Influence of Grapevine Cultivar on Population Levels of Lobesia botrana (Lepidoptera: Tortricidae) and Effectiveness of Insecticides in Controlling This Pest
Abstract
1. Introduction
2. Materials and Methods
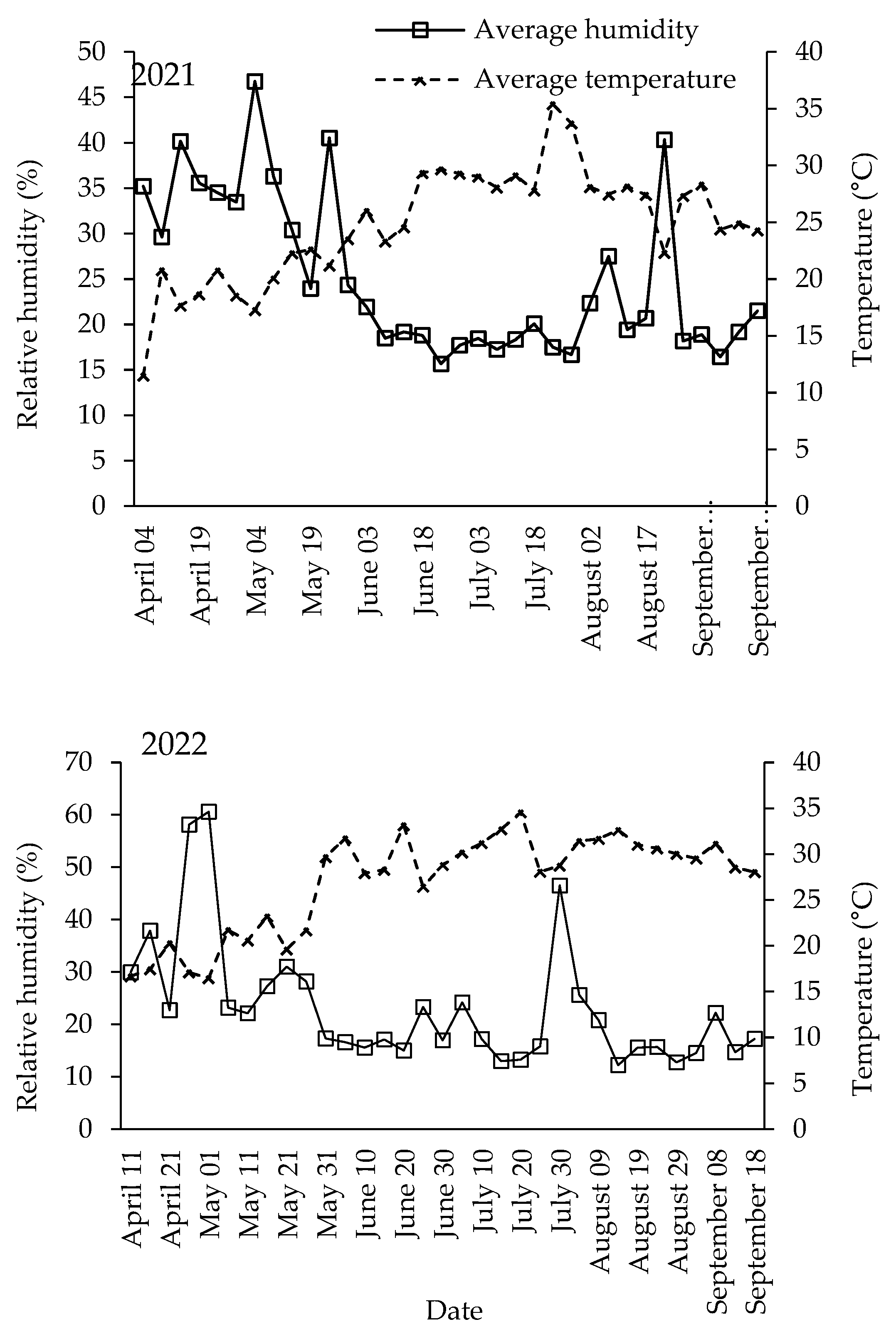
2.1. Vineyards
2.2. Population Dynamics of Lobesia botrana Different Life Stages
2.3. Cluster Analysis
2.4. Application of Insecticides for Controlling Lobesia botrana
2.5. Statistical Analysis
3. Results
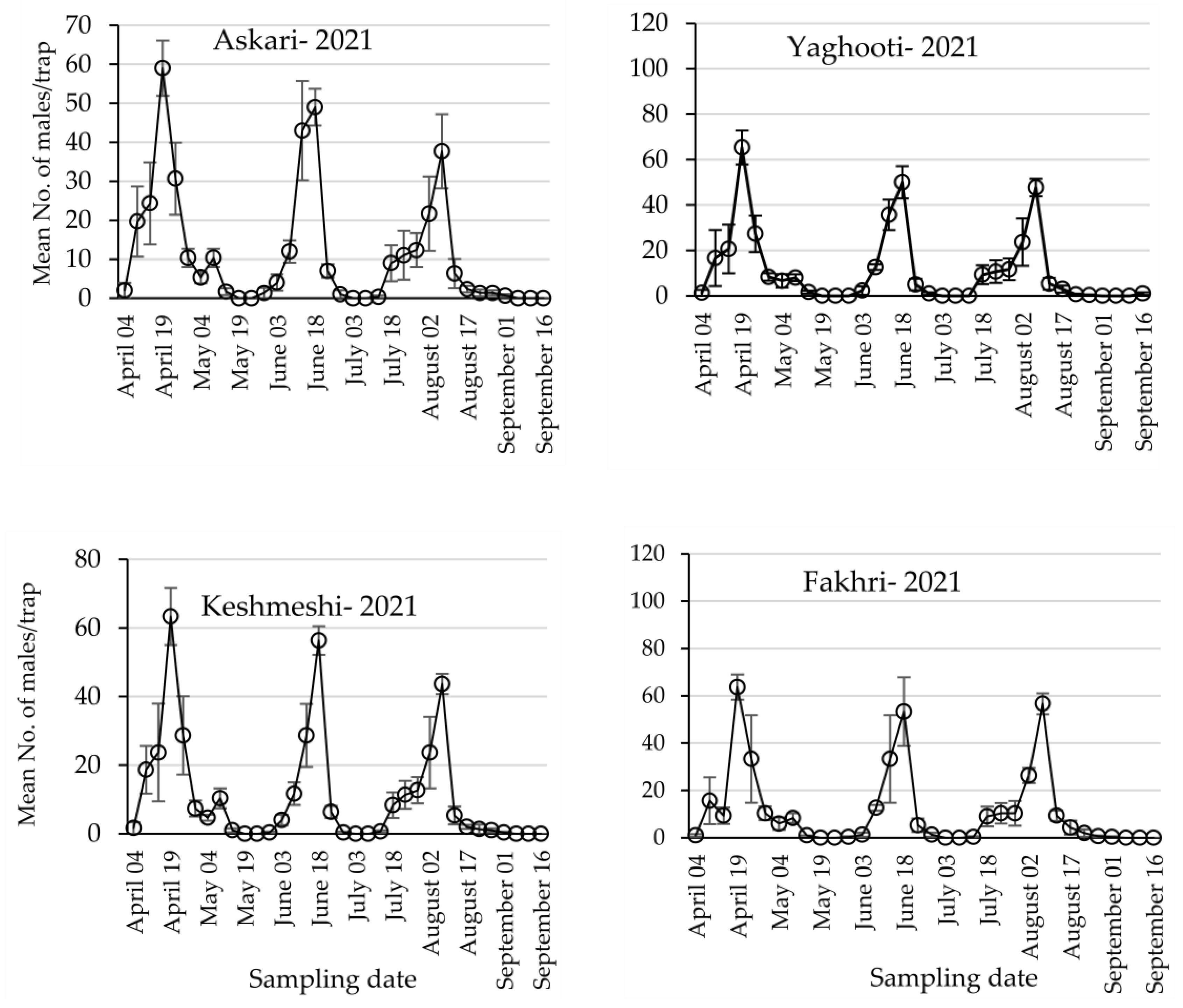
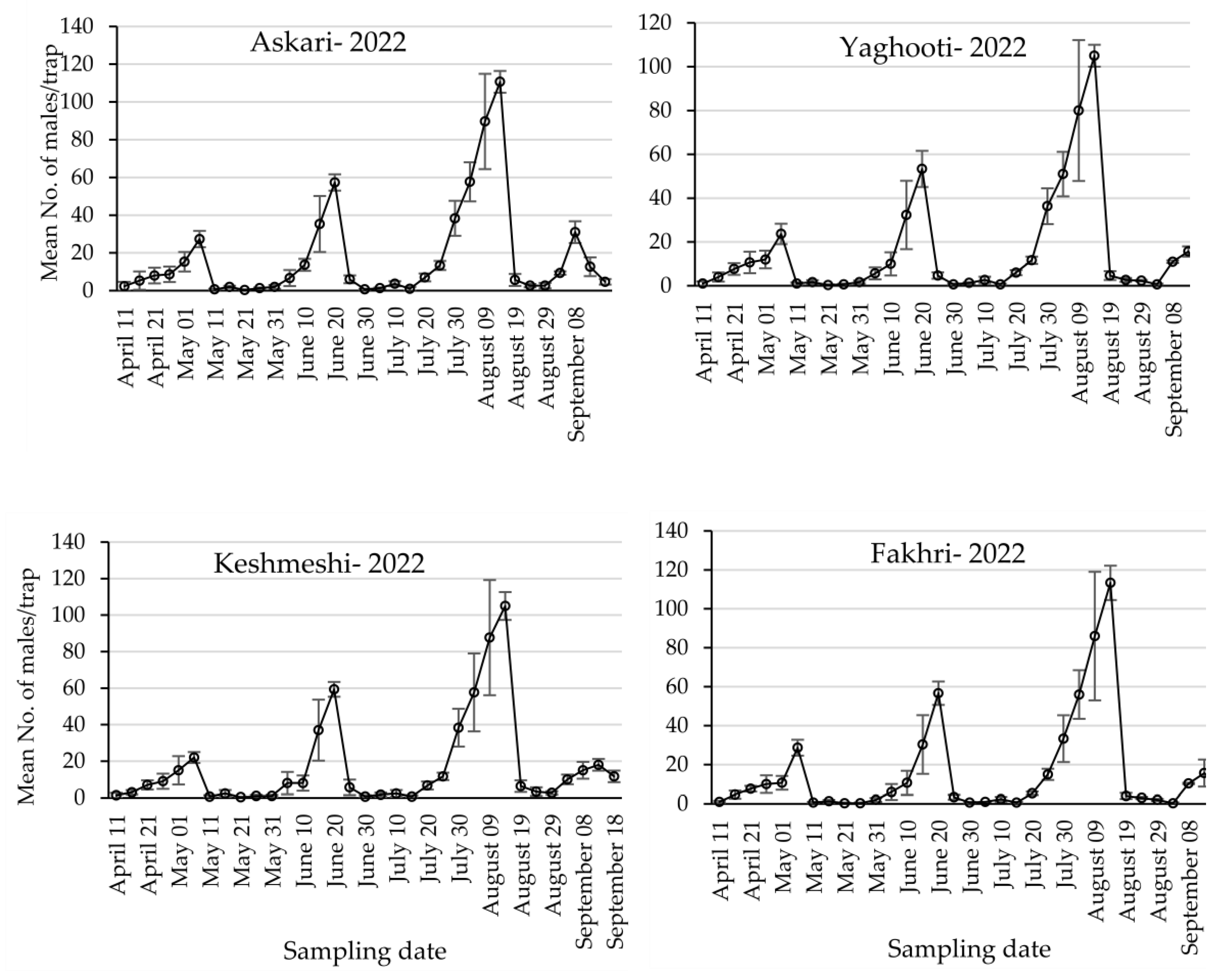
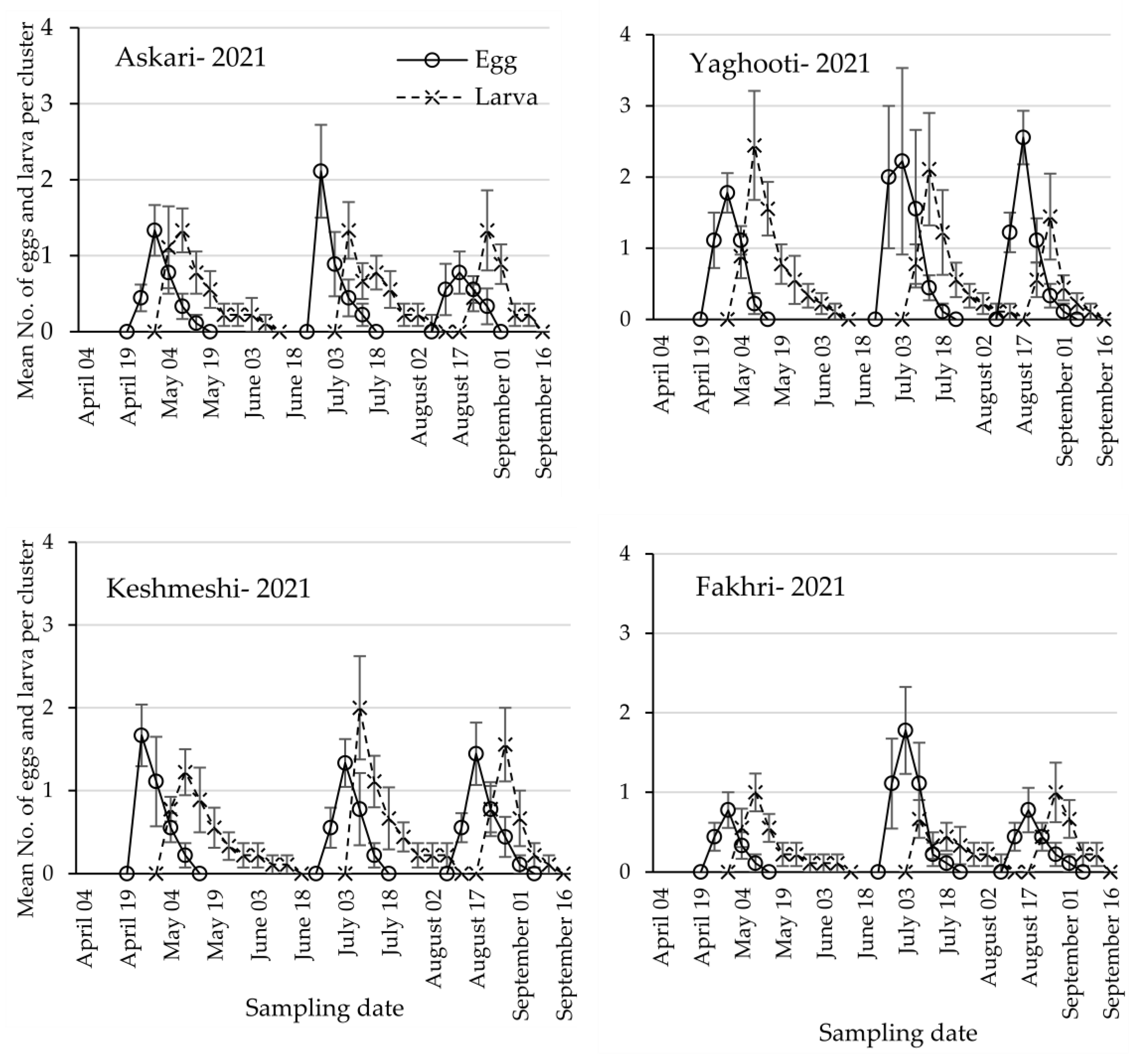
3.1. Population Dynamics of Lobesia botrana Different Life Stages
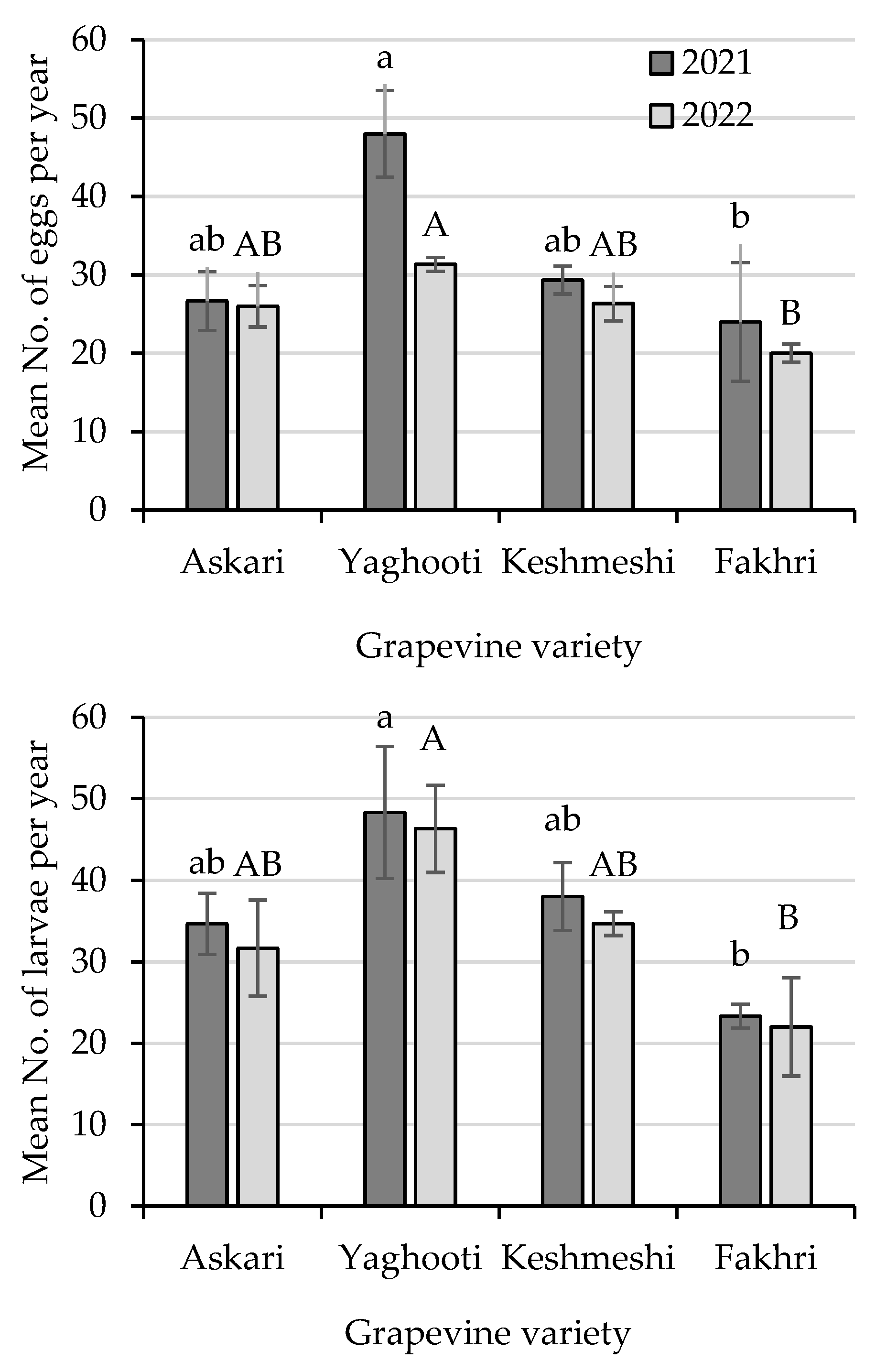
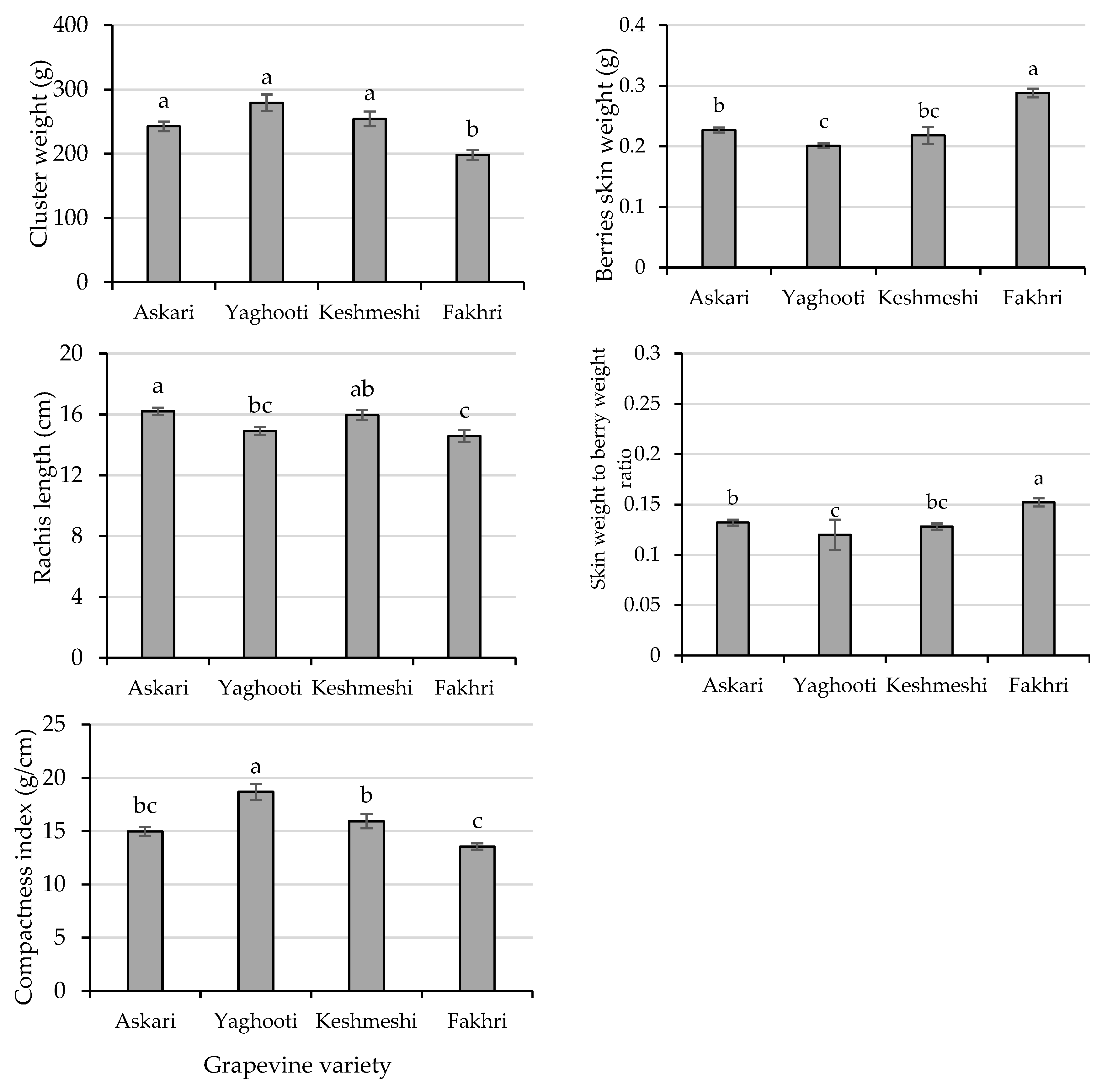
3.2. Cluster Analysis
3.3. Application of Insecticides for Controlling Lobesia botrana
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valera, J.; Matilla-Seiquer, G.; Obón, C.; Alcaraz, F.; Rivera, D. Grapevine in the ancient upper euphrates: Horticultural implications of a bayesian morphometric study of archaeological seeds. Horticulturae 2023, 9, 803. [Google Scholar] [CrossRef]
- Reisch, B.I.; Owens, C.L.; Cousins, P.S. Grape. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 225–262. [Google Scholar]
- Afkari-Sayyah, A.H.; Khorramifar, A.; Karami, H. Identification and classification of different grape cultivars using cultivar leaves by electroni nose. J. Environ. Sci. Stud. 2021, 6, 4382–4389. [Google Scholar]
- Tafazzoli, A.; Hekmati, J.; FIrouzeh, M. Grape, 2nd ed.; Shiraz University Press: Shiraz, Iran, 1993; p. 343. [Google Scholar]
- Moschos, T. Yield loss quantification and assessment of economic injury level for the anthophagous generation of the European grapevine moth Lobesia botrana Den. et Schiff.(Lepidoptera: Tortricidae). Int. J. Pest. Manag. 2005, 51, 81–89. [Google Scholar] [CrossRef]
- Varela, L.G.; Smith, R.J.; Cooper, M.L.; Hoenisch, R.W. European grapevine moth, Lobesia botrana Napa Valley vineyards. Pract. Winery Vineyard 2010, 2010, 1–5. [Google Scholar]
- Shojaaddini, M.; Amiri, R.; Babaei, S. An investigation of the susceptibility of 10 iranian grape cultivars to Lobesia botrana (Lep.: Tortricidae). Karafan Q. Sci. J. 2020, 17, 35–53. [Google Scholar]
- Moschos, T. Yield loss quantification and economic injury level estimation for the carpophagous generations of the European grapevine moth Lobesia botrana Den. et Schiff.(Lepidoptera: Tortricidae). Int. J. Pest. Manag. 2006, 52, 141–147. [Google Scholar] [CrossRef]
- Vassiliou, V.A. Control of Lobesia botrana (Lepidoptera: Tortricidae) in vineyards in Cyprus using the mating disruption technique. Crop Prot. 2009, 28, 145–150. [Google Scholar] [CrossRef]
- Altimira, F.; De La Barra, N.; Godoy, P.; Roa, J.; Godoy, S.; Vitta, N.; Tapia, E. Lobesia botrana: A biological control approach with a biopesticide based on entomopathogenic fungi in the winter season in chile. Insects 2022, 13, 8. [Google Scholar] [CrossRef]
- Akbarzadeh, S.G. Larval parasitoids of Lobesia botrana (Denis and Schiffermüller, 1775) (Lepidoptera: Tortricidae) in Orumieh vineyards. J. Agric. Sci. Technol. 2012, 14, 267–274. [Google Scholar]
- Pavan, F.; Stefanelli, G.; Villani, A.; Cargnus, E. Influence of grapevine cultivar on the second generations of Lobesia botrana and Eupoecilia ambiguella. Insects 2018, 9, 8. [Google Scholar] [CrossRef]
- Heit, G.; Sione, W.; Cortese, P. Three years analysis of Lobesia botrana (Lepidoptera: Tortricidae) flight activity in a quarantined area. J. Crop Prot. 2015, 4, 605–615. [Google Scholar]
- Castex, V.; de Cortázar-Atauri, I.G.; Beniston, M.; Moreau, J.; Semenov, M.; Stoffel, M.; Calanca, P. Exploring future changes in synchrony between grapevine (Vitis vinifera) and its major insect pest, Lobesia botrana. OENO One 2023, 57, 161–174. [Google Scholar] [CrossRef]
- Sharon, R.; Zahavi, T.; Soroker, V.; Harari, A.R. The effect of grape vine cultivars on Lobesia botrana (Lepidoptera: Tortricidae) population levels. J. Pest Sci. 2009, 82, 187–193. [Google Scholar] [CrossRef]
- Fermaud, M. Cultivar susceptibility of grape berry clusters to larvae of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 1998, 91, 974–980. [Google Scholar] [CrossRef]
- Altimira, F.; Vitta, N.; Tapia, E. Integrated pest management of Lobesia botrana with microorganism in vineyards: An alternative for clean grapes production. In Grapes and Wine; IntechOpen: London, UK, 2021. [Google Scholar]
- Ferracini, C.; Pogolotti, C.; Lentini, G.; Saitta, V.; Busato, E.; Rama, F.; Alma, A. Performance of pheromone-baited traps to monitor the seasonal abundance of tortrix moths in chestnut groves. Insects 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Ioriatti, C.; Anfora, G.; Tasin, M.; De Cristofaro, A.; Witzgall, P.; Lucchi, A. Chemical ecology and management of Lobesia botrana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2011, 104, 1125–1137. [Google Scholar] [CrossRef]
- Vassiliou, V.A. Effectiveness of insecticides in controlling the first and second generations of the Lobesia botrana (Lepidoptera: Tortricidae) in table grapes. J. Econ. Entomol. 2011, 104, 580–585. [Google Scholar] [CrossRef]
- Ioriatti, C.; Lucchi, A.; Bagnoli, B.; Koul, O.; Cuperus, G.; Elliot, N. Grape areawide pest management in Italy. In Areawide Pest Management: Theory and Implementation; Koul, O., Cuperus, G.W., Elliott, N., Eds.; CAB International: Wallingford, UK, 2008; pp. 208–225. [Google Scholar]
- Sáenz-de-Cabezón Irigaray, F.J.; Marco, V.; Zalom, F.G.; Pérez-Moreno, I. Effects of methoxyfenozide on Lobesia botrana Den & Schiff (Lepidoptera: Tortricidae) egg, larval and adult stages. Pest Manag. Sci. 2005, 61, 1133–1137. [Google Scholar]
- Sáenz-de-Cabezón, F.J.; Pérez-Moreno, I.; Zalom, F.G.; Marco, V. Effects of lufenuron on Lobesia botrana (Lepidoptera: Tortricidae) egg, larval, and adult stages. J. Econ. Entomol. 2006, 99, 427–431. [Google Scholar] [CrossRef]
- Hosseinzadeh, J.; Karimpour, Y.; Farazmand, H. Effect of lufox, on Lobesia botrana Den. & Schiff.(Lepidoptera: Tortricidae). Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2011, 3, 11–17. [Google Scholar]
- Duso, C.; Pozzebon, A.; Lorenzon, M.; Fornasiero, D.; Tirello, P.; Simoni, S.; Bagnoli, B. The impact of microbial and botanical insecticides on grape berry moths and their effects on secondary pests and beneficials. Agronomy 2022, 12, 217. [Google Scholar] [CrossRef]
- Shahini, S.; Kullaj, E.; Çakalli, A.; Çakalli, M.; Lazarevska, S.; Pfeiffer, D.G.; Gumeni, F. Population dynamics and biological control of European grapevine moth (Lobesia botrana: Lepidoptera: Tortricidae) in Albania using different strains of Bacillus thuringiensis. Int. J. Pest. Manag. 2010, 56, 281–286. [Google Scholar] [CrossRef]
- Irigaray, F.J.S.-D.-C.; Moreno-Grijalba, F.; Marco, V.; Pérez-Moreno, I. Acute and reproductive effects of Align, an insecticide containing azadirachtin, on the grape berry moth, Lobesia botrana. J. Insect Sci. 2010, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Pease, C.E.; López-Olguín, J.F.; Pérez-Moreno, I.; Marco-Mancebón, V. Effects of kaolin on Lobesia botrana (Lepidoptera: Tortricidae) and its compatibility with the natural enemy, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2016, 109, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Syngenta AG (Basel). Manual for Field Trials in Crop Protection, 4th ed.; Syngenta International AG: Basel, Switzerland, 2004; p. 444. [Google Scholar]
- Moreau, J.; Rahme, J.; Benrey, B.; Thiery, D. Larval host plant origin modifies the adult oviposition preference of the female European grapevine moth Lobesia botrana. Die Naturwissenschaften 2008, 95, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Richard, A.; Benrey, B.; Thiéry, D. Host plant cultivar of the grapevine moth Lobesia botrana affects the life history traits of an egg parasitoid. Biol. Control 2009, 50, 117–122. [Google Scholar] [CrossRef]
- Civolani, S.; Boselli, M.; Butturini, A.; Chicca, M.; Fano, E.A.; Cassanelli, S. Assessment of insecticide resistance of Lobesia botrana (Lepidoptera: Tortricidae) in Emilia-Romagna region. J. Econ. Entomol. 2014, 107, 1245–1249. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows Version 16.0; Spss Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Zanuncio, J.C.; Lemes, P.G.; Santos, G.P.; Soares, M.A.; Wilcken, C.F.; Serrão, J.E. Population dynamics of lepidoptera pests in Eucalyptus urophylla plantations in the Brazilian Amazonia. Forests 2014, 5, 72–87. [Google Scholar] [CrossRef]
- Sciarretta, A.; Zinni, A.; Mazzocchetti, A.; Trematerra, P. Spatial analysis of Lobesia botrana (Lepidoptera: Tortricidae) male population in a mediterranean agricultural landscape in Central Italy. Environ. Entomol. 2008, 37, 382–390. [Google Scholar] [CrossRef][Green Version]
- Gabel, B.; Mocko, V. Forecasting the cyclical timing of the grape vine moth, Lobesia botrana (Lepidoptera, Tortricidae). Acta Entomol. Bohemoslov. 1984, 81, 1–14. [Google Scholar]
- Moravie, M.A.; Davison, A.C.; Pasquier, D.; Charmillot, P.J. Bayesian forecasting of grape moth emergence. Ecol. Modell. 2006, 197, 478–489. [Google Scholar] [CrossRef]
- Ali, M.; Abdel-Lateef, F.; Awadallah, A.; Korashy, M. The effect of temperature and humidity on the development of vine grape moth Lobesia botrana Schiff [Egypt]. In Proceedings of the The Fourth Conference of Pest Control, Cairo, Egypt, 30 September 1978. [Google Scholar]
- Kaplan, M.; Özgen, I.; Kılıç, M. Determination of adult population fluctuation and infestation rate of bunch of grapes european grapevine moth [Lobesia botrana (Denis & Schiffermüller) (Lepidoptera: Tortricidae)] in the vineyards in Mazıdağı (Mardin). Meyve Bilimi 2016, 3, 10–16. [Google Scholar]
- Martín-Vertedor, D.; Ferrero-García, J.J.; Torres-Vila, L.M. Global warming affects phenology and voltinism of Lobesia botrana in Spain. Agric. For. Entomol. 2010, 12, 169–176. [Google Scholar] [CrossRef]
- Gallardo, A.; Ocete, R.; López, M.A.; Maistrello, L.; Ortega, F.; Semedo, A.; Soria, F.J. Forecasting the flight activity of Lobesia botrana (Denis & Schiffermüller) (Lepidoptera, Tortricidae) in Southwestern Spain. J. Appl. Entomol. 2009, 133, 626–632. [Google Scholar]
- Gutierrez, A.P.; Ponti, L.; Cooper, M.L.; Gilioli, G.; Baumgärtner, J.; Duso, C. Prospective analysis of the invasive potential of the European grapevine moth Lobesia botrana (Den. & Schiff.) in California. Agric. For. Entomol. 2012, 14, 225–238. [Google Scholar]
- van der Geest, L.P.; Evenhuis, H.H. Tortricid Pests: Their Biology, Natural Enemies and Control; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Thiéry, D.; Moreau, J. Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 2005, 143, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Stockel, J.; Roehrich, R.; Rodríguez-Molina, M.C. The relation between dispersal and survival of Lobesia botrana larvae and their density in vine inflorescences. Entomol. Exp. Appl. 1997, 84, 109–114. [Google Scholar] [CrossRef]
- Tonina, L.; Giomi, F.; Sancassani, M.; Ajelli, M.; Mori, N.; Giongo, L. Texture features explain the susceptibility of grapevine cultivars to Drosophila suzukii (Diptera: Drosophilidae) infestation in ripening and drying grapes. Sci. Rep. 2020, 10, 10245. [Google Scholar] [CrossRef]
- Amo-Salas, M.; Ortega-López, V.; Harman, R.; Alonso-González, A. A new model for predicting the flight activity of Lobesia botrana (Lepidoptera: Tortricidae). Crop Prot. 2011, 30, 1586–1593. [Google Scholar] [CrossRef]
- Georghiou, G.P. Overview of insecticide resistance. In Managing Resistance to Agrochemicals; Green, M.B., LeBaron, H.M., Moberg, W.K., Eds.; ACS Publications: Washington, DC, USA, 1990; Volume 421, pp. 18–41. [Google Scholar]
- Sudo, M.; Takahashi, D.; Andow, D.A.; Suzuki, Y.; Yamanaka, T. Optimal management strategy of insecticide resistance under various insect life histories: Heterogeneous timing of selection and interpatch dispersal. Evol. Appl. 2018, 11, 271–283. [Google Scholar] [CrossRef]


| Insecticides | Formulation | Trade Name | Recommended Concentrations (ppm) | Company |
|---|---|---|---|---|
| Bacillus thuringiensis subsp. Kurstaki (Bt) | SC 24% | Biolep | 2000 | Biorun, Iran |
| Alpha cypermethrin + teflubenzuron | SC15% | Imunit | 750 | BASF, Germany |
| Trichlorfon | SP 80% | Dipterex | 1500 | Arman Sabz Adineh, Iran |
| Phosalone | EC 35% | Zolone | 1500 | Mahan, Iran |
| Lufenuron + fenoxycarb | EC 10.5% | Lufox | 300 | Syngenta, Germany |
| Treatments | Second Generation | Third Generation |
|---|---|---|
| 1 | Control (water) | Control (water) |
| 2 | Bt | Alpha cypermethrin + teflubenzuron |
| 3 | Bt | Trichlorfon |
| 4 | Bt | Phosalone |
| 5 | Lufenuron + fenoxycarb | Alpha cypermethrin + teflubenzuron |
| 6 | Lufenuron + fenoxycarb | Trichlorfon |
| Year | Generation | Askari | Yaghooti | Keshmeshi | Fakhri | F3,8, p |
|---|---|---|---|---|---|---|
| 2021 | First | 59.00 ± 7.09 | 65.33 ± 7.53 | 63.33 ± 8.33 | 63.66 ± 6.33 | 0.135, 0.937 |
| Second | 49.00 ± 4.72 | 50.00 ± 7.09 | 56.33 ± 4.17 | 53.33 ± 6.00 | 0.353, 0.788 | |
| Third | 37.66 ± 9.52 | 47.66 ± 3.84 | 43.66 ± 2.96 | 56.66 ± 6.00 | 1.691, 0.246 | |
| 2022 | First | 27.33 ± 4.33 | 23.66 ± 4.66 | 22.00 ± 3.00 | 28.66 ± 4.09 | 0.582, 0.643 |
| Second | 57.33 ± 4.33 | 53.33 ± 8.25 | 59.33 ± 4.05 | 56.66 ± 6.00 | 0.178, 0.908 | |
| Third | 110.67 ± 5.78 | 105.00 ± 5.00 | 105.00 ± 7.63 | 113.33 ± 8.82 | 0.360, 0.784 |
| Source | df | Mean Square | F | p |
|---|---|---|---|---|
| Time | 2 | 4.699 | 0.617 | 0.543 |
| Treatment | 5 | 766.245 | 100.547 | <0.001 ** |
| Generation | 1 | 4.572 | 0.600 | 0.441 |
| Time × Treatment | 10 | 155.896 | 20.457 | <0.001** |
| Treatment × Generation | 5 | 13.108 | 1.720 | 0.141 |
| Time × Treatment × Generation | 12 | 6.732 | 0.883 | 0.567 |
| Error | 72 | 7.621 | ||
| Total | 108 |
| Time after Spray (d) | Generation | Treatments | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | Second | 8.64 ± 1.23 a | 7.40 ± 0.00 ab | 8.64 ± 1.23 a | 3.70 ± 0.00 b | 3.70 ± 0.00 b | 4.93 ± 1.23 ab |
| Third | 7.40 ± 2.13 | 2.46 ± 1.23 | 3.7. ± 0.00 | 4.93 ± 3.26 | 7.40 ± 2.13 | 6.17 ± 3.26 | |
| 7 | Second | 22.22 ± 2.13 a | 3.70 ± 0.00 b | 1.23 ± 1.23 b | 4.93 ± 3.26 b | 2.46 ± 2.46 b | 2.46 ± 1.23 b |
| Third | 19.75 ± 1.23 a | 1.23 ± 1.23 b | 1.23 ± 1.23 b | 2.46 ± 1.23 b | 4.93 ± 1.23 b | 6.17 ± 1.23 b | |
| 14 | Second | 28.39 ± 1.23 a | 0.00 ± 0.00 b | 3.70 ± 0.00 b | 3.70 ± 2.10 b | 1.23 ± 1.23 b | 2.46 ± 1.23 b |
| Third | 29.62 ± 2.13 a | 1.23 ± 1.23 b | 1.23 ± 1.23 b | 1.23 ± 1.23 b | 2.46 ± 1.23 b | 2.46 ± 1.23 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepahvand, Z.; Ziaee, M.; Ghorbani, R.; Hemmati, S.A.; Francikowski, J. Influence of Grapevine Cultivar on Population Levels of Lobesia botrana (Lepidoptera: Tortricidae) and Effectiveness of Insecticides in Controlling This Pest. Agriculture 2023, 13, 2286. https://doi.org/10.3390/agriculture13122286
Sepahvand Z, Ziaee M, Ghorbani R, Hemmati SA, Francikowski J. Influence of Grapevine Cultivar on Population Levels of Lobesia botrana (Lepidoptera: Tortricidae) and Effectiveness of Insecticides in Controlling This Pest. Agriculture. 2023; 13(12):2286. https://doi.org/10.3390/agriculture13122286
Chicago/Turabian StyleSepahvand, Zahra, Masumeh Ziaee, Roshanak Ghorbani, Seyed Ali Hemmati, and Jacek Francikowski. 2023. "Influence of Grapevine Cultivar on Population Levels of Lobesia botrana (Lepidoptera: Tortricidae) and Effectiveness of Insecticides in Controlling This Pest" Agriculture 13, no. 12: 2286. https://doi.org/10.3390/agriculture13122286
APA StyleSepahvand, Z., Ziaee, M., Ghorbani, R., Hemmati, S. A., & Francikowski, J. (2023). Influence of Grapevine Cultivar on Population Levels of Lobesia botrana (Lepidoptera: Tortricidae) and Effectiveness of Insecticides in Controlling This Pest. Agriculture, 13(12), 2286. https://doi.org/10.3390/agriculture13122286







