Investigating Genetic Characteristics of Chinese Holstein Cow’s Milk Somatic Cell Score by Genetic Parameter Estimation and Genome-Wide Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal, Phenotype, and Genotype Data
2.3. Genetic Parameter Estimation
2.4. Analysis of Principal Components
2.5. Genome-Wide Association Analysis
2.6. Function Annotation Analysis of Candidate Genes
2.7. Cell Culture and Lipopolysaccharide (LPS) Treatment
2.8. Extraction of RNA and Quantitative Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Genetic Parameter Evaluation
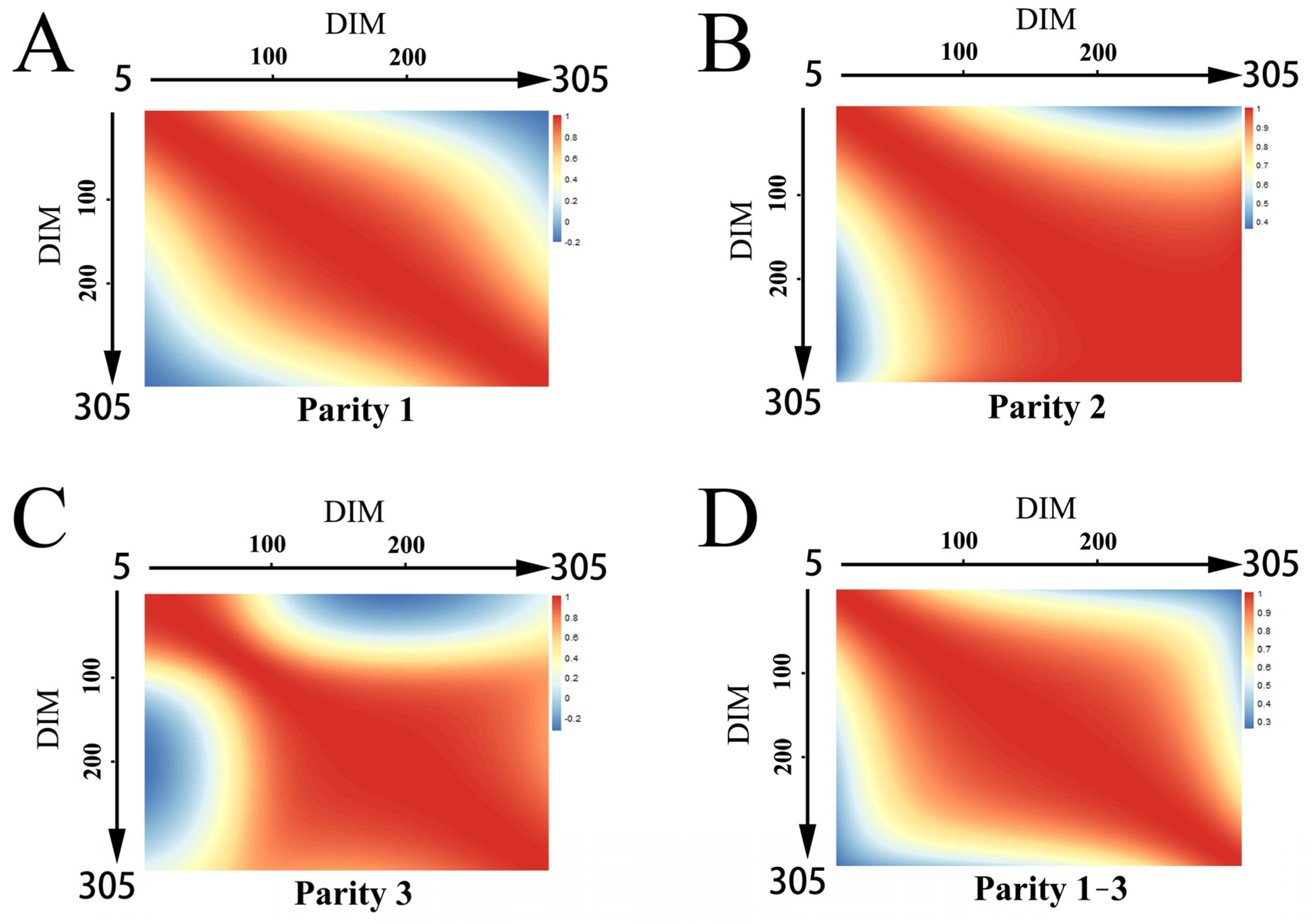
3.2. Genetic and Permanent Environmental Correlations
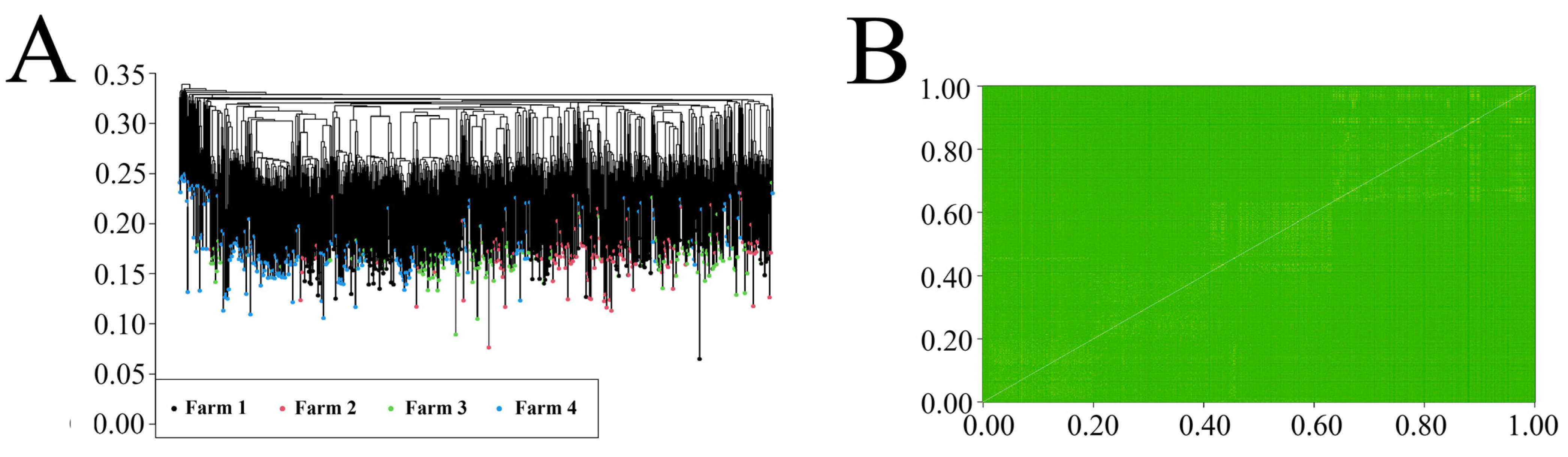
3.3. Population Structure Analysis
3.4. Genome-Wide Association Study for SCS
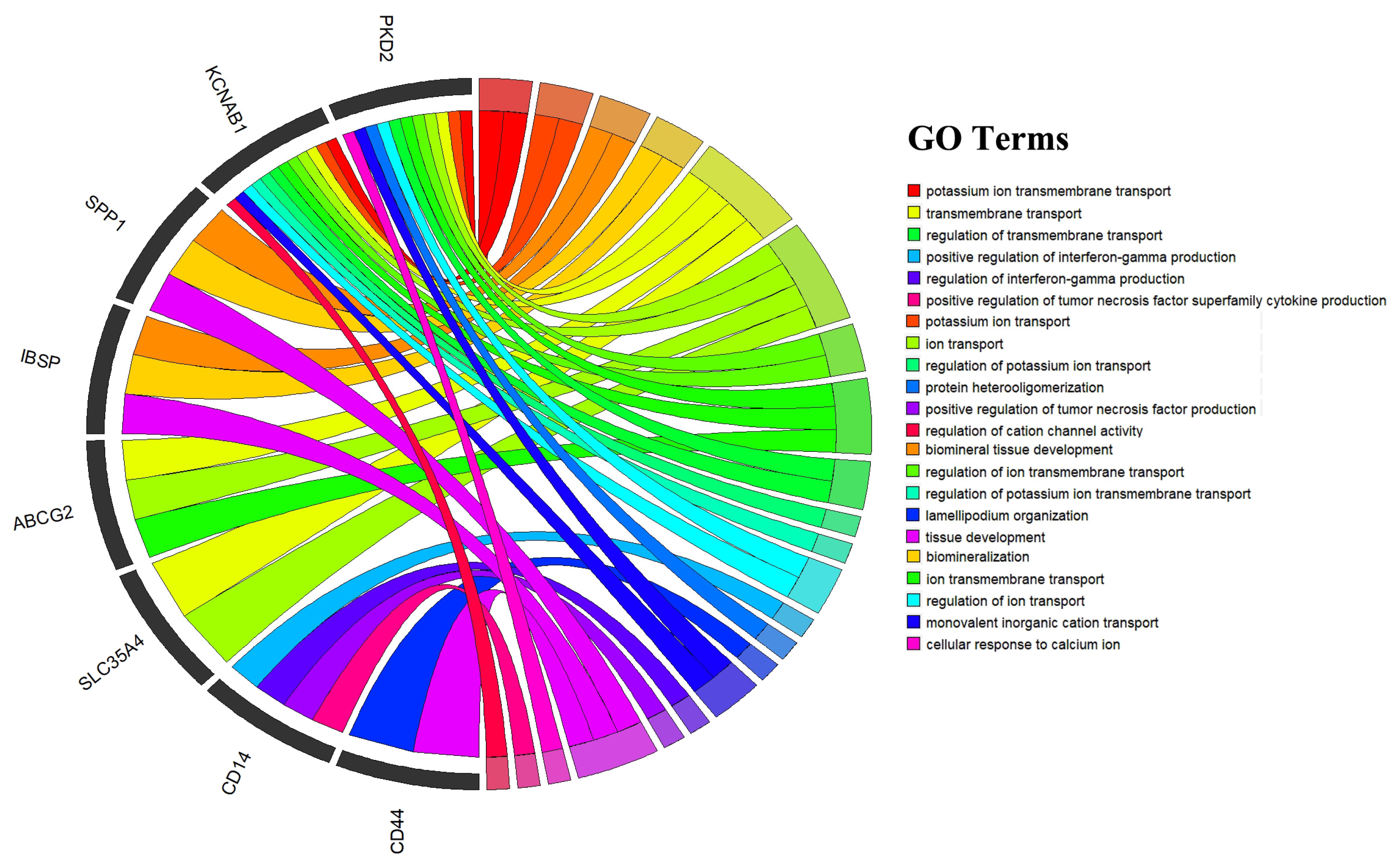
3.5. Annotation and Enrichment Analysis of Candidate Genes
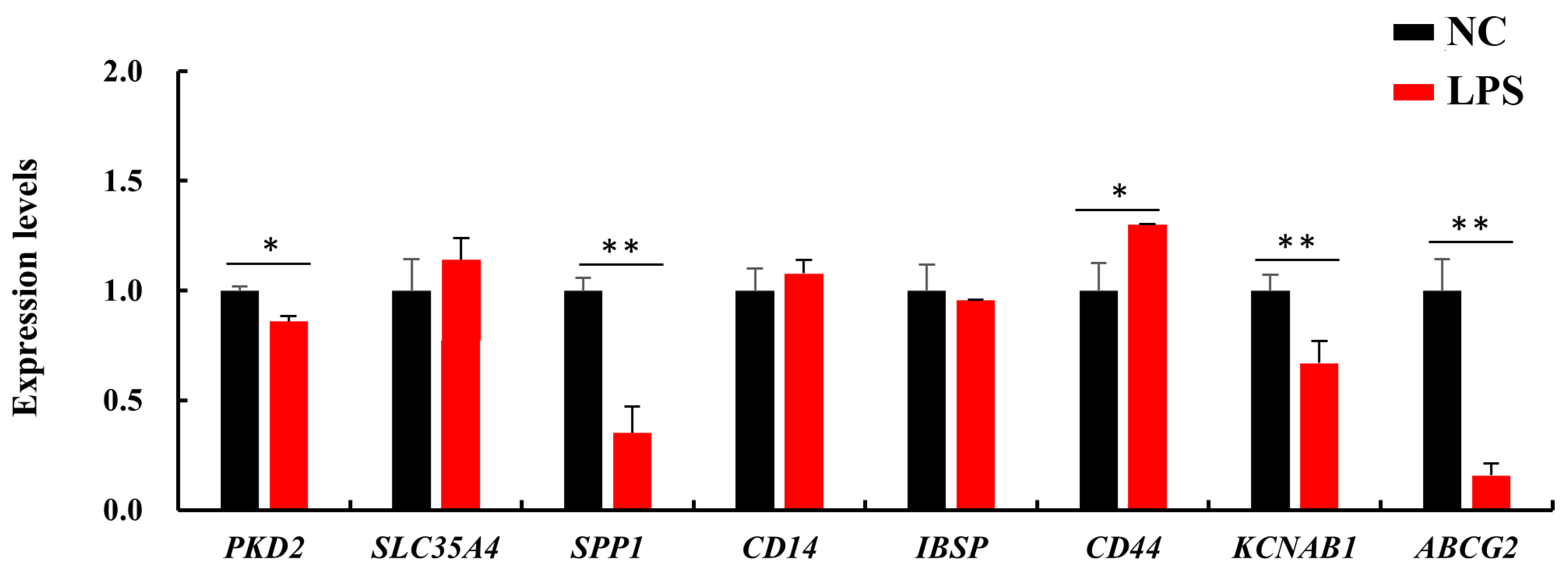
3.6. Expressions of Candidate Genes in LPS-Challenged bMECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi-Sefidmazgi, A.; Moradi-Shahrbabak, M.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.R.; Amer, P.R. Estimation of economic values and financial losses associated with clinical mastitis and somatic cell score in Holstein dairy cattle. Animal 2011, 5, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, C.B.; Jensen, M.B.; de Passille, A.M.; Hanninen, L.; Rushen, J. Lying time and the welfare of dairy cows. J. Dairy Sci. 2021, 104, 20–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Zhang, W.; Chen, S.; Wen, X.; Ran, X.; Wang, H.; Zhao, J.; Qi, Y.; Xue, N. Prevalence of subclinical mastitis among dairy cattle and associated risks factors in China during 2012–2021: A systematic review and meta-analysis. Res. Vet. Sci. 2022, 148, 65–73. [Google Scholar] [CrossRef]
- Kerslake, J.I.; Amer, P.R.; O’Neill, P.L.; Wong, S.L.; Roche, J.R.; Phyn, C.V.C. Economic costs of recorded reasons for cow mortality and culling in a pasture-based dairy industry. J. Dairy Sci. 2018, 101, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeck, A.; Loker, S.; Miglior, F.; Kelton, D.F.; Jamrozik, J.; Schenkel, F.S. Genetic relationships of clinical mastitis, cystic ovaries, and lameness with milk yield and somatic cell score in first-lactation Canadian Holsteins. J. Dairy Sci. 2014, 97, 5806–5813. [Google Scholar] [CrossRef] [Green Version]
- Shook, G.E.; Schutz, M.M. Selection on Somatic Cell Score to Improve Resistance to Mastitis in the United States. J. Dairy Sci. 1994, 77, 648–658. [Google Scholar] [CrossRef]
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef]
- de Oliveira, H.R.; Fonseca e Silva, F.; Gualberto Barbosa da Silva, M.V.; Barbosa Dias de Siqueira, O.H.G.; Machado, M.A.; do Carmo Panetto, J.C.; Gloria, L.S.; Brito, L.F. Bayesian Models combining Legendre and B-spline polynomials for genetic analysis of multiple lactations in Gyr cattle. Livest. Sci. 2017, 201, 78–84. [Google Scholar] [CrossRef]
- Strucken, E.M.; Bortfeldt, R.H.; de Koning, D.J.; Brockmann, G.A. Genome-wide associations for investigating time-dependent genetic effects for milk production traits in dairy cattle. Anim. Genet. 2012, 43, 375–382. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Madsen, P.; Li, R.; Liu, W.; Bao, P.; Xue, G.; Gao, Y.; Di, X.; Su, G. Impact of the Order of Legendre Polynomials in Random Regression Model on Genetic Evaluation for Milk Yield in Dairy Cattle Population. Front. Genet. 2020, 11, 586155. [Google Scholar] [CrossRef]
- Schaeffer, L.R.; Jamrozik, J.; Kistemaker, G.J.; Van Doormaal, B.J. Experience with a test-day model. J. Dairy Sci. 2000, 83, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, G.R.; Sonstegard, T.S.; VanRaden, P.M.; Matukumalli, L.K.; Schnabel, R.D.; Taylor, J.F.; Schenkel, F.S.; van Tassell, C.P. Selection of single-nucleotide polymorphisms and quality of genotypes used in genomic evaluation of dairy cattle in the united States and Canada. J. Dairy Sci. 2009, 92, 3431–3436. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, Y.; Zhou, J.; Lu, L.; Wang, X.; Liang, Y.; Loor, J.J.; Gou, D.; Xu, H.; Yang, Z. Tea tree oil prevents mastitis-associated inflammation in lipopolysaccharide-stimulated bovine mammary epithelial cells. Front. Vet. Sci. 2020, 7, 496. [Google Scholar] [CrossRef]
- Thomas, F.C.; Mullen, W.; Tassi, R.; Ramírez-Torres, A.; Mudaliar, M.; McNeilly, T.N.; Zadoks, R.N.; Burchmore, R.; Eckersall, P.D. Mastitomics, the integrated omics of bovine milk in an experimental model of Streptococcus uberis mastitis: 1. High abundance proteins, acute phase proteins and peptidomics. Mol. Biosyst. 2016, 12, 2735–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnzon, C.-F.; Dahlberg, J.; Gustafson, A.-M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The effect of lipopolysaccharide-induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: A kinetic approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, T.; Penasa, M.; Finocchiaro, R.; Visentin, G.; Cassandro, M. Alternative somatic cell count traits exploitable in genetic selection for mastitis resistance in Italian Holsteins. J. Dairy Sci. 2018, 101, 10001–10010. [Google Scholar] [CrossRef] [Green Version]
- Harmon, R. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Lu, X.; Abdalla, I.M.; Nazar, M.; Fan, Y.; Zhang, Z.; Wu, X.; Xu, T.; Yang, Z. Genome-Wide Association Study on Reproduction-Related Body-Shape Traits of Chinese Holstein Cows. Animals 2021, 11, 1927. [Google Scholar] [CrossRef]
- Lu, X.; Arbab, A.A.I.; Abdalla, I.M.; Liu, D.; Zhang, Z.; Xu, T.; Su, G.; Yang, Z. Genetic Parameter Estimation and Genome-Wide Association Study-Based Loci Identification of Milk-Related Traits in Chinese Holstein. Front. Genet. 2022, 12, 799664. [Google Scholar] [CrossRef]
- Madsen, P.; Milkevych, V.; Gao, H.; Christensen, O.F.; Jensen, J. DMU-A Package for Analyzing Multivariate Mixed Models in Quantitative Genetics and Genomics. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11 February 2018. [Google Scholar]
- Garrick, D.J.; Taylor, J.F.; Fernando, R.L. Deregressing estimated breeding values and weighting information for genomic regression analyses. Genet. Sel. Evol. 2009, 41, 55. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Mota, L.F.M.; Lopes, F.B.; Fernandes Júnior, G.A.; Rosa, G.J.M.; Magalhães, A.F.B.; Carvalheiro, R.; Albuquerque, L.G. Genome-wide scan highlights the role of candidate genes on phenotypic plasticity for age at first calving in Nellore heifers. Sci. Rep. 2020, 10, 6481. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Tian, F.; Pan, Y.; Buckler, E.S.; Zhang, Z. A SUPER Powerful Method for Genome Wide Association Study. PLoS ONE 2014, 9, e107684. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.-P.; Govignon-Gion, A.; Croiseau, P.; Fritz, S.; Hozé, C.; Miranda, G.; Martin, P.; Barbat-Leterrier, A.; Letaïef, R.; Rocha, D.; et al. Within-breed and multi-breed GWAS on imputed whole-genome sequence variants reveal candidate mutations affecting milk protein composition in dairy cattle. Genet. Sel. Evol. 2017, 49, 68. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Heneberg, P.; Mao, Y.; Jiang, Q.; Loor, J.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2022, 100, skab345. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, X.; Lu, X.; Liang, Y.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin activated AMPK signaling contributes to the alleviation of LPS-induced inflammatory responses in bovine mammary epithelial cells. BMC Vet. Res. 2021, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Janovick-Guretzky, N.A.; Dann, H.M.; Carlson, D.B.; Murphy, M.R.; Loor, J.J.; Drackley, J.K. Housekeeping gene expression in bovine liver is affected by physiological state, feed intake, and dietary treatment. J. Dairy Sci. 2007, 90, 2246–2252. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Peng, J.; Loor, J. Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca2+ in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 11354–11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadhurst, D.I.; Kell, D.B. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef] [Green Version]
- Bohmanova, J.; Miglior, F.; Jamrozik, J.; Misztal, I.; Sullivan, P. Comparison of random regression models with Legendre polynomials and linear splines for production traits and somatic cell score of Canadian Holstein cows. J. Dairy Sci. 2008, 91, 3627–3638. [Google Scholar] [CrossRef] [Green Version]
- Adriaens, I.; Van Den Brulle, I.; Geerinckx, K.; D’Anvers, L.; De Vliegher, S.; Aernouts, B. Milk losses linked to mastitis treatments at dairy farms with automatic milking systems. Prev. Vet. Med. 2021, 194, 105420. [Google Scholar] [CrossRef]
- Banos, G.; Shook, G.E. Genotype by Environment Interaction and Genetic Correlations Among Parities for Somatic Cell Count and Milk Yield. J. Dairy Sci. 1990, 73, 2563–2573. [Google Scholar] [CrossRef] [Green Version]
- Wahinya, P.; Jeyaruban, M.; Swan, A.; Gilmour, A.; Magothe, T. Genetic parameters for test-day milk yield, lactation persistency, and fertility in low-, medium-, and high-production systems in Kenya. J. Dairy Sci. 2020, 103, 10399–10413. [Google Scholar] [CrossRef]
- Frioni, N.; Rovere, G.; Aguilar, I.; Urioste, J.I. Genetic parameters and correlations between days open and production traits across lactations in pasture based dairy production systems. Livest. Sci. 2017, 204, 104–109. [Google Scholar] [CrossRef]
- Carlén, E.; Strandberg, E.; Roth, A. Genetic Parameters for Clinical Mastitis, Somatic Cell Score, and Production in the First Three Lactations of Swedish Holstein Cows. J. Dairy Sci. 2004, 87, 3062–3070. [Google Scholar] [CrossRef] [Green Version]
- Ojango, J.M.; Mrode, R.; Rege, J.; Mujibi, D.; Strucken, E.; Gibson, J.; Mwai, O. Genetic evaluation of test-day milk yields from smallholder dairy production systems in Kenya using genomic relationships. J. Dairy Sci. 2019, 102, 5266–5278. [Google Scholar] [CrossRef]
- Sul, J.H.; Martin, L.S.; Eskin, E. Population structure in genetic studies: Confounding factors and mixed models. PLoS Genet. 2018, 14, e1007309. [Google Scholar] [CrossRef] [PubMed]
- Macciotta, N.P.P.; Biffani, S.; Bernabucci, U.; Lacetera, N.; Vitali, A.; Ajmone-Marsan, P.; Nardone, A. Derivation and genome-wide association study of a principal component-based measure of heat tolerance in dairy cattle. J. Dairy Sci. 2017, 100, 4683–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, T.; König, S. Genome-wide associations and detection of potential candidate genes for direct genetic and maternal genetic effects influencing dairy cattle body weight at different ages. Genet. Sel. Evol. 2019, 51, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, A.L.; Zaitlen, N.A.; Reich, D.; Patterson, N. New approaches to population stratification in genome-wide association studies. Nat. Rev. Genet. 2010, 11, 459–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Cook, A.C.; Kappil, M.; Günthert, U.; Chambers, A.F.; Tuck, A.B.; Denhardt, D.T. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: Novel post-transcriptional, post-translational regulation. Clin. Exp. Metastasis 2005, 22, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska-Łuczak, I.; Kulig, H. Polymorphism of the FAM13A, ABCG2, OPN, LAP3, HCAP-G, PPARGC1A genes and somatic cell count of Jersey cows–Preliminary study. Res. Vet. Sci. 2013, 94, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, C.; Kuang, S.-D.; Su, C.; Zhao, X.; Xiong, Y.-l.; Li, Y.-S.; Gao, S.-G. OPN Deficiency Increases the Severity of Osteoarthritis Associated with Aberrant Chondrocyte Senescence and Apoptosis and Upregulates the Expression of Osteoarthritis-Associated Genes. Pain Res. Manag. 2020, 2020, 3428587. [Google Scholar] [CrossRef]
- Sharma, B.S.; Leyva, I.; Schenkel, F.; Karrow, N.A. Association of Toll-Like Receptor 4 Polymorphisms with Somatic Cell Score and Lactation Persistency in Holstein Bulls. J. Dairy Sci. 2006, 89, 3626–3635. [Google Scholar] [CrossRef] [Green Version]
- Kanneganti, T.D.; Lamkanfi, M.; Núñez, G. Intracellular NOD-like Receptors in Host Defense and Disease. Immunity 2007, 27, 549–559. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Calzado, M.; Bacher, S.; Schmitz, M.L. NF-κB Inhibitors for the Treatment of Inflammatory Diseases and Cancer. Curr. Med. Chem. 2007, 14, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Jeong, C.H.; Cheng, W.N.; Bae, H.; Lee, K.W.; Han, S.M.; Petriello, M.C.; Lee, H.G.; Seo, H.G.; Han, S.G. Bee venom decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J. Microbiol. Biotechnol. 2017, 27, 1827–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougarn, S.; Cunha, P.; Gilbert, F.; Meurens, F.; Rainard, P. Validation of candidate reference genes for normalization of quantitative PCR in bovine mammary epithelial cells responding to inflammatory stimuli. J. Dairy Sci. 2011, 94, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments-A review. Asian-Australasian. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar]
- He, X.; Liu, W.; Shi, M.; Yang, Z.; Zhang, X.; Gong, P. Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells. Res. Vet. Sci. 2017, 112, 7–12. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X.; Liu, Z.; Zhang, W.; Fang, J.; Xue, J.; Bao, H. Hydroxysafflor yellow A inhibits staphylococcus aureus-induced mouse endometrial inflammation via TLR2-mediated NF-kB and MAPK pathway. Inflammation 2021, 44, 835–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.-J. Selenium deficiency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef] [PubMed]



| Parity | N | RN | Mean | SE | Median | Min | Max | Skew | Kurtosis | Heritability (SE) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5127 | 37,179 | 2.57 | 0.01 | 2.26 | 0.67 | 9.00 | 0.75 | 0.76 | 0.24 (0.01) |
| 2 | 3777 | 27,432 | 2.83 | 0.01 | 2.68 | 0.68 | 9.00 | 0.58 | 0.25 | 0.14 (0.03) |
| 3 | 2982 | 21,670 | 2.88 | 0.02 | 2.68 | 0.54 | 9.00 | 0.61 | 0.21 | 0.07 (0.02) |
| 1–3 | 8580 | 86,281 | 2.70 | 0.01 | 2.49 | 0.54 | 9.00 | 0.69 | 0.50 | 0.18 (0.01) |
| PC | Variance (%) | Twstat | p-Value |
|---|---|---|---|
| 1 | 11.822 | 3.613 | 0.001 |
| 2 | 9.811 | 2.831 | 0.002 |
| 3 | 7.303 | 0.552 | 0.088 |
| 4 | 6.393 | 0.699 | 0.073 |
| 5 | 5.511 | 0.587 | 0.084 |
| 6 | 4.553 | −0.366 | 0.244 |
| 7 | 4.302 | 0.800 | 0.064 |
| 8 | 3.503 | −0.168 | 0.200 |
| SNP | CHR | Position | Nearest Gene | Distance (kb) | MAF | EVP | p-Value |
|---|---|---|---|---|---|---|---|
| rs135806474 | 6 | 36725781 | SPP1 | within (intron) | 0.251174 | 2.46% | 1.26 × 10−9 |
| rs41256968 | 15 | 65736591 | CD44 | within (exon) | 0.224178 | 2.17% | 1.50 × 10−8 |
| rs41566683 | 1 | 37299219 | EPHA3 | within (intron) | 0.113850 | 2.06% | 1.15 × 10−7 |
| rs109267271 | 7 | 51754749 | CD14 | −8146 | 0.409976 | 1.17% | 1.22 × 10−7 |
| rs134115197 | 1 | 110980155 | TIPARP | +24,264 | 0.433099 | 1.25% | 1.40 × 10−7 |
| rs109756462 | 6 | 101037292 | MAPK10 | within (intron) | 0.397887 | 1.26% | 4.33 × 10−7 |
| Pathway | Description | Gene Name | p-Value |
|---|---|---|---|
| bta04512 | ECM–receptor interaction | SPP1, IBSP, CD44 | 0.0003 |
| bta04620 | Toll-like receptor signaling pathway | SPP1, CD14, MAPK10 | 0.0016 |
| bta04510 | Focal adhesion | SPP1, IBSP, MAPK10 | 0.0028 |
| bta00970 | Aminoacyl-tRNA biosynthesis | HARS1, HARS2 | 0.0050 |
| bta05169 | Epstein–Barr virus infection | CD44, MAPK10 | 0.0178 |
| bta05417 | Lipids and atherosclerosis | CD14, MAPK10 | 0.0387 |
| bta04215 | Multiple species of apoptosis | MAPK10 | 0.0408 |
| bta04010 | MAPK signaling pathway | CD14, MAPK10 | 0.0475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Jiang, H.; Arbab, A.A.I.; Wang, B.; Liu, D.; Abdalla, I.M.; Xu, T.; Sun, Y.; Liu, Z.; Yang, Z. Investigating Genetic Characteristics of Chinese Holstein Cow’s Milk Somatic Cell Score by Genetic Parameter Estimation and Genome-Wide Association. Agriculture 2023, 13, 267. https://doi.org/10.3390/agriculture13020267
Lu X, Jiang H, Arbab AAI, Wang B, Liu D, Abdalla IM, Xu T, Sun Y, Liu Z, Yang Z. Investigating Genetic Characteristics of Chinese Holstein Cow’s Milk Somatic Cell Score by Genetic Parameter Estimation and Genome-Wide Association. Agriculture. 2023; 13(2):267. https://doi.org/10.3390/agriculture13020267
Chicago/Turabian StyleLu, Xubin, Hui Jiang, Abdelaziz Adam Idriss Arbab, Bo Wang, Dingding Liu, Ismail Mohamed Abdalla, Tianle Xu, Yujia Sun, Zongping Liu, and Zhangping Yang. 2023. "Investigating Genetic Characteristics of Chinese Holstein Cow’s Milk Somatic Cell Score by Genetic Parameter Estimation and Genome-Wide Association" Agriculture 13, no. 2: 267. https://doi.org/10.3390/agriculture13020267
APA StyleLu, X., Jiang, H., Arbab, A. A. I., Wang, B., Liu, D., Abdalla, I. M., Xu, T., Sun, Y., Liu, Z., & Yang, Z. (2023). Investigating Genetic Characteristics of Chinese Holstein Cow’s Milk Somatic Cell Score by Genetic Parameter Estimation and Genome-Wide Association. Agriculture, 13(2), 267. https://doi.org/10.3390/agriculture13020267






