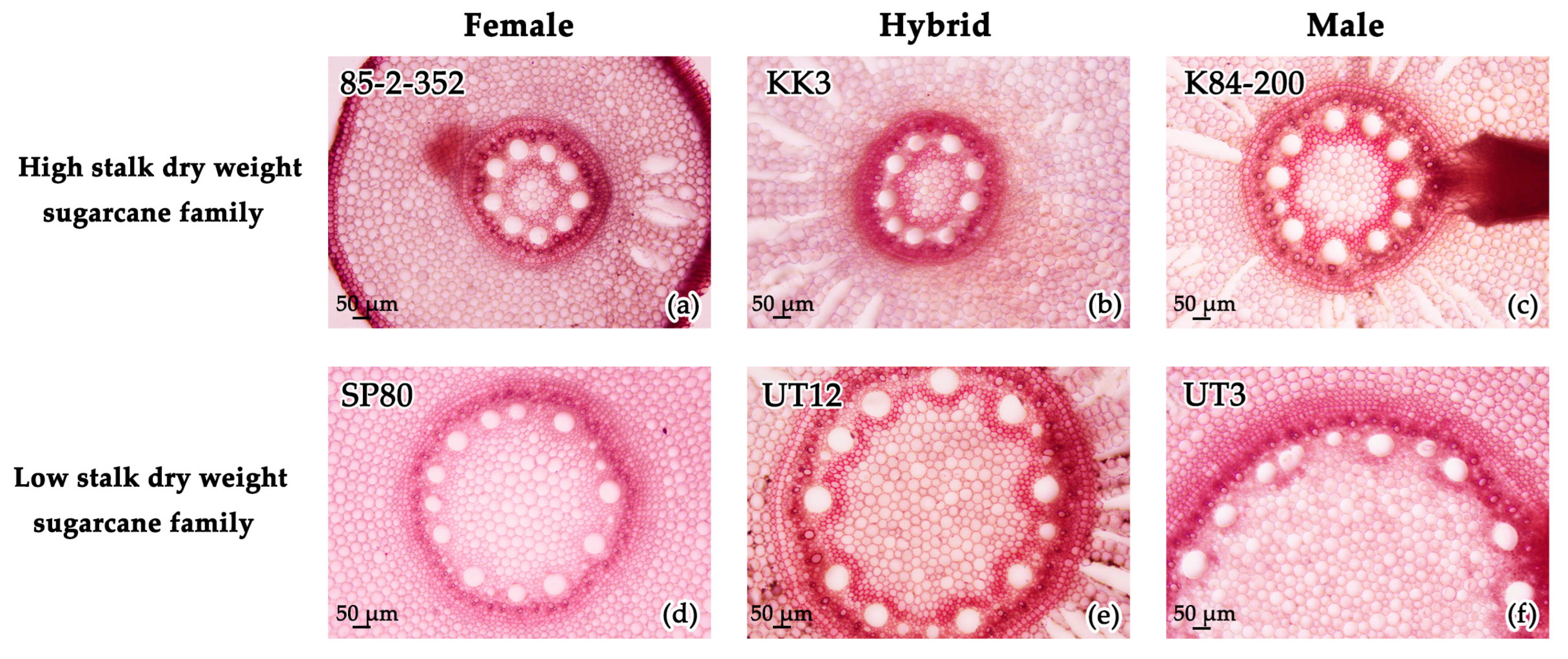


| Cultivar | Xylem Vessel Number | Total Xylem Vessel Size | Average Xylem Vessel Size | Percentage of Xylem Vessel Area/Stele Area |
|---|---|---|---|---|
| (µm) | (µm) | (%) | ||
| High stalk dry weight sugarcane family | ||||
| 85-2-352 (female parent) | 9.9 | 19,616 b | 2092 b | 13.1 |
| KK3 (hybrid) | 10.4 | 18,934 b | 2023 b | 13.2 |
| K84-200 (male parent) | 10.0 | 33,171 a | 2795 a | 11.6 |
| Mean | 10.1 | 23,907 | 2303 | 12.6 |
| F-test | ns | ** | ** | ns |
| Low stalk dry weight sugarcane family | ||||
| SP80 (female parent) | 12.3 b | 29,291 b | 2870 b | 12.9 a |
| UT12 (hybrid) | 12.9 b | 41,346 b | 3055 b | 8.6 b |
| UT3 (male parent) | 20.0 a | 90,070 a | 4489 a | 8.7 b |
| Mean | 15.1 | 53,569 | 3471 | 10.0 |
| F-test | ** | ** | * | ** |
| High and low stalk dry weight sugarcane cultivars | ||||
| KK3 | 10.4 b | 18,934 b | 2023 b | 13.2a |
| UT12 | 12.9 a | 41,346 a | 3055 a | 8.6 b |
| Mean | 11.7 | 30,140 | 2539 | 10.9 |
| F-test | * | ** | ** | ** |
| Characteristic | High Stalk Dry Weight Sugarcane Family | ||||
|---|---|---|---|---|---|
| 85-2-352 | KK3 | K84-200 | Mean | F-test | |
| Female Parent | Hybrid | Male Parent | |||
| Laminar thickness (μm) | 244.6 | 242.7 | 224.2 | 237.2 | ns |
| Xylem vertical length (μm) | 42.0 | 36.4 | 34.7 | 37.7 | ns |
| Xylem horizontal length (μm) | 53.0 a | 50.1 a | 43.5 b | 48.9 | * |
| Phloem vertical length (μm) | 53.9 a | 57.0 a | 40.6 b | 50.5 | ** |
| Phloem horizontal length (μm) | 76.3 a | 66.3 ab | 53.8 b | 65.4 | * |
| Bundle sheath horizontal length (μm) | 73.5 a | 67.0 b | 79.3 a | 73.3 | ** |
| Bundle sheath vertical length (μm) | 159.4 a | 158.9 a | 143.1 b | 153.8 | * |
| Bulliform cell horizontal length (μm) | 46.6 a | 47.2 a | 38.9 b | 44.3 | * |
| Bulliform cell vertical length (μm) | 78.0 a | 74.4 a | 59.9 b | 70.7 | ** |
| Bulliform size (μm2) | 3682.0 a | 3665.4 a | 2063.9 b | 3137.1 | ** |
| Epidermis vertical length (μm) | 9.8 | 9.1 | 10.2 | 9.7 | ns |
| Characteristics | Low stalk dry weight sugarcane family | ||||
| SP80 | UT12 | UT3 | Mean | F-test | |
| Female parent | Hybrid | Male parent | |||
| Leaf thickness (μm) | 246.3 b | 287.2 a | 256.6 b | 263.4 | * |
| Xylem vertical length (μm) | 41.5 ab | 44.5 a | 36.9 b | 40.9 | * |
| Xylem horizontal length (μm) | 52.1 | 52.3 | 48.0 | 50.8 | ns |
| Phloem vertical length (μm) | 50.5 | 54.3 | 57.3 | 54.0 | ns |
| Phloem horizontal length (μm) | 60.1 | 67.9 | 63.2 | 63.7 | ns |
| Bundle sheath horizontal length (μm) | 82.4 | 83.3 | 81.6 | 82.4 | ns |
| Bundle sheath vertical length (μm) | 167.6 ab | 186.8 a | 155.0 b | 169.8 | * |
| Bulliform cell horizontal length (μm) | 39.7 | 50.2 | 50.3 | 46.7 | ns |
| Bulliform cell vertical length (μm) | 72.8 | 81.0 | 77.2 | 77.0 | ns |
| Bulliform size (μm2) | 3096.4 b | 4442.6 a | 4464.5 a | 4001.2 | ** |
| Epidermis vertical length (μm) | 9.8 | 10.3 | 10.8 | 10.3 | ns |
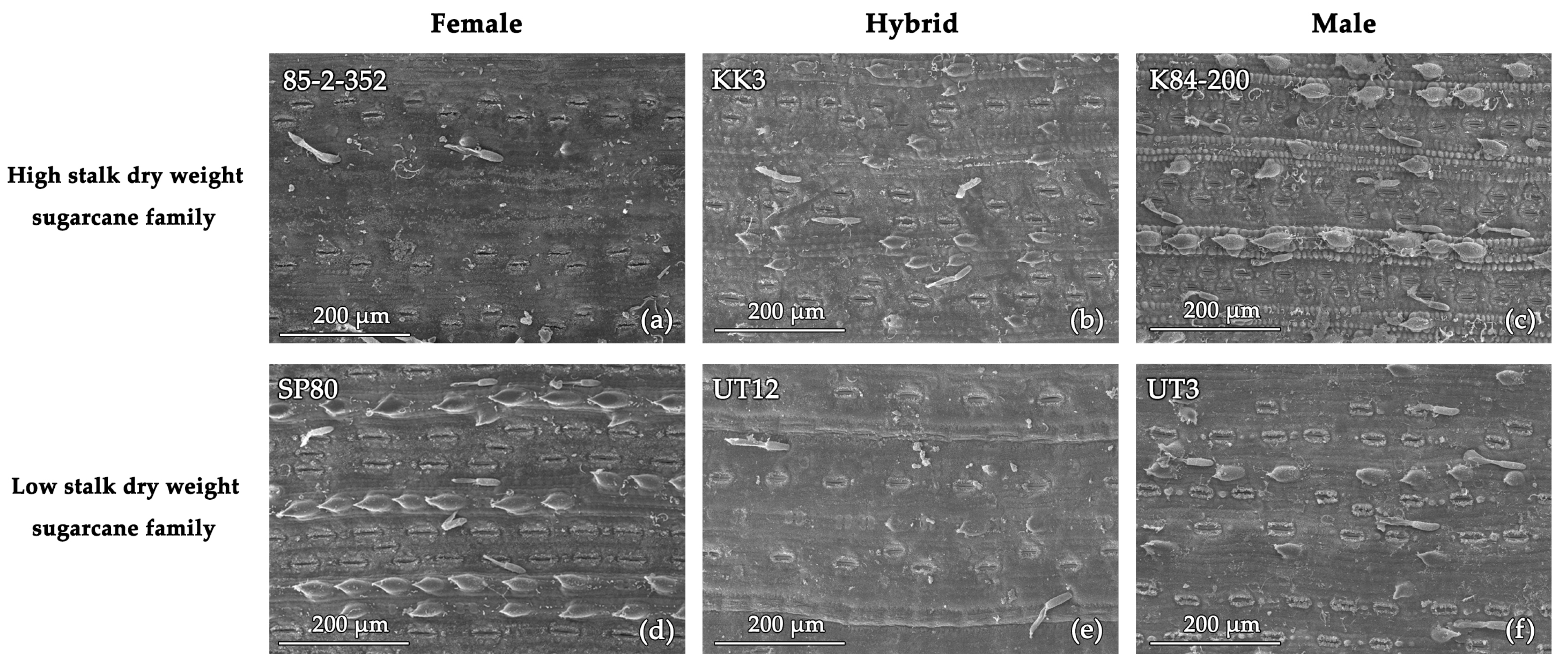
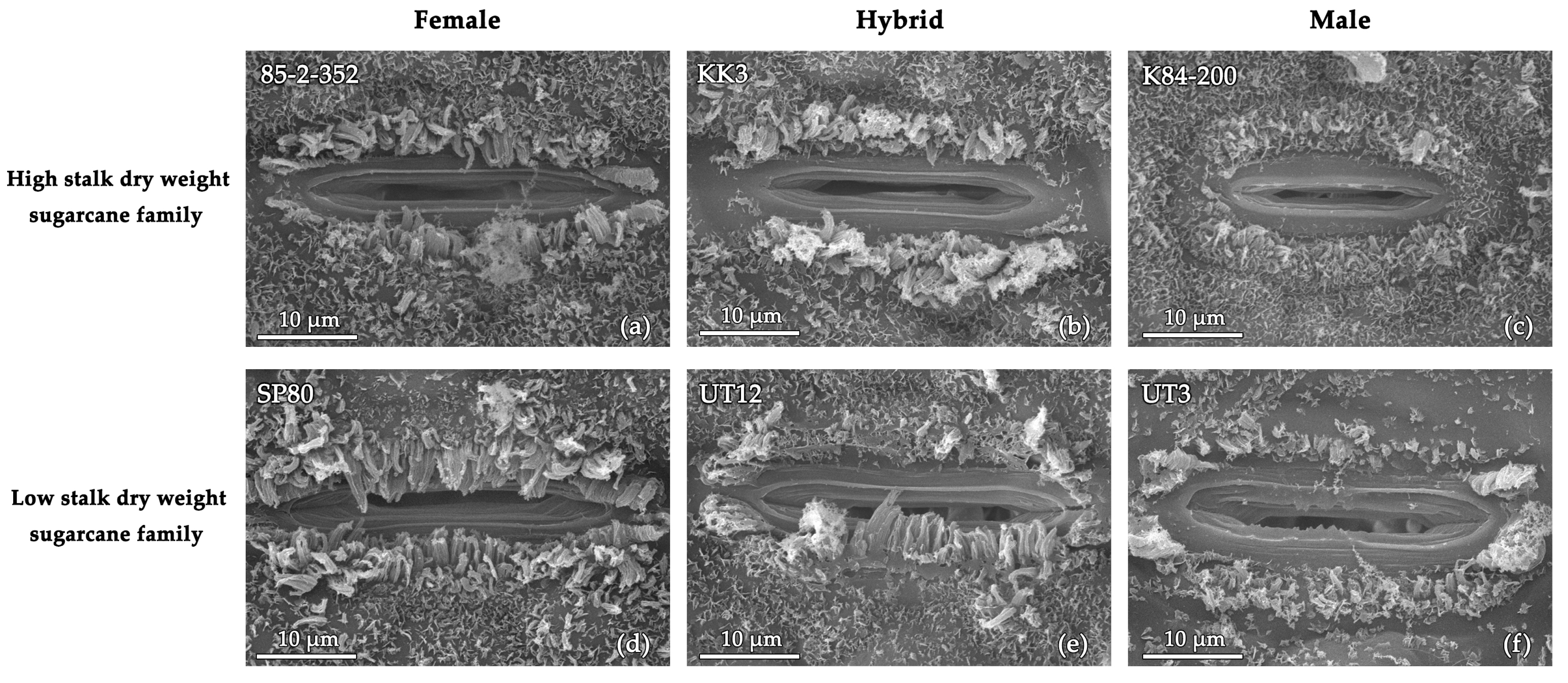
| Cultivars | Stomatal Length | Stomatal Width | Pore Length | Guard Cell |
|---|---|---|---|---|
| (µm) | (µm) | (µm) | (µm) | |
| High stalk dry weight sugarcane family | ||||
| 85-2-352 (female parent) | 40.6 | 24.0 | 28.0 | 10.6 |
| KK3 (hybrid) | 40.7 | 22.6 | 25.6 | 9.3 |
| K84-200 (male parent) | 37.5 | 23.1 | 21.8 | 10.0 |
| Mean | 39.6 | 23.2 | 25.1 | 10.0 |
| F-test | ns | ns | ns | ns |
| Low stalk dry weight sugarcane family | ||||
| SP80 (female parent) | 41.6 | 22.9 | 28.5 | 10.0 |
| UT12 (hybrid) | 42.4 | 26.5 | 30.7 | 11.8 |
| UT3 (male parent) | 42.5 | 25.0 | 30.4 | 10.0 |
| Mean | 42.2 | 24.8 | 29.8 | 10.6 |
| F-test | ns | ns | ns | ns |
| High and low stalk dry weight sugarcane cultivars | ||||
| KK3 | 40.7 | 22.6 | 25.6 | 9.3 |
| UT12 | 42.4 | 26.5 | 30.7 | 11.8 |
| Mean | 41.5 | 24.5 | 28.1 | 10.5 |
| F-test | ns | ns | ns | * |
| Cultivar | gs | E | PN | WUE |
|---|---|---|---|---|
| (mol H2O m−2 s−1) | (mmol H2O m−2 s−1) | (µmol CO2 m−2 s−1) | (µmol mmol−1) | |
| High stalk dry weight sugarcane family | ||||
| 85-2-352 (female parent) | 0.15 | 2.93 | 35.94 ab | 15.68 |
| KK3 (hybrid) | 0.12 | 2.44 | 34.57 b | 16.12 |
| K84-200 (male parent) | 0.17 | 3.03 | 40.21 a | 12.71 |
| Mean | 0.15 | 2.80 | 36.91 | 14.83 |
| F-test | ns | ns | * | ns |
| Low stalk dry weight sugarcane family | ||||
| SP80 (female parent) | 0.22 a | 3.68 | 41.38 a | 11.95 |
| UT12 (hybrid) | 0.12 b | 2.60 | 32.43 b | 11.63 |
| UT3 (male parent) | 0.19 ab | 3.53 | 43.34 a | 13.30 |
| Mean | 0.18 | 3.27 | 39.05 | 12.29 |
| F-test | * | ns | * | ns |
| High and low stalk dry weight sugarcane cultivars | ||||
| KK3 | 0.12 | 2.44 | 34.57 | 16.12 |
| UT12 | 0.12 | 2.60 | 32.43 | 11.64 |
| Mean | 0.12 | 2.52 | 33.50 | 13.88 |
| F-test | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Comparison of Physiological, Anatomical, and Morphological Traits between Sugarcane Hybrids and Their Parents with Different Stalk Dry Weights in the Early Growth Stage under Hydroponic Conditions. Agriculture 2023, 13, 2234. https://doi.org/10.3390/agriculture13122234
Khonghintaisong J, Songsri P, Jongrungklang N. Comparison of Physiological, Anatomical, and Morphological Traits between Sugarcane Hybrids and Their Parents with Different Stalk Dry Weights in the Early Growth Stage under Hydroponic Conditions. Agriculture. 2023; 13(12):2234. https://doi.org/10.3390/agriculture13122234
Chicago/Turabian StyleKhonghintaisong, Jidapa, Patcharin Songsri, and Nakorn Jongrungklang. 2023. "Comparison of Physiological, Anatomical, and Morphological Traits between Sugarcane Hybrids and Their Parents with Different Stalk Dry Weights in the Early Growth Stage under Hydroponic Conditions" Agriculture 13, no. 12: 2234. https://doi.org/10.3390/agriculture13122234







