Regulation of Inorganic Zinc Supplementation on Intestinal Absorption, Metabolism, and Muscle Development in Broilers Fed Low-Protein Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Broilers, Feeding, and Management
2.3. Growth Performance and Carcass Trait Analysis
2.4. Meat Quality Determination
2.5. Preparation and Analysis of Blood Samples
2.6. Tissue Sampling for Intestinal Morphological Observation
2.7. Fecal Trace Elements
2.8. Total RNA Extraction, cDNA Synthesis, and Quantitative PCR
2.9. Statistical Analyses
3. Results
3.1. Inorganic Zinc Supplementation Content of Low-Protein Diets
3.2. Effects of Inorganic Zinc Supplementation in Low-Protein Diets on Growth Performance of Broilers
3.3. Effect of Inorganic Zinc Supplementation in Low-Protein Diets on Carcass Traits and Meat Quality of Broilers
3.4. Effects of Inorganic Zinc Supplementation in Low-Protein Diets on Serum Biochemical Indexes of Broilers
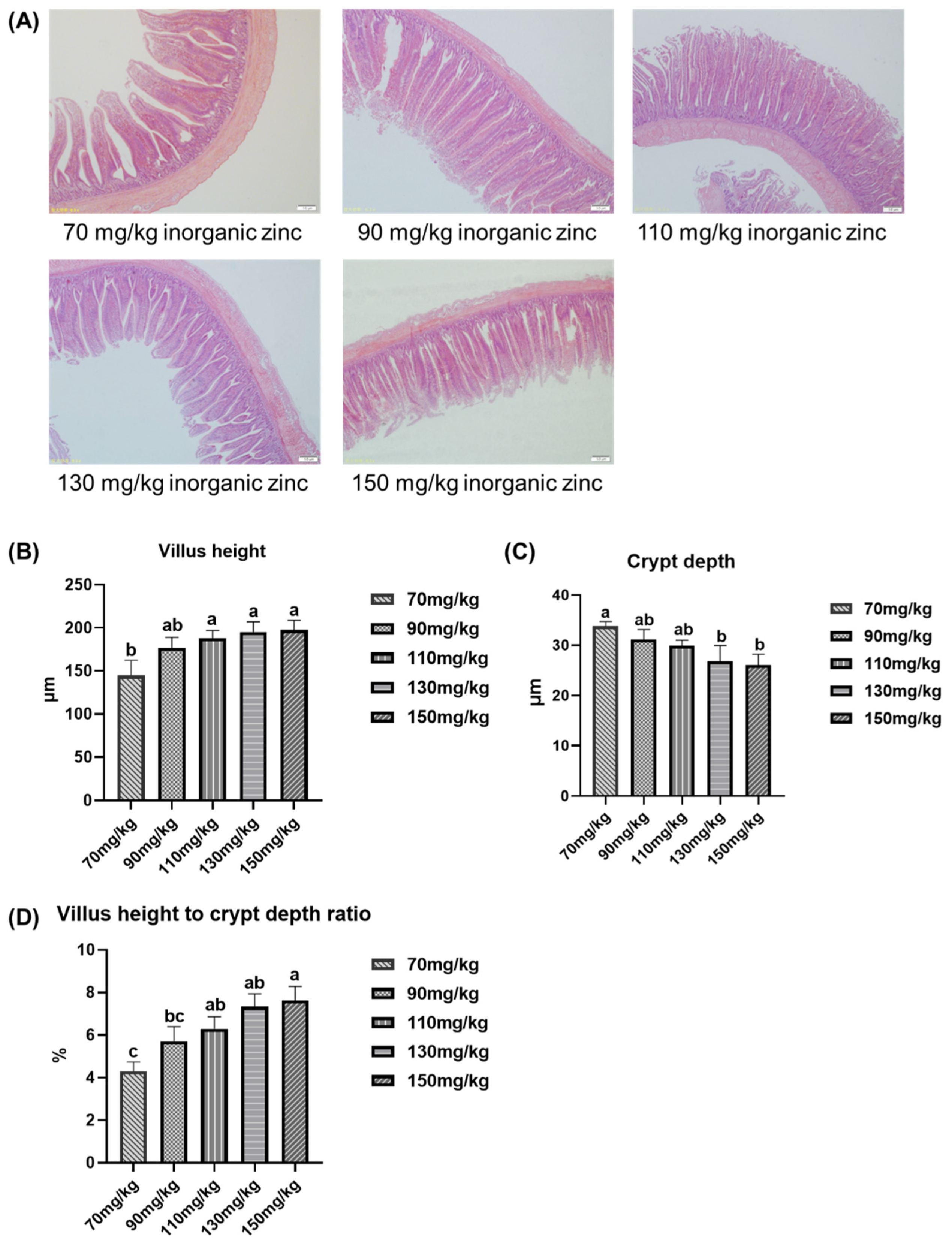
3.5. Effect of Inorganic Zinc Supplementation in Low-Protein Diets on Intestinal Tissue Morphology of Broilers
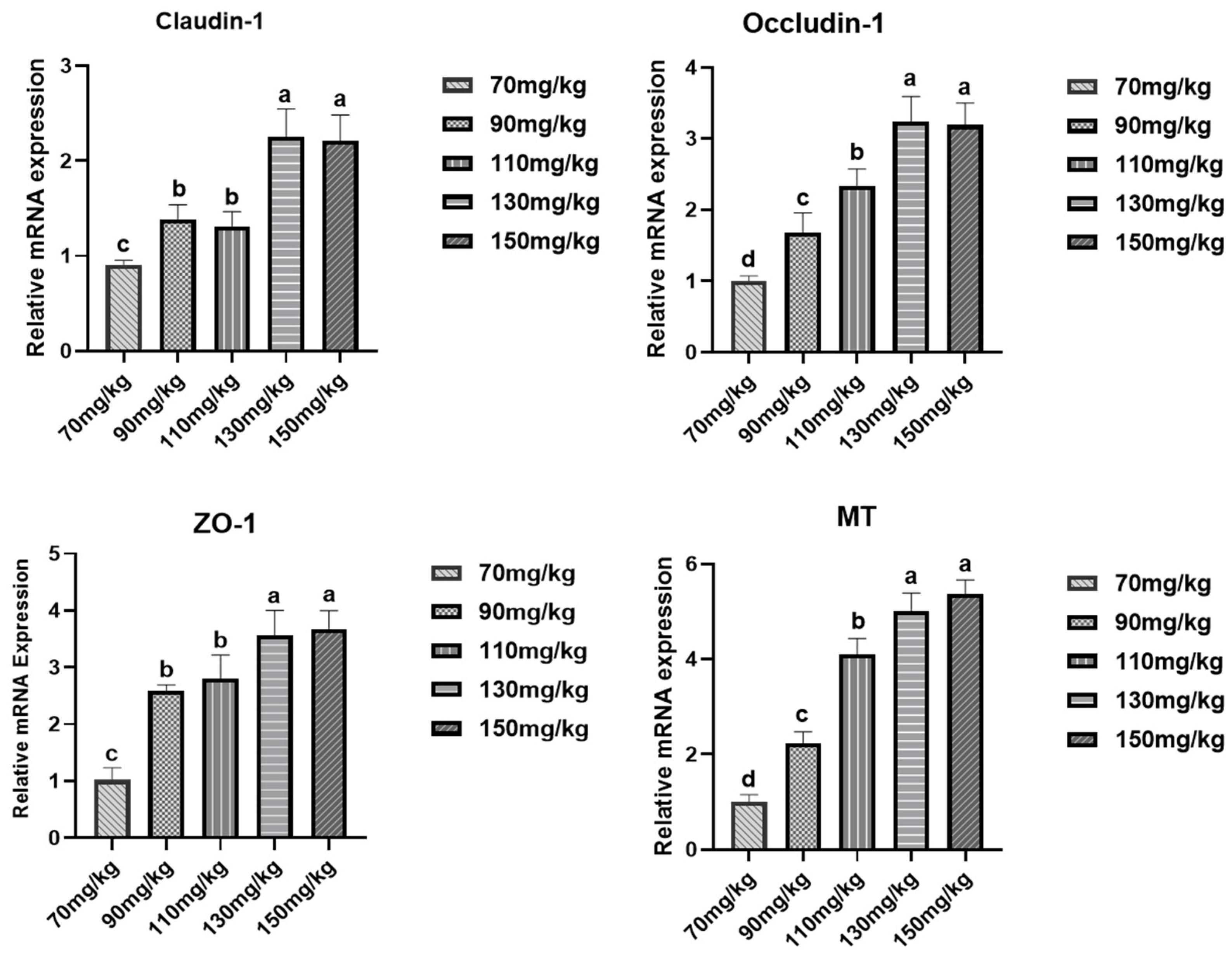
3.6. Effects of Inorganic Zinc Supplementation in Low-Protein Diets on Gene Expression in Gut, Liver, and Muscles of Broilers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2015, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Diedrichsen, R.M. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein, amino acid-supplemented diets. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.A.; Fadlullah, M.; Zulkifli, I.; Farjam, A.S.; Chwen, L.T. Amino acids fortification of low-protein diet for broilers under tropical climate: Ideal essential amino acids profile. Ital. J. Anim. Sci. 2015, 94, 2772–2777. [Google Scholar] [CrossRef]
- Lemme, A.; Hiller, P.; Klahsen, M.; Taube, V.; Simon, I. Reduction of dietary protein in broiler diets not only reduces n-emissions but is also accompanied by several further benefits. J. Appl. Poult. Res. 2019, 28, 867–880. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Ahmed, H.; Ketkat, S.; Selim, S. Supplementation of a low-protein diet with tryptophan, threonine, and valine and its impact on growth performance, blood biochemical constituents, immune parameters, and carcass traits in broiler chickens. Vet. World 2020, 13, 1234–1244. [Google Scholar] [CrossRef]
- Mayer, A.N.; Vieira, S.L.; Berwanger, E.; Angel, C.R.; Kindlein, L.; França, I.; Noetzold, T.L. Zinc requirements of broiler breeder hens. Poult. Sci. 2019, 98, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat Stress Impacts on Broiler Performance: A Systematic Review and Meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.L.; Adenuga, G.A.; Sandhir, R. Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci. 2016, 152, 145–155. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Modisaojang-Mojanaga, M.M.C.; Mokolopi, B.G.; Mbajiorgu, C.A. A Meta-analysis of Responses of Broiler Chickens to Dietary Zinc Supplementation: Feed Intake, Feed Conversion Ratio and Average Daily Gain. Biol. Trace Elem. Res. 2022, 201, 2491–2502. [Google Scholar] [CrossRef]
- Sandström, B.; Cederblad, A. Zinc absorption from composite meals. II. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 739–745. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Lily, S. Zinc intestinal absorption in rats: Specificity of amino acids as ligands. J. Nutr. 1986, 116, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, C.; Sumiati Jayanegara, A.; Wina, E. Effect of zinc on the immune response and production performance of broilers: A meta-analysis. Asian-Australas. J. Anim. Sci. 2020, 33, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Paulk, C.B.; Burnett, D.D.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S. Effect of added zinc in diets with ractopamine hydrochloride on growth performance, carcass characteristics, and ileal mucosal inflammation mRNA expression of finishing pigs. J. Anim. Sci. 2015, 93, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, M.P.; Casagrande-Proietti, P.; Forte, C.; Beghelli, D.; Acuti, G.; Zanichelli, D.; Bosco, A.D.; Castellini, C.; Trabalza-Marinucci, M. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous extracts on broiler performance, immune function and intestinal microbial population. J. Appl. Anim. Res. 2016, 44, 474–479. [Google Scholar] [CrossRef]
- Duff, M.; Ettarh, R.R. Crypt cell production rate in the small intestine of the zinc-supplemented mouse. Cells Tissues Organs 2002, 172, 21–28. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, Z.; Yuan, J.; Yang, Y.; Guo, Y.; Zhang, B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged withSalmonella entericaserovar typhimurium. J. Microbiol. 2014, 52, 1002–1011. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Piacenza, F.; Basso, A.; Malavolta, M. Zinc, metallothioneins and immunosenescence: Effect of zinc supply as nutrigenomic approach. Biogerontology 2011, 12, 455–465. [Google Scholar] [CrossRef]
- Nattrass, G.S.; Quigley, S.P.; Gardner, G.E.; Bawden, C.S.; Greenwood, P.L. Genotypic and nutritional regulation of gene expression in two sheep hindlimb muscles with distinct myofibre and metabolic characteristics. Crop Pasture Sci. 2006, 57, 691–698. [Google Scholar] [CrossRef]
- Kim, W.K.; Patterson, P.H. Effects of dietary zinc supplementation on broiler performance and nitrogen loss from manure. Poult. Sci. 2004, 83, 34–38. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014, 12, 3668. [Google Scholar] [CrossRef]
- Dai, S.F.; Wang, L.K.; Wen, A.Y.; Wang, L.X.; Jin, G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bai, X.; Wen, A.; Shah, A.A.; Dai, S.; Ren, Q.; Wang, S.; He, S.; Wang, L. Assessment of interactions between glutamine and glucose on meat quality, AMPK, and glutamine concentrations in pectoralis major meat of broilers under acute heat stress. J. Appl. Poult. Res. 2016, 25, 370–378. [Google Scholar] [CrossRef]
- Animal Feeding Stuffs. Determination of the contents of calcium, copper, iron, magnesium, manganese, potassium, sodium and zinc. In Method Using Atomic Absorption Spectrometry; ISO International Standard (ISO): Geneva, Switzerland, 2000. [Google Scholar]
- Siegert, W.; Wild, K.J.; Schollenberger, M.; Helmbrecht, A.; Rodehutscord, M. Effect of glycine supplementation in low protein diets with amino acids from soy protein isolate or free amino acids on broiler growth and nitrogen utilisation. Br. Poult. Sci. 2016, 57, 424–434. [Google Scholar] [CrossRef]
- Abiona, O.O.; Sandra, V.K.; Yanming, H. Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poult. Sci. 2018, 97, 3891–3898. [Google Scholar] [CrossRef]
- Franklin, S.B.; Young, M.B.; Ciacciariello, M. The Impact of Different Sources of Zinc, Manganese, and Copper on Broiler Performance and Excreta Output. Animals 2022, 12, 1067. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Khani, D.E.; Ann, B.M.; Fima, L. Absorption of zinc by the rat ileum: Effects of histidine and other low-molecular-weight ligands. J. Nutr. 1983, 113, 1346–1354. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Mbajiorgu, C.A. Potentials of Dietary Zinc Supplementation in Improving Growth Performance, Health Status, and Meat Quality of Broiler Chickens. Biol. Trace Elem. Res. 2023, 201, 1418–1431. [Google Scholar] [CrossRef]
- Ramiah, S.K.; Awad, E.A.; Mookiah, S.; Idrus, Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult. Sci. 2019, 98, 3828–3838. [Google Scholar] [CrossRef]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar] [CrossRef]
- Saenmahayak, B.; Bilgili, S.F.; Hess, J.B.; Singh, M. Live and processing performance of broiler chickens fed diets supplemented with complexed zinc. J. Appl. Poult. Res. 2010, 19, 334–340. [Google Scholar] [CrossRef]
- Qudsieh, R.I.; Smith, D.P.; Brake, J. Effect of elevated dietary inorganic zinc on live performance, carcass yield, and quality of male and female broilers. Poult. Sci. 2018, 97, 4122–4130. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Jing, M.Y.; Weng, X.Y.; Fu, L.J.; Wang, J.F. Effects of dietary zinc levels on the activities of enzymes, weights of organs, and the concentrations of zinc and copper in growing rats. Biol. Trace Elem. Res. 2005, 107, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.S.; Wang, Q.; Li, K.-X.; Guo, T.-Y.; Xiao, X.; Wang, Y.-X.; Zhan, X.-A. Effects of Maternal Zinc Glycine on Mortality, Zinc Concentration, and Antioxidant Status in a Developing Embryo and 1-Day-Old Chick. Biol. Trace Elem. Res. 2018, 181, 323–330. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Mu, C.; Gao, K.; Pi, Y.; Zhu, W. Low crude protein diets supplemented with casein hydrolysate enhance the intestinal barrier function and decrease the pro-inflammatory cytokine expression in the small intestine of pigs-ScienceDirect. Anim. Nutr. 2021, 7, 770–778. [Google Scholar] [CrossRef]
- Kumar, A.; Hosseindoust, A.; Kim, M.J.; Kim, K.; Choi, Y.; Lee, S.; Lee, S.; Lee, J.; Cho, H.; Kang, W.S.; et al. Nano-sized Zinc in Broiler Chickens: Effects on Growth Performance, Zinc Concentration in Organs, and Intestinal Morphology. J. Poult. Sci. 2021, 58, 21–29. [Google Scholar] [CrossRef]
- Mwangi, S.; Timmons, J.; Ao, T.; Paul, M.; Macalintal, L.; Pescatore, A.; Cantor, A.; Ford, M.; Dawson, K.A. Effect of zinc imprinting and replacing inorganic zinc with organic zinc on early performance of broiler chicks. Poult. Sci. 2017, 96, 861–868. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Zhang, J.; Zhang, N.; Yang, X.; Qu, H.; Xi, L.; Han, J. Effects of Dietary Zinc Levels on the Growth Performance, Organ Zinc Content, and Zinc Retention in Broiler Chickens. Revista Brasileira de Ciência Avícola 2018, 20, 127–132. [Google Scholar] [CrossRef]
- Lu, R.Y.; Yang, W.X.; Hu, Y.J. The role of epithelial tight junctions involved in pathogen infections. Mol. Biol. Rep. 2014, 41, 6591–6610. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Morgan, N.; Roberts, J.; Wu, S.-B.; Swick, R.; Toghyani, M. Zinc hydroxychloride supplementation improves tibia bone development and intestinal health of broiler chickens-ScienceDirect. Poult. Sci. 2021, 100, 101254. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Qureshi, M.A.; Dietert, R.R.; Mccormick, C.C. Tissue-specific accumulation of metallothionein in chickens as influenced by the route of zinc administration. J. Nutr. 1988, 118, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Abraham, C. Activation of Pattern Recognition Receptors Up-Regulates Metallothioneins, Thereby Increasing Intracellular Accumulation of Zinc, Autophagy, and Bacterial Clearance by Macrophages. Gastroenterology 2014, 147, 835–846. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Li, X.; Zhao, D.; Qin, S.; Shi, Z.; Wang, Z. Dietary zinc supplementation in breeding pigeons improves the carcass traits of squabs through regulating antioxidant capacity and myogenic regulatory factor expression. Poult Sci. 2023, 102, 102809. [Google Scholar] [CrossRef]


| Feed Ingredients (%) | Feeding Stage | ||
|---|---|---|---|
| 0–14 Days | 15–28 Days | 29–42 Days | |
| Corn | 61.21 | 69.39 | 73.08 |
| Soyabean meal | 31.70 | 23.50 | 20.10 |
| Soya oil | 0.80 | 1.10 | 1.50 |
| Corn protein flour | 2.50 | 2.50 | 2.40 |
| Dicalcium phosphate | 1.59 | 1.66 | 1.34 |
| Stone powder | 0.98 | 0.54 | 0.41 |
| NaCl | 0.28 | 0.30 | 0.21 |
| DL-Methionine | 0.21 | 0.19 | 0.18 |
| Baking soda | 0.21 | 0.21 | 0.20 |
| L-Lysine hydrochloride | 0.10 | 0.17 | 0.18 |
| Trace element premix 1 | 0.10 | 0.10 | 0.10 |
| Choline chloride-50 | 0.10 | 0.10 | 0.10 |
| Vitamin–mineral premix 2 | 0.05 | 0.05 | 0.05 |
| 12% salinomycin | 0.05 | 0.05 | 0.00 |
| Thermostable phytase | 0.01 | 0.01 | 0.01 |
| L-Threonine | 0.00 | 0.02 | 0.03 |
| Compound enzyme preparation | 0.01 | 0.01 | 0.01 |
| Substitute antibodies a | 0.10 | 0.10 | 0.10 |
| Total % | 100 | 100 | 100 |
| Nutrient composition Chemical analysis 3 (%) | |||
| Crude protein (CP) (DM%) | 21.31 | 17.96 | 16.47 |
| Coarse ash (DM%) | 5.73 | 5.32 | 5.20 |
| Crude fat (DM%) | 2.16 | 2.47 | 2.22 |
| Crude fiber (DM%) | 3.81 | 3.54 | 3.77 |
| Moisture (DM%) | 14.59 | 15.10 | 13.05 |
| Nitrogen-free extract (DM%) | 63.36 | 67.51 | 69.87 |
| Calcium (DM%) | 1.04 | 1.31 | 1.67 |
| Phosphorus (DM%) | 1.34 | 1.38 | 1.59 |
| Lysine (DM%) | 0.70 | 0.80 | 0.90 |
| Methionine (DM%) | 1.57 | 1.76 | 1.91 |
| Zn (mg/kg) b | 110 | 110 | 110 |
| ME (MJ/kg) | 11.67 | 12.01 | 12.31 |
| Stage | Content of Inorganic Zinc in a Low-Protein Diet | SEM | ||||
|---|---|---|---|---|---|---|
| 70 mg/kg | 90 mg/kg | 110 mg/kg | 130 mg/kg | 150 mg/kg | ||
| Day 0–14 | 72.82 | 88.17 | 115.50 | 127.13 | 143.61 | 1.979 |
| Day 15–28 | 69.55 | 83.21 | 115.20 | 128.35 | 143.16 | 0.768 |
| Day 29–42 | 72.37 | 84.21 | 106.23 | 126.61 | 145.99 | 2.389 |
| Gene Names | Accession No. | Primer Sequences (5′—3′) | Annealing Temp (°C) |
|---|---|---|---|
| Beta-actin | NM_205518 | ATCCGGACCCTCCATTGTC AGCCATGCCAATCTCGTCTT | 55 |
| GADPH | NM_204305.1 | GGAGAAACCAGCCAAGTAT CCATTGAAGTCACAGGAGA | 55 |
| Myf5 | NM_001030363.2 | TTCGAGACCTTGAAGAGGTGC TGTACCTGATGGCGTTCCTC | 55 |
| Myf6 | NM_001030746.3 | GCTCTGAAAAGGCGGACTGT TCCTGCAGCCTCTCGATGTA | 60 |
| MyoD | NM_204214.3 | ACCCAAAGCATGGGAAGAGT AGGCAGTATGGGACATGTGG | 55 |
| MyOG | NM_204305.1 | GTGACCCTGTGCCCTGAAAG TCGATGGACACGGTTTTGCG | 60 |
| MT | NM_205275.1 | CTCCTGCTCCTGTGCTGGGTCGTGC CGGTTCCTTGCAGACACAGCCCTT | 55 |
| Claudin-1 | NM_001013611.2 | ATCCAGTGCAAGGTGTACGA ACCAACCAGACCCAGGAGTA | 55 |
| Occludin-1 | NM_205128.1 | TCTGTGCTGAGATGGACAGC TCCTCTGCCACATCCTGGTA | 55 |
| ZO-1 | XM_413.773 | CTTCAGGTGTTTCTCTTCCTCCTC CTGTGGTTTCATGGCTGGATC | 55 |
| Items | Content of Inorganic Zinc in a Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 70 mg/kg (n = 54) | 90 mg/kg (n = 54) | 110 mg/kg (n = 54) | 130 mg/kg (n = 54) | 150 mg/kg (n = 54) | |||
| Body weight gain (g) | |||||||
| Week 1 | 143.67 | 145.08 | 146.10 | 146.64 | 152.65 | 0.780 | 0.103 |
| Week 2 | 262.72 | 261.43 | 270.97 | 276.22 | 286.80 | 1.222 | 0.047 |
| Week 3 | 334.26 b | 338.55 b | 337.26 b | 373.26 ab | 384.70 a | 5.058 | 0.009 |
| Week 4 | 493.70 | 491.67 | 504.92 | 506.25 | 568.49 | 7.571 | 0.149 |
| Week 5 | 541.69c | 578.12 bc | 590.25 bc | 616.17 ab | 676.89 a | 10.260 | <0.001 |
| Week 6 | 683.00 | 648.60 | 671.77 | 639.39 | 690.78 | 9.102 | 0.812 |
| Feed intake (g) | |||||||
| Week 1 | 170.43 | 170.92 | 175.33 | 167.83 | 176.27 | 1.786 | 0.347 |
| Week 2 | 366.76 | 371.52 | 378.08 | 379.18 | 380.46 | 1.043 | 0.103 |
| Week 3 | 556.76 | 549.55 | 534.46 | 575.40 | 598.06 | 4.389 | 0.167 |
| Week 4 | 754.24 | 742.05 | 741.76 | 717.96 | 810.66 | 11.037 | 0.612 |
| Week 5 | 1000.94 | 1038.47 | 1045.52 | 1011.64 | 1105.98 | 3.489 | 0.351 |
| Week 6 | 1295.34 | 1202.36 | 1207.14 | 1083.97 | 1174.06 | 24.751 | 0.361 |
| FCR | |||||||
| Week 1 | 1.19 | 1.18 | 1.20 | 1.15 | 1.15 | 0.012 | 0.290 |
| Week 2 | 1.40 | 1.42 | 1.40 | 1.37 | 1.33 | 0.015 | 0.452 |
| Week 3 | 1.66 | 1.62 | 1.59 | 1.54 | 1.55 | 0.015 | 0.239 |
| Week 4 | 1.53 a | 1.51 ab | 1.47 ab | 1.42 b | 1.43 b | 0.004 | 0.015 |
| Week 5 | 1.85 | 1.80 | 1.77 | 1.64 | 1.63 | 0.034 | 0.039 |
| Week 6 | 1.90 a | 1.85 ab | 1.79 ab | 1.70 b | 1.70 b | 0.004 | 0.006 |
| Items | Content of Inorganic Zinc in a Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 70 mg/kg (n = 6) | 90 mg/kg (n = 6) | 110 mg/kg (n = 6) | 130 mg/kg (n = 6) | 150 mg/kg (n = 6) | |||
| Dressed percentage | 90.3 | 90.2 | 90.4 | 92.1 | 91.5 | 0.57 | 0.058 |
| Percentage of half-eviscerated yield with giblet | 81.4 | 81.3 | 81.5 | 82.8 | 81.5 | 0.62 | 0.080 |
| Percentage of eviscerated yield | 69.2 b | 69.9 ab | 70.1 ab | 72.3 a | 70.8 ab | 0.92 | 0.044 |
| Percentage of breast muscle yield | 22.1 b | 23.5 ab | 23.6 ab | 24.7 a | 24.0 ab | 0.76 | 0.037 |
| Percentage of leg muscle yield | 22.9 | 23.9 | 24.0 | 24.8 | 24.3 | 0.98 | 0.197 |
| Percentage of abdominal fat yield | 2.8 a | 2.8 a | 2.6 a | 2.3 ab | 2.0 b | 0.20 | 0.002 |
| Items | Place | Content of Inorganic Zinc in a Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 70 mg/kg (n = 6) | 90 mg/kg (n = 6) | 110 mg/kg (n = 6) | 130 mg/kg (n = 6) | 150 mg/kg (n = 6) | ||||
| Flesh (L*) | Pectorals | 77.8 a | 76.8 a | 75.2 ab | 70.1 b | 70.5 b | 2.17 | <0.001 |
| Leg muscles | 71.6 | 71.8 | 70.6 | 67.4 | 67.5 | 1.84 | 0.030 | |
| PH45min | Pectorals | 5.81 | 5.92 | 5.96 | 6.09 | 6.06 | 0.170 | 0.391 |
| Leg muscles | 6.21 | 6.21 | 6.26 | 6.29 | 6.39 | 0.080 | 0.309 | |
| Shearing force | Pectorals | 30.3 a | 28.4 ab | 28.0 ab | 24.6 bc | 22.9 c | 1.10 | <0.001 |
| Leg muscles | 27.6 a | 26.1 a | 25.5 ab | 21.9 b | 22.4 b | 1.19 | <0.001 | |
| Water holding capacity | Pectorals | 17.5 c | 19.2 bc | 19.4 abc | 22.7 a | 22.6 ab | 0.98 | <0.001 |
| Leg muscles | 14.1 c | 16.7 c | 16.9 bc | 20.2 ab | 20.7 a | 1.02 | <0.001 | |
| Drip loss | Pectorals | 17.7 a | 17.6 a | 14.0 b | 11.6 b | 11.4 b | 0.79 | <0.001 |
| Leg muscles | 18.3 a | 17.6 ab | 18.6 a | 15.0 bc | 14.7 c | 0.88 | <0.001 | |
| Items | Content of Inorganic Zinc in a Low-Protein Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 70 mg/kg (n = 6) | 90 mg/kg (n = 6) | 110 mg/kg (n = 6) | 130 mg/kg (n = 6) | 150 mg/kg (n = 6) | |||
| ALP | 2038.87 | 2270.70 | 2245.22 | 2308.92 | 2061.53 | 182.626 | 0.887 |
| TP | 23.47 | 23.55 | 24.33 | 26.62 | 24.77 | 1.481 | 0.063 |
| ALB | 10.80 c | 11.05 bc | 11.38 abc | 12.43 a | 12.20 ab | 0.362 | 0.004 |
| GLO | 12.75 | 12.93 | 13.03 | 13.96 | 12.83 | 0.843 | 0.385 |
| A/G | 0.85 | 0.87 | 0.89 | 0.90 | 0.96 | 0.044 | 0.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Zhou, C.; Jia, Y.; Li, Y.; He, Y.; Che, H.; Zhang, Y.; Zhang, J.; Peng, D. Regulation of Inorganic Zinc Supplementation on Intestinal Absorption, Metabolism, and Muscle Development in Broilers Fed Low-Protein Diets. Agriculture 2023, 13, 2239. https://doi.org/10.3390/agriculture13122239
Sun R, Zhou C, Jia Y, Li Y, He Y, Che H, Zhang Y, Zhang J, Peng D. Regulation of Inorganic Zinc Supplementation on Intestinal Absorption, Metabolism, and Muscle Development in Broilers Fed Low-Protein Diets. Agriculture. 2023; 13(12):2239. https://doi.org/10.3390/agriculture13122239
Chicago/Turabian StyleSun, Ruihong, Changhai Zhou, Yougang Jia, Yumei Li, Yuntong He, Haoyu Che, Yonghong Zhang, Jing Zhang, and Dongqiao Peng. 2023. "Regulation of Inorganic Zinc Supplementation on Intestinal Absorption, Metabolism, and Muscle Development in Broilers Fed Low-Protein Diets" Agriculture 13, no. 12: 2239. https://doi.org/10.3390/agriculture13122239





