Abstract
The application of pulp and paper mill sludge to agricultural soils is commonly considered as a strategy to improve soil properties, promote plant growth, and reduce the demand for costly chemical fertilization. The aim of this study was to evaluate if sodium lignosulfonate (sLS), one of the sludges of pulp production, may affect the biomass production, the respiration (R) and net CO2 assimilation rate (An) at the leaf level, and the content and accumulation of trace elements in the leaves of cucumbers grown under a sufficient nutrient supply or soil nutrient deficit. A pot culture experiment was conducted using sLS application rates of 0, 1.0, 2.5, 5.0, and 10 vol% to sandy loam soil. The decline in nutrient availability caused an increase in the R/An ratio and dramatically depressed biomass accumulation. The leaf Fe, Ni, Cr, Co, Al, and Pb contents were lower under low nutrient availability than under sufficient nutrient supply. Although sLS was not very effective in lessening the negative effect of nutrient deficiency on biomass accumulation, it reduced respiratory carbon losses and cell membrane permeability in the leaves of cucumbers grown under nutrient deficit. The reduction in the toxic level of leaf Mn in seedlings grown under sufficient nutrient availability and the toxic level of leaf Fe under a nutrient deficit might also be considered as a positive effect of the sLS application to sandy soil.
1. Introduction
Currently, research in plant breeding is focused on increasing efficiency and reducing the cost of agricultural production [1,2,3,4,5]. As soil improvers, a wide range of substances has been studied, including lignosulfonates (LSs) [6,7,8], which are byproducts of lignin processing using the sulfite method of pulp production. LSs contain sulfonyl, carboxyl, phenolic hydroxyl, and alcoholic hydroxy groups, which are responsible for their chelating, buffering, and cation exchange properties [9]. These properties allow us to consider LSs as conditioners of soil’s physical properties [8] and chelate fertilizer [10] with their positive effect on the physical, chemical, and biological properties of soil [11]. This is especially important for soils with low natural fertility, such as sandy loam soil. This type of soil is widely used for agricultural purposes but is typically nutrient-deficient soil with a low ability of macro- and micronutrients for plants [12]. It can be hypothesized that the application of LSs to nutrient poor soils can improve availability of nutrients for plants, which, in turn, can positively affect the main physiological traits, including growth, photosynthetic CO2 assimilation (A), and respiration (R), as well as the coupling between these processes. It is well known that plant productivity and crop yield are strongly controlled by the balance between respiration and photosynthesis [13]. The R/A ratio is considered an important indicator of plant carbon balance and is widely used in studies of the ability of plants to acclimate to stress factors [14,15], including soil nutrient deficiency [16]. Several lines of evidence suggest that the R/A ratio tends to increase under stress [17,18]. This increase can be caused by accelerated demand for respiratory energy, an increase in dissipative processes, and/or a reduction of the photosynthetic rate [19]. An increase in the R/A ratio indicates the loss of carbon by the plant and, as a result, a decrease in the growth rate and accumulation of plant biomass. The studies of LSs application on plant growth have shown that plant response depends on multiple factors, such as LS cations and types, application dose, plant species, and plant development stages [20,21,22]. The positive impact of LS application on plant biomass accumulation has been found for some cultivated plants [9,21,23], but not for others [24,25]. Experimental evidence shows that LSs can promote root activity and elongation [26] and increase the content of proteins and sugar [9,23]. Kok et al. [23] showed that sodium LS [sLS] application caused an increase in rubisco activity, total chlorophyll, and photosynthesis-related protein content, resulting in enhanced photosynthesis activities and plant biomass accumulation for the indica rice cultivar. Along with the sLS-mediated increase in the photosynthetic rate, the reduction of reactive oxygen species can improve plant growth as was found by Kok et al. [23]. Much less information is available, however, about the impact of LSs on plant respiratory metabolism, which is an important physiological process controlling plant growth and productivity, especially under stress conditions, including soil nutrient deficiency. In addition, less is known about the LSs-mediated modification of the coupling between photosynthesis and respiration and its effect on plant biomass accumulation. It is well known that nutrient-containing fertilizers increase plant growth rate and yield, as well as affect plant nutrient uptake and plant element concentrations [27]. As well as macronutrients, micronutrients, including iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn), are essential for multiple biological processes that affect plant agricultural productivity and crop yield [27]. LSs include polysaccharides, monosaccharides, minerals, organic acids, and macro- and microelements [28,29,30], which makes it possible to use them as a fertilizer to increase the nutrient element contents of soils [31,32]. However, not only the concentration of soil nutrients but also the availability of elements to plants affects nutrient uptake and element accumulation in plant tissue. Chelating agents can be used to control the availability of soil micronutrients by complexing them with soil metal cations. The ability of LSs to chelate micronutrient ions, such as ions of Fe, Mn, and Zn, and form ion-LS complexes has been widely studied in recent years [10,33,34]. These studies suggest that using of LSs as ion-LS complexes might be an eco-compatible and cost-friendly micronutrients source, as compared to synthetic chelates, although ion-LS complexes can show a lower efficiency than synthetic chelates and different use among plant species [31,32,34]. Since element uptake by plants strongly depends on nutrient content and availability in soils [27], it can be hypothesized that the application of LS can affect microelement content and accumulation in plant organs. A recent study [25] showed that low doses of sodium LS caused an increase in the content of several macronutrients in cucumber plants grown under conditions of sufficient nutrient availability. However, this was not found for plants grown under soil nutrient deficiency. Much less information is available, however, about the effects of LSs application on trace element content and their distribution and accumulation in plants. Moreover, it is uncertain whether the effect of LSs application on the uptake of microelements by plants depends on the soil nutrient supply.
Our study aimed to investigate sLS application’s impact on growth parameters, as well as on the accumulation of biomass and trace elements in Cucumus sativus L. leaves. Since plant growth is closely related to photosynthesis and respiration, these physiological processes were also studied. Cucumbers are one of the most widely cultivated vegetable crops in the world. Since cucumber plants are sensitive to the impact of stress factors, including soil nutrient deficiency, they are often the object of many scientific studies aimed at increasing cucumber crop yield and stress resistance [35,36]. We studied not only micronutrients, such as Fe, Mn, Cu, and Zn, which are needed for the successful growth of plants, but also nickel (Ni), chromium (Cr), and cobalt (Co), which are essential microelements only at extremely low concentrations. Also, we studied leaf aluminum (Al) and lead (Pb) to understand whether the sLS application affects the content of these non-essential elements. All these studied elements are toxic to crop plants at high tissue concentrations. In this study, cucumber seedlings were grown under contrasting conditions of nutrient availability—adequate and low soil nutrient supplies. We assessed whether the sLS application would affect the main physiological processes, as well as microelement content, and if this effect is more pronounced under soil nutrient deficiency.
2. Materials and Methods
2.1. Soil Substrate Preparation
For the experimental study, sandy loam soil has been collected at the Korza field station of the Karelian Research Center, located in Karelia, the Northwest of Russia. Under natural forest vegetation, this soil is acidic (pH values between 3.5 and 6.0) and has low nutrient content, including nitrogen (total N concentration between 0.01 and 0.03%) [12]. The collected soil was air-dried and sieved with a 2 mm sieve. We divided the soil sample into five parts and mixed each of the parts with sodium lignosulfonate (sLS) (Kondopoga Pulp and Paper Mill, Karelia, Russia) to achieve its content equal 0, 1.0, 2.5, 5.0, and 10 vol% (0sLS, 1sLS, 2.5sLS, 5sLS, and 10sLS treatments, respectively). Sodium lignosulfonate collected from Kondopoga Pulp and Paper Mill had the following total chemical composition: O—48%, C—42%, S—7%, and H—4% with ash percentage of 17% [29]. Moreover, 1.6 g kg−1 of N, 0.1 g kg−1 of both P and K, 0.8 g kg−1 of Ca and Mg, and 2.2 g kg−1 of Na were recently found in this type of LS [25]. All soil samples containing sLS were incubated in large pots in a temperature-controlled room under 21−23 °C for 90 days. During incubation, once a week the pots were weighted, and the substrates were watered up to 70−80% of the maximum soil water holding capacity to prevent soil water deficit [37]. Following the incubation period, we parked the substrates into plastic pots (volume equal to 0.80 L) with an approximate soil bulk density of 1.4 g cm−3 for subsequent planting of germinated seeds. Each treatment (0sLS, 1sLS, 2.5sLS, 5sLS, and 10sLS) included eight pots.
2.2. Plant Material and Growth Conditions
Prior to sowing, we imbibed uniform seeds of cucumber (C. sativus L., var. Kurag) in distilled water for 24 h; afterwards, we sowed four seeds in a pot. We put the pots into two climate chambers and maintained temperature at 25/22 °C day/night, 16-h photoperiod, 300 μmol m−2 s−1 of photosynthetic photon flux density (PPFD), and 60−70% relative air humidity. The pots of each sLS treatment were divided into two blocs. For the first bloc, the nutrient solution composed of 1 g L−1 Ca(NO3)2, 0.25 g L−1 KH2PO4, 0.25 g L−1 MgSO4 7H2O, 0.25 g L−1 KNO3, a trace amount of FeSO4 with pH 6.2−6.4 and EC 2.0 mS cm−1 was supplied every two days. For watering of second part seedlings, we used distilled water every two days. Consequently, we designated the first bloc for growing seedlings under sufficient nutrient availability (SNA) and the second bloc for growing seedlings under low soil nutrient availability (LNA). One week after the beginning of the experiment, we thinned the seedlings one seedling per pot. To avoid plant location effects, we moved the pots daily and randomly within each chamber and every week between the chambers.
2.3. Chlorophyll Fluorescence and Chlorophyll Content Parameters
Chlorophyll fluorescence parameters) were measured with MINI-PAM (Walz, Effeltrich, Germany). These parameters include Fv/Fm—maximum photochemical quantum efficiency of PSII—and φPSII—actual photochemical quantum efficiency. Before measuring minimum and maximum fluorescence (Fo and Fm, respectively) of leaves, they were adapted to dark for 30 min using leaf clips. The Fo and Fm were taken by illumination with saturated flash light and used to calculate maximal photochemical efficiency of PSII (Fv/Fm= (Fm− Fo)/Fm). After the measurement of dark-adapted parameters, the cucumber seedlings were exposed to growth irradiance for light adaptation for 30 min. The minimum and maximum fluorescence of light-adapted leaves (Ft and Fm′, respectively) were measured in the same way as for dark-adapted leaves. The φPSII values were calculated as (Fm′ − Ft)/Fm′ according to Genty et al. [38]. Leaf chlorophyll content index was measured on the same leaves as the chlorophyll fluorescence parameters using a portable SPAD (Soil Plant Analysis Development)-502® chlorophyll meter (Minolta Camera Co. Ltd., Tokyo, Japan). At least three measurements were made along a blade of one single leaf. The chlorophyll fluorescence parameters and chlorophyll content index were measured for all cucumber seedlings among all treatments on days 17, 21, 25, and 28 after sowing.
2.4. CO2 Gas Exchange
Measurements of leaf gas exchange commenced after at least two hours of photosynthesis under the light [39]. Light response curves were measured on the youngest fully expanded leaves using a portable photosynthesis system (HCM-1000, Walz, Effeltrich, Germany) equipped with a 2.5 cm2 leaf chamber with a red–blue light source. The measurements were started at 27 days after germination and performed at the leaf temperature of 25 °C, relative air humidity of 60–70%, 400 ± 20 ppm of CO2, and the flow rate through the leaf chamber of 600 μmol s−1. The leaves were first exposed to growth irradiance of 300 PPFD, and thereafter, we measured the irradiance response of net CO2 at 1200 μmol PPFD m−2 s−1. Then, we determined the same parameter at 1000, 800, 300, 60, 40, 20, and zero PPFD. Readings were taken after steady state rates of CO2 exchange were reached. The rates of leaf respiration (R) were taken after 30 min of zero irradiance. The apparent quantum yield of photosynthesis (α) was calculated as the slope of the net CO2 assimilation rate An versus irradiance of 20, 40, and 60 μmol m−2 s−1. The ratios of R to An at leaf level were calculated for the An rates at 1200 (light saturation level) and 300 (growth irradiance) PPFD and designated as R/An1200 and R/An300, respectively.
2.5. Electrolyte Leakage and Leaf Relative Water Content
Electrolyte leakage (EC) was measured using an electrical conductivity meter by estimating the ion leaching from the leaves into water according to [40]. About 0.5 g fresh weight of washed leaves was cut into small tubes with 10 mL distilled water and incubated at 23 °C for 4 h. The suspension medium was measured for the initial electrical conductivity (EC1). The samples were then boiled at 100 °C for 20 min to release all the electrolytes, cooled and the final electrical conductivity (EC2) was measured. The percent leakage of electrolytes was calculated as (EC1/EC2) × 100%.
For the relative water content (RWC) measurements, leaf discs were collected from randomly selected leaves of each treatment. Fresh weights of the leaf discs (FW) were determined, and the discs were placed in Petri dishes containing distilled water to rehydrate. After 24 h under 4 °C leaf turgid weight (TW) was determined. The leaf discs’ leaves were oven-dried at 70 °C for dry weight (DW) determination. Leaf RWC was calculated as (FW−DW)/(TW−DW) × 100% according to [41].
2.6. Plant Growth Parameters
After the completion of CO2 exchange measurements, we harvested the 30-day-old cucumber seedlings and determined the dry weight of plants separated into leaves, stems, and roots at 70 °C to weight constancy. The following growth parameters were calculated: total dry biomass as the sum of roots, stems, and leaves’ dry weight; leaf weight ratio (LWR) as the ratio between leaf weight and total biomass; root weight ratio (RWR) as the ratio between root weight and total biomass; and root shoot ratio (RSR) as the ratio between root weight and shoot (leaves plus stems) biomass.
2.7. Chemical Analyses
After the measurement of growth parameters, we analyzed the chemical composition of dry leaves. Four seedlings of each treatment grown under condition of SNA were used for determining the content of microelements in leaves. For the seedlings grown under LNA, we combined all leaves of four seedlings of each of the treatments in bulk sample. The leaf subsamples of 0.2–0.3 g were homogenized and then digested with HNO3 and HCl. Samples were mineralized using the «Speedwave four» microwave sample preparation system (Berghof Products + Instruments GmbH, Eningen unter Achalm, Germany) at 135 °C for 30 min. Leaf concentrations of Fe, Mn, Cu, and Zn were determined by spectrophotometric atomic absorption (Shimadzu AA-7000, Kioto, Japan) and the content of Cr, Co, Ni, Al, and Pb was determined by absorption method, according to [42], with electrothermal atomization on an AA-6800 spectrometer (Shimadzu, Japan) in the Core Facility “Analytical laboratory” of the Forest Institute of KarRC of RAS. Certified multi-element solutions (Inorganic Ventures) are used as calibration standards. The element accumulation in leaves was determined by multiplying the dry leaf biomass per plant by the leaf element content, while the content of the element was expressed in milligrams per kilogram (mg kg−1) and element accumulation was expressed in milligrams per plant (mg plant−1).
2.8. Statistical Analyses
All data were shown as mean ± standard error. The effects of soil sLS concentration, nutrient availability for plants, and their interaction were analyzed using a two-way ANOVA. To assess the significant difference between the treatments, the least significant difference (LSD) of ANOVA was used for plant growth and chemical parameters. We reported differences at the p < 0.05 level as significant. A correlation matrix value was made to evaluate the strength and direction of the relation between leaf element content and plant growth parameters. All statistical tests were carried out with Statistica software (v. 8.0.550.0, StatSoft, Inc.).
3. Results
3.1. Effect of Nutrient Availability on Plant Biomass Parameters
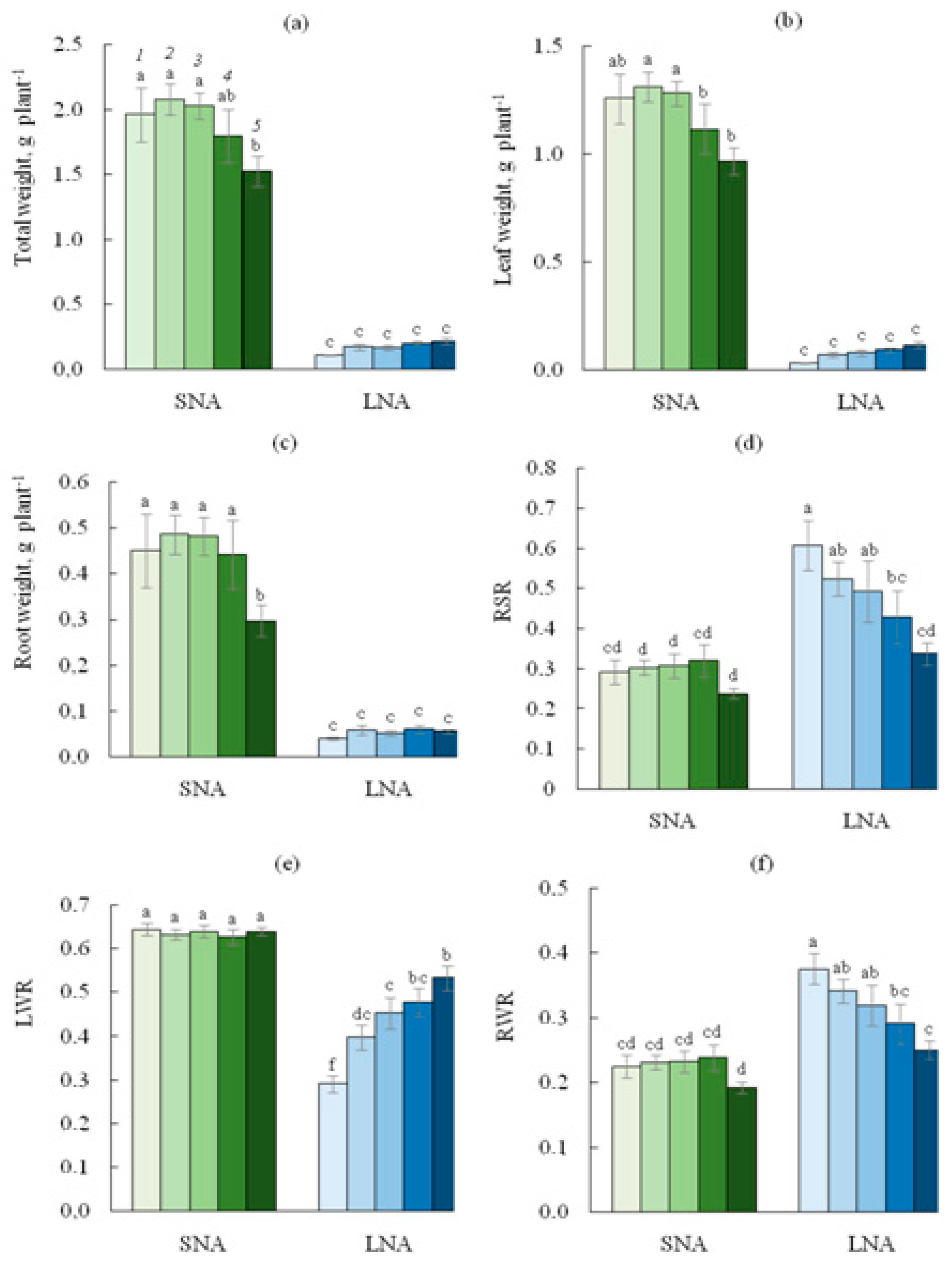
As shown in Table 1, nutrition availability for cucumber seedlings significantly affected all growth parameters observed in this study. As expected, for the 0sLS seedlings, the reduced nutrient availability dramatically depressed seedling growth, with decreases in total, leaf, and root biomass by almost 18, 40, and 11 times, respectively (Figure 1). For the 0sLS leaves, the LWR value was lower, and RWR was higher under LNA than under the SNA condition.

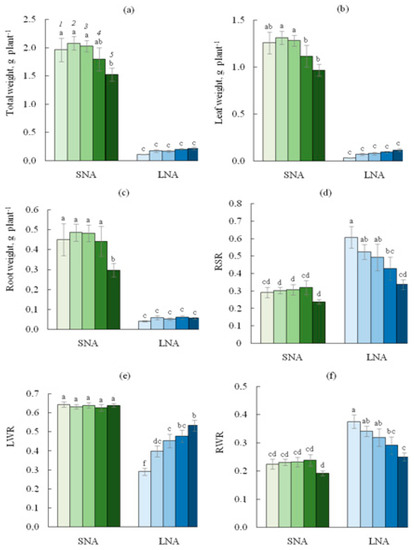
Figure 1.
Total (a), leaf (b), root (c) weight, root shoot ratio (RSR, (d)), leaf weight ratio (LWR, (e)), root weight ratio (RWR, (f)), for cucumber seedlings grown on the sandy loam soil with a sodium lignosulfonate concentration of 0 (1), 1 (2), 2.5 (3), 5 (4), and 10 (5) vol % under sufficient nutrient availability (SNA) or low nutrient availability (LNA). Significant differences at p < 0.05 indicated with different letters.
3.2. Effect of sLS on Plant Biomass Parameters
The two-way ANOVA treatment results show that the effect of sLS application was significant for all biomass parameters (Table 1). While soil sLS contents of 1.0, 2.5, and 5.0% did not affect the total and organ biomass of seedlings grown under SNA, the sLS content of 10% decreased biomass values except for the LWR value (Figure 1). In accordance with the increase in soil sLS content under LNA conditions, total, leaf, and root biomass tended to increase, but this increase was too small to restore these parameters to the level of SNA-grown seedlings. Under LNA conditions, the LWR values increased, and RWR decreased with the increase in the soil sLS content.
3.3. Effect of Nutrient Availability and sLS Application on Photosynthetic Parameters, EC and RWC
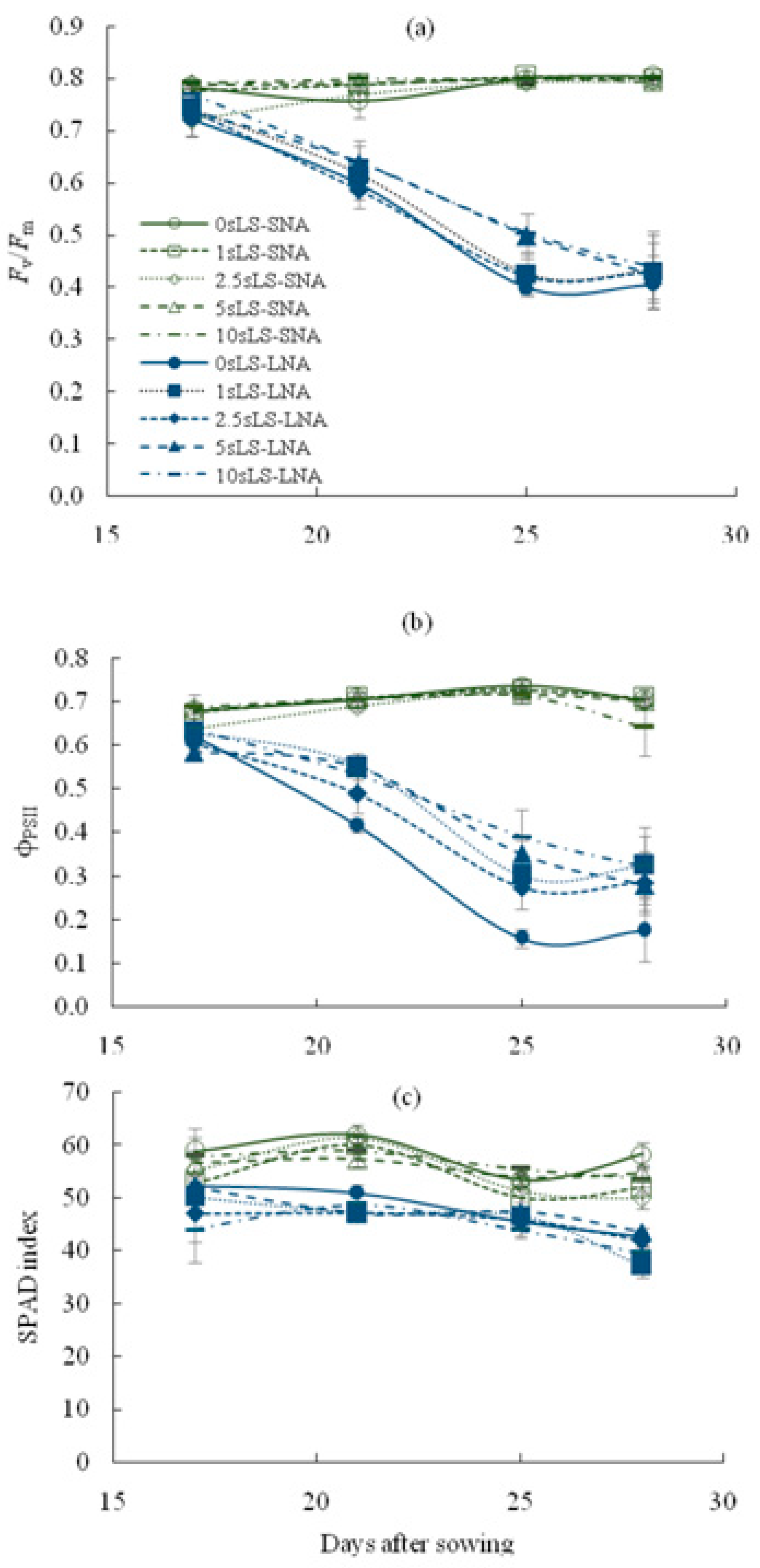
As shown in Table 1, the nutrient availability for plants significantly affected the parameters of chlorophyll fluorescence (Fv/Fm and φPSII) and chlorophyll content, as well as the α value, and rates of An and R. The decline in nutrient availability decreased the values of these parameters, and sLS application did not lead to an increase in Fv/Fm and SPAD index regardless of the soil sLS content (Figure 2a,c). A statistically significant sLS-mediated increase was found for the φPSII values (Figure 2b), but the increase was not sufficient to restore these values to the level of SNA-grown seedlings.
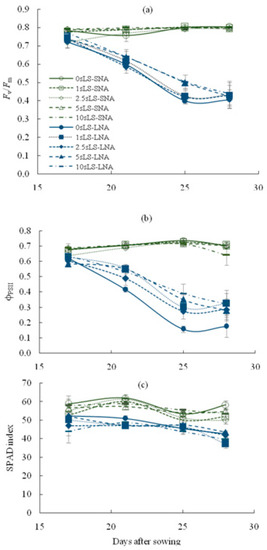
Figure 2.
Temporal dynamics of the maximal quantum yield of PSII photochemistry ((a), Fv/Fm), actual quantum yield of PSII photochemistry ((b), ϕPSII) and SPAD index (c) for cucumber seedlings grown on the sandy loam soil with a sodium lignosulfonate concentration of 0 (0sLS), 1 (1sLS), 2.5 (2.5sLS), 5 (5sLS), and 10 (10sLS) vol % under sufficient nutrient availability (SNA) or low nutrient availability (LNA).
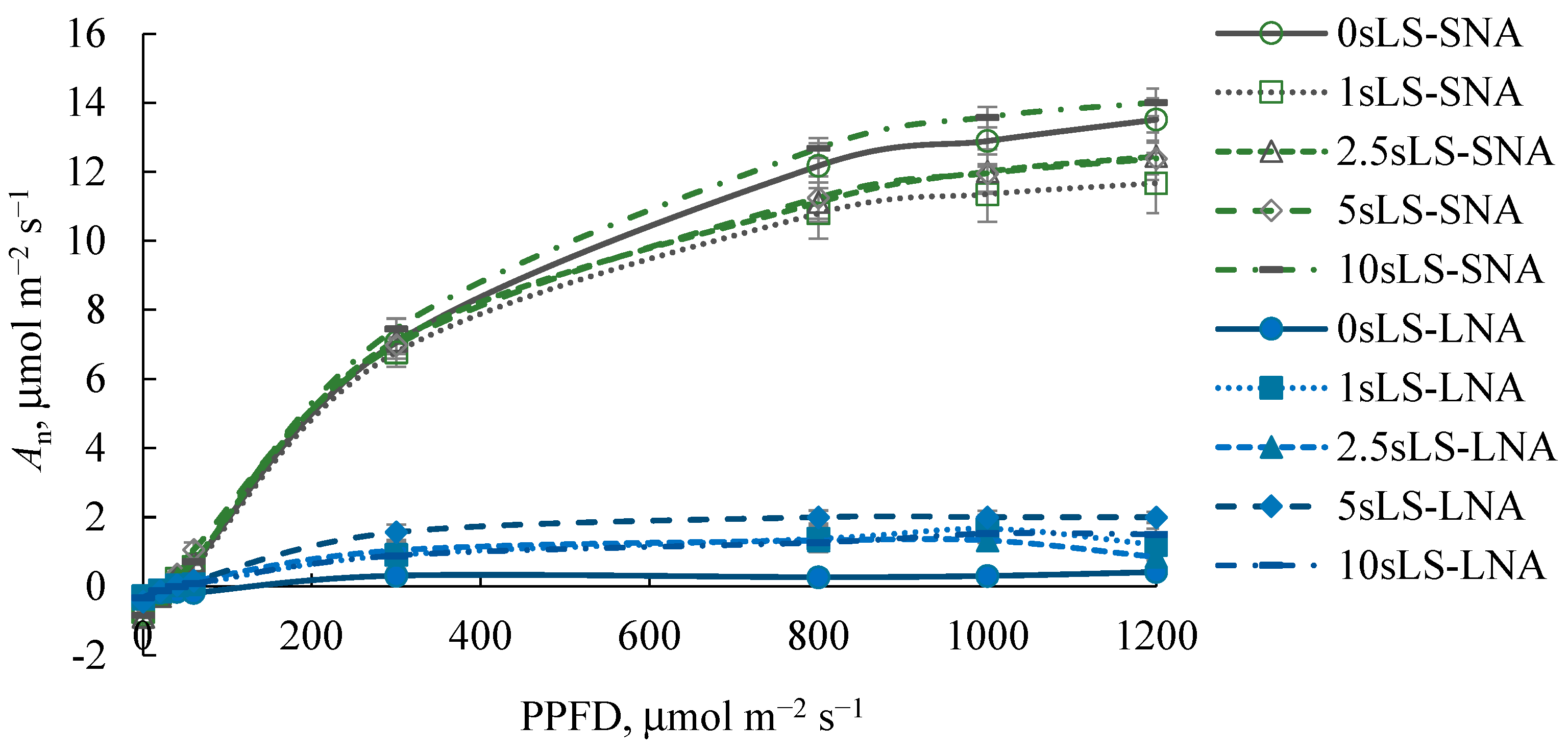
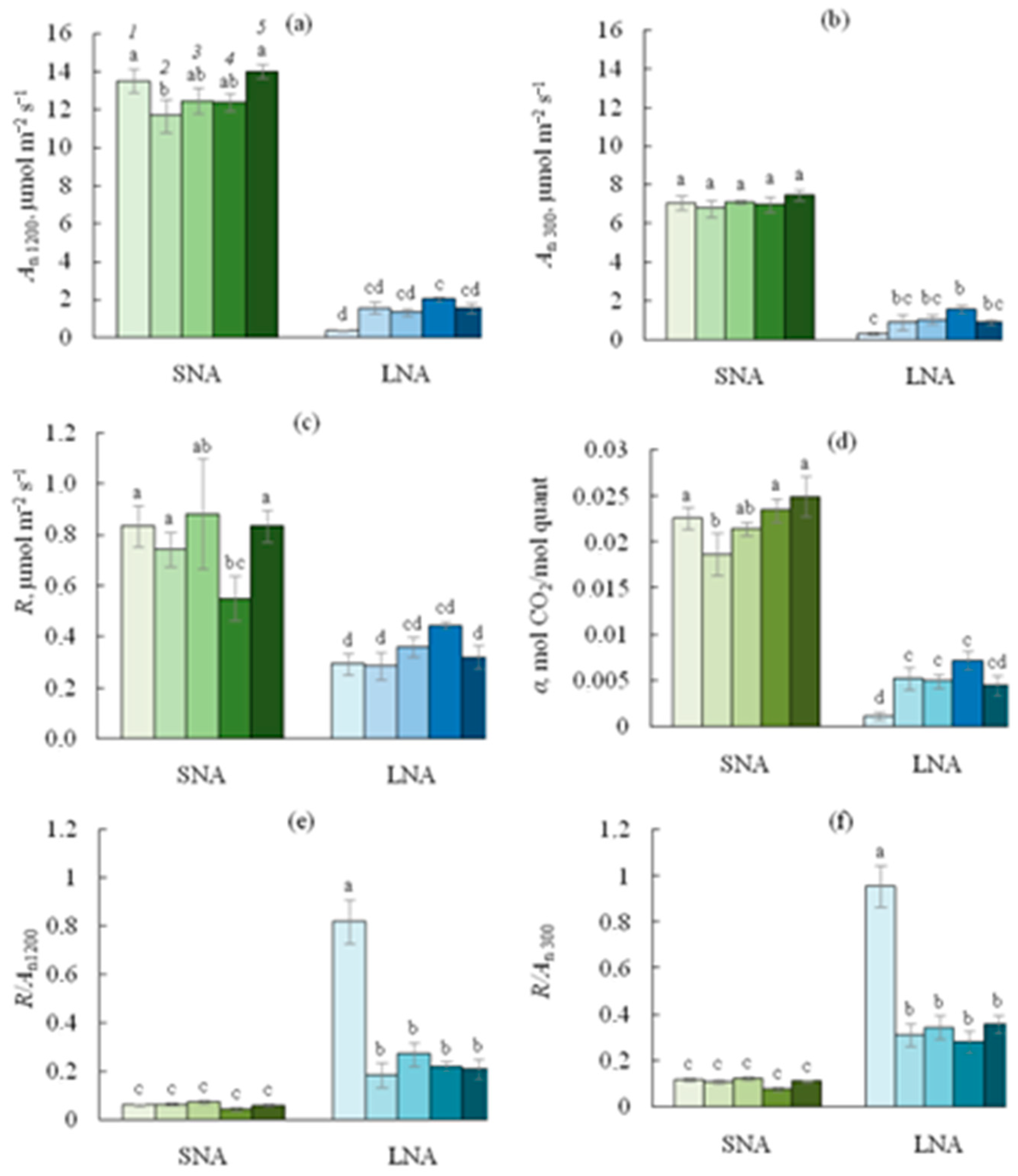
As expected, the An rates were higher in SNA-grown seedlings than in their LNA-grown counterparts, regardless of the PPFD level at which An was measured (Figure 3). For the SNA-seedlings, sLS did not affect the An300 rates (Figure 4b), but the depression of An1200 was pronounced when 1% of sLS was added into the soil (Figure 4a). For the LNA-grown seedlings, the sLS application increased both An1200 and An300 rates, regardless of the sLS content, but this increase was not significant enough. The effect of sLS on the α value of the both SNA- and LNA-grown seedlings repeated its impact on the An1200 values (Figure 4a,d).
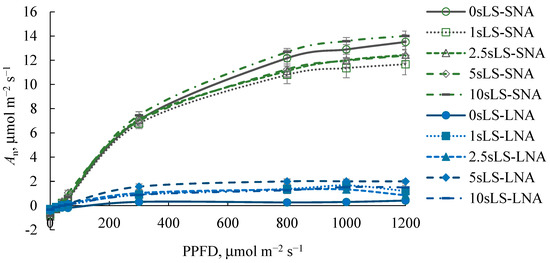
Figure 3.
Light response curves of leaf CO2 gas exchange of cucumber seedlings grown on the sandy loam soil with a sodium lignosulfonate concentration of 0 (0sLS), 1 (1sLS), 2.5 (2.5sLS), 5 (5sLS), and 10 (10sLS) vol % under sufficient nutrient availability (SNA) or low nutrient availability (LNA).
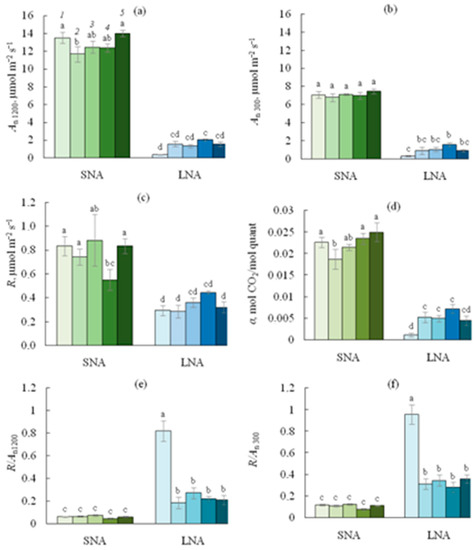
Figure 4.
Net CO2 assimilation rate at 1200 ((a), An1200) or 300 ((b), An300) PPFD, leaf respiration ((c), R), apparent quantum yield of photosynthesis ((d), α), R/An ratio at 1200 ((e), R/An1200) or 300 ((f), R/An300) PPFD for cucumber seedlings grown on the sandy loam soil with a sodium lignosulfonate concentration of 0 (1), 1 (2), 2.5 (3), 5 (4), and 10 (5) vol % under sufficient nutrient availability (SNA) or low nutrient availability (LNA). Significant differences at p < 0.05 indicated with different letters.
The R rates were higher in SNA than in LNA leaves (Figure 4c). While the sLS application did not affect leaf R in the LNA-grown seedlings, for their SNA-grown counterparts, the 5sLS leaves had the lowest R rates. The ratios R/An at the leaf level were lower in SNA than in the LNA seedlings, regardless of whether An1200 or An300 was used in the ratio (Figure 4e,f). The nutrient deficiency dramatically increased both the R/An1200 and R/An300 ratios, but sLS application significantly decreased these values among all treatments.
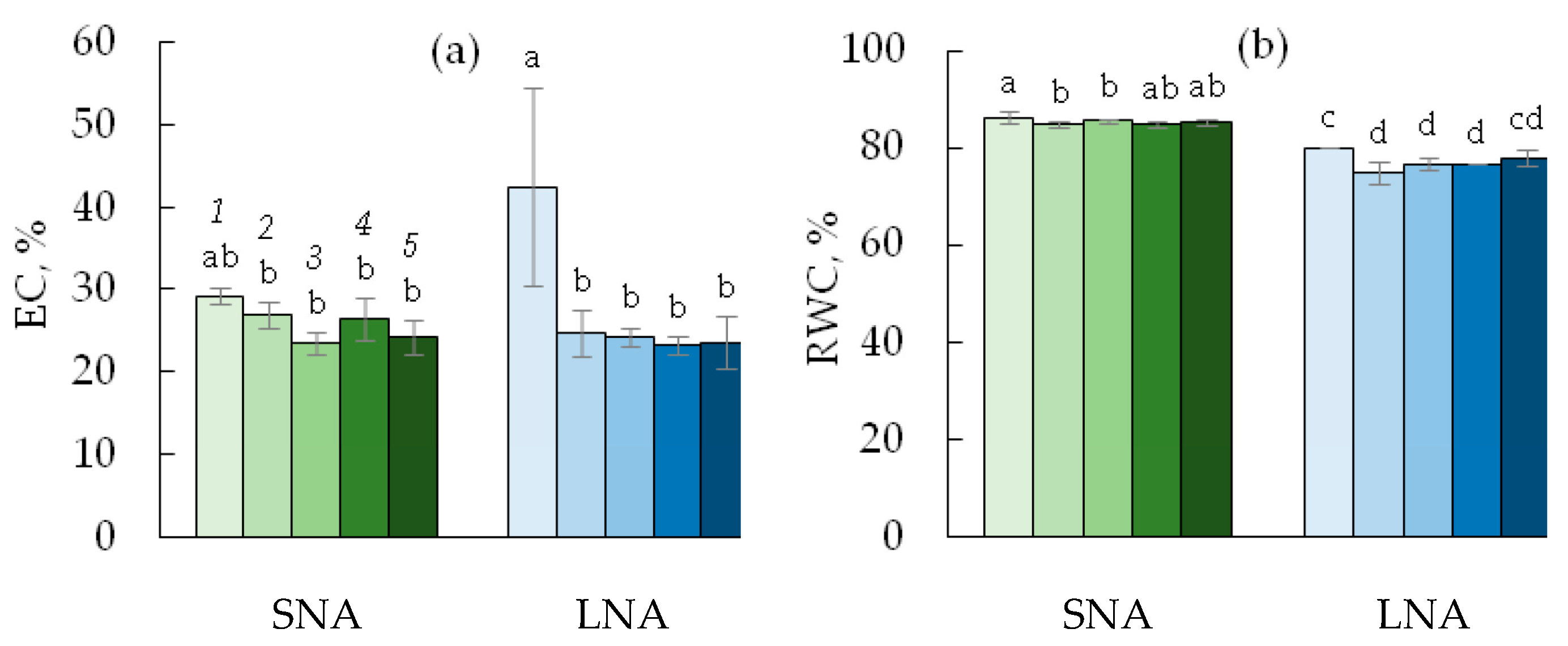
The two-way ANOVA analysis revealed the significant effect of nutrient availability for plants on the electrolyte leakage (EC), but not on the relative water content (RWC) in cucumber leaves (Table 1). For the 0sLS seedlings, the decline in nutrient availability caused an increase in EC (Figure 5a) and a decrease in RWC (Figure 5b). Under SNA conditions, the EC values tended to be higher in leaves grown on the soil without sLS than on the soils containing it, but these differences were not supported by statistical tests (p > 0.05). The sLS application significantly decreased EC in LNA-grown leaves with the reduction rate independent of the sLS content in the soil. The RWC values were lower in leaves grown on the soil without sLS, regardless of its content in the soil and the condition of nutrient availability for plants.
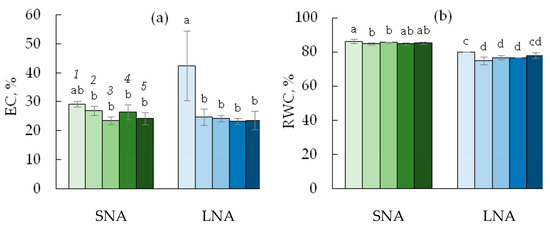
Figure 5.
Electrolyte leakage (EC, (a)) and relative water content (RWC, (b)) in leaves of cucumber seedlings grown on the sandy loam soil with a sodium lignosulfonate concentration of 0 (1), 1 (2), 2.5 (3), 5 (4), and 10 (5) vol % under sufficient nutrient availability (SNA) or low nutrient availability (LNA). Significant differences at p < 0.05 indicated with different letters.
3.4. Leaf Microelements Content
According to the two-way ANOVA, the effect of nutrient availability for plants was significant for leaf Fe, Zn, Ni, Cr, Co, Al, and Pb but insignificant for Mn and Cu content (Table 1). The decline in nutrient availability caused an increase in Fe, Ni, Cr, Al, Co, and Pb concentration in 0sLS leaves by 21, 4.4, 8.0, 5.8, 6.1, and 4.9 times, respectively, and a decrease in Zn content by 9% (Table 2). As shown in Table 1, sLS application significantly affected the Fe, Mn, Ni, Al, Cr, and Co content in the leaves of cucumber seedlings. The effect of sLS on the concentrations of some microelements in the leaves depended on nutrient availability for seedlings. While the increase in the soil sLS concentration decreased leaf Fe, Cr, and Pb content under LNA, this tendency was not recorded for seedlings grown under SNA conditions. Lignosulfonate application decreased the leaf Al concentration under SNA but tended to increase it in the leaves of LNA-grown seedlings. In accordance with the increase in soil sLS content, leaf Mn, Zn, Ni, and Co concentrations decreased regardless of the conditions of nutrient availability for plants.

Table 2.
Concentration and accumulation of microelements in leaves of cucumber seedlings grown on sandy loam soil with lignosulfonate concentrations of 0 (0sLS), 1 (1sLS), 2.5 (2.5sLS), 5 (5sLS), and 10 (10sLS) % under sufficient (SNA) or low (LNA) nutrient availability.
3.5. Correlation between Leaf Microelement Content and Plant Growth Parameters
For most of the studied trace elements (Fe, Ni, Cr, Co, Al, and Pb), a strong negative correlation between most growth parameters and leaf element concentration was found for the united SNA and LNA conditions (Table 3). The RWR values were positively correlated with the concentration of these elements in the leaves. A statistically significant relation was determined between the biomass values and leaf content of Cu and Zn, but no relation between Mn concentration and growth parameters was found.

Table 3.
Correlation coefficients between plant growth parameters and microelements content in leaves of cucumber seedlings.
3.6. Leaf Trace Element Accumulation
For all the studied trace elements, their accumulation was lower in the leaves of cucumber seedlings grown under LNA than under SNA conditions (Table 2). The effect of sLS application on the accumulation of some elements depended on the nutrient availability for plants. While the increase in the soil sLS concentration decreased leaf Mn, Ni, and Co accumulation under SNA, this tendency was not recorded for LNA-grown seedlings. In accordance with the increase in soil sLS content, the Al accumulation decreased in SNA leaves and increased in LNA ones except for 10sLS leaves. While sLS application did not affect Cu, Zn, and Cr accumulation in LNA leaves, for SNA-grown seedlings, 1sLS leaves had the highest accumulation of these elements. While leaf accumulation of Fe tended to decrease following the increase in soil sLS content under LNA conditions, this tendency was not so clear for seedlings grown under SNA. The Pb accumulation in cucumber leaves was not affected by sLS application, regardless of the conditions of nutrient availability.
4. Discussion
Since lignosulfonates contain chemical groups that form chelate complexes with metal cations, many of which are essential elements, it was expected that LSs can be useful to improve the fertility of agricultural soil [6,31,43] and enhance plant growth and productivity [1,20,26]. In our study, we sought to recognize how the addition of sLS in sandy loam soil affects biomass and leaf-essential and non-essential element accumulation in cucumber seedlings. Therefore, we quantified the impact of nutrient availability for plants on leaf microelements composition to understand whether the response of plant growth and leaf element content to sLS application depends on this factor. The results of our study showed that the effect of sLS on the growth parameters and leaf microelements content strongly depends on soil nutrient availability. As we expected, the low level of nutrient availability for the cucumber seedlings resulted in a dramatic decline in plant growth and biomass accumulation (Figure 1), connected with the low rates of An and R (Figure 3 and Figure 4). These results are in agreement with the findings that photosynthesis is the main driver for plant biomass production, which is negatively affected by soil nutrient deficiency [44,45,46].
Early studies showed that the beneficial effects of LSs on plant growth vary depending on the cation contained in LSs, soil lignosulfonate concentrations, or plant species [9,20]. In our study, the effect of sLS application on growth parameters was not as strong as we expected, regardless of soil sLS content and the condition of soil nutrient availability (Figure 1). Under the sufficient nutrient condition, the seedlings grown on soil with low content of sLS did not demonstrate any different response of biomass accumulation compared with the soil without sLS. Our finding that high levels of sLS in soil cause a decrease in total, leaf, and root dry biomass in SNA seedlings is consistent with a study demonstrating a negative effect of high levels of LS on plant traits [24]. Presumably, this negative effect is largely associated with lignosulfonate-mediated sodium salinization of the soil under this study. As mentioned in the Introduction section, we hypothesized that sLS might increase nutrient availability for cucumber seedlings, which, in turn, can have a positive effect on plant biomass accumulation. However, we found no evidence of a significant positive impact of sLS on growth parameters. Thus, sodium LS application was not successful in buffering the negative response of plants to nutrient deficiency in soil.
The plant biomass and carbon accumulation strongly relate to the plant carbon balance which is the difference between A and R [47]. Under optimal growth conditions, respiration loss of carbon assimilated during photosynthesis(R/A) tended to be constant, but stress is known to increase the R/A ratio [17], which was confirmed by our study (Figure 4e, f). Increased R/A can be caused by emerging respiratory energy demand or suppressed photosynthesis [17,48]. In this study, both R and An were depressed by soil nutrient deficiency, with An being depressed to a higher extent than R (Figure 4a–c). The shift in the carbon balance toward carbon losses seems to be one of the main reasons for the inhibited growth of cucumber seedlings grown under soil nutrient limitation in this study. The nutrient deficit-mediated decrease in An was connected to decreased values of chlorophyll fluorescence parameters (Fv/Fm and φPSII), which may be used as a direct indicator of photosynthetic activity and plant resistance to stress [39]. Our finding that the decline in nutrient availability strongly influences the photosynthetic apparatus, including PSII photochemistry and leaf chlorophyll content, is according to the studies that have found functional changes in the photosynthetic machinery under deficiency in soil nutrients [49,50]. Electrolyte leakage is a key index of plant cell membrane permeability, which increases under stress [51], including nutrient deficiency, as was found in this study (Figure 5a). The sLS application decreased the EC values, and this decrease was more pronounced for the leaves of cucumbers grown under low nutrient availability. Van der Paal et al. [52] showed that cell membranes’ permeability was closely connected with lipid peroxidation in the cell membranes. It can be assumed that the beneficial effect of sLS application on the permeability of cell membranes is associated with a lignosulfonate-mediated decrease in lipid peroxidation in plant membranes.
Nutrient deficiency did not cause a decrease in leaf microelement content, but, on the contrary, increased the concentration of Fe, Ni, Cr, Co, Al, and Pb with a toxic level for Fe, and close to a toxic level for Ni, Al ᴎ Co. In this study, the toxic level of Fe could be one of the factors that reduced plant growth. Fe toxicity often occurs in plants grown on soils with low pH, leading to increases in ferrous ion concentration, disrupting cell homeostasis, and impairing plant growth and yield [53]. Since the sandy loam soil used in this study contains a high Fe content and high soil acidity associated with a high Fe availability [12], ion uptake by plant roots was not limited. The deficit of soil mineral nutrients causes a decrease in nutrient accumulation in plants, but the uptake of microelements by plants can be increased [54]. A deficiency of soil macronutrients can be considered the main cause of excess iron in cucumber leaves grown under LNA. Along with other reasons, the toxic level of leaf Fe could be due to a disturbance of membrane integrity or mechanisms of blocking of iron transport under LNA conditions [53]. Although the positive direct or indirect effects of Fe and low concentrations of Ni, Cr, and Co on plant growth processes are known [54], in this study, the correlation analysis revealed a negative relationship between biomass accumulation and the concentration of these elements in leaves (Table 3). A positive correlation between the leaf Zn or Cu content and plant growth parameters was found in this study, which confirms the physiological significance of these micronutrients in growth processes. However, for the LNA-grown seedling, this correlation was negative. Therefore, it can be assumed that the significance of some trace elements for plant biomass accumulation may be controlled by such environmental factors as soil nutrient availability.
The capability of LSs to complex different cations, including Fe, Mn, and Zn, is well documented [6,55,56]. These complexes can play important role in ion uptake and accumulation in plants, but our study did not reveal any significant sLS-mediated increase in the content of micronutrients in cucumber leaves, regardless of the level of nutrient availability for plants (Table 2). The low efficiency of sLS as a chelating agent may be due to its weak bonds and low complex stability [33]. However, under a low rate of sLS application (1.0%), the concentration of Zn and Cu tended to increase, and leaf Zn and Cu accumulation increased, which can be associated with the chelating capacity of lignosulfonate under the study. A recent study [57] has shown a decrease in soil acidity with sLS application. Decreased microelement mobility under the condition of increased soil pH can be one of the reasons for the decreased contents of Mn, Ni, Co, and Al in leaves under both SNA and LNA conditions and the decreased contents of Fe, Zn, and Cr in the leaves grown under LNA. This result is consistent with the well-documented negative correlation between soil pH and leaf microelements content [58]. The sLS-mediated decline in the content of important micronutrients, such as Mn, can be one of the reasons for the increased LWR and decreased RWR for LNA-grown seedlings (Figure 1). This biomass distribution may be one of the mechanisms for maintaining the main physiological processes of plants such as respiration, photosynthesis, and transpiration that ensure the survival of plants under stressful conditions of low nutrient availability. The reduction in the content of toxic levels of Mn in SNA-grown and Fe in LNG-grown leaves can be considered a positive effect of the sLS application. It should be noted that the negative effect of sLS application on leaf concentration and accumulation of toxic levels of Ni, Cr, Co, Al, and Pb was not established in our study.
5. Conclusions
In summary, for sandy loamy soil used in this study, the effect of nutrient availability on the accumulation of dry matter by cucumber seedlings, as well as on the content and accumulation of microelements was more significant than the effect of sodium lignosulfonate application. The decline in nutrient availability caused a significant decrease in the photosynthetic activity and accumulation of plant biomass and leaf microelements, but sLS was not successful in eliminating this negative effect regardless of the sLS application rate. The sLS application with the rate of 5–10% decreased the content of most microelements, including toxic levels of Mn under SNA and Fe under LNA conditions. The sLS-mediated decrease of leaf microelements content corresponded to the decrease in dry plant biomass under sufficient nutrient availability and redistribution of biomass between the root and leaves in the direction of increasing leaf mass under low nutrient availability conditions.
Author Contributions
Conceptualization, M.Y. and P.K.; methodology, N.K.; formal analysis, N.K.; investigation, E.I.; writing—original draft preparation, E.I.; writing—review and editing, N.K.; supervision, P.K.; project administration, P.K.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-16-00145. The study of light curves of CO2-gas exchange was supported by the Ministry of Science and Higher Education of the Russian Federation, grant number FMEN-2022-0004.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Experimental facilities for this study were offered by the Core Facility of the Karelian Research Centre of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lv, T.; Yang, H.; Zhang, R.; Fan, W.; Xu, Y.; Cao, H.; Ning, L.; Zhou, C.; Wang, L. Effects of lignin on root activity and soil nutrients of Malus hupehensis var. pingyiensis under the use of organic fertilizer. Agric. Sci. 2017, 8, 341–347. [Google Scholar]
- Zhao, H.-T.; Li, T.-P.; Zhang, Y.; Hu, J.; Bai, Y.-C.; Shan, Y.-H.; Ke, F. Effects of vermicompost amendment as a basal fertilizer on soil properties and cucumber yield and quality under continuous cropping conditions in a greenhouse. J. Soil Sediment 2017, 17, 2718–2730. [Google Scholar] [CrossRef]
- Tenic, E.; Ghogare, R.; Dhingra, A. Biochar—A Panacea for Agriculture or Just Carbon? Horticulturae 2020, 6, 37. [Google Scholar] [CrossRef]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus biochar application enhances Ca uptake of upland rice, soil available P, exchangeable K, yield, and N use efficiency of sugarcane in a crop rotation system. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Ikkonen, E.; Chazhengina, S.; Butilkina, M.; Sidorova, V. Physiological response of onion (Allium cepa L.) seedlings to shungite application under two soil water regimes. Acta Physiol. Plant. 2021, 43, 75–88. [Google Scholar] [CrossRef]
- Carrasco, J.; Kovács, K.; Czech, V.; Fodor, F.; Lucena, J.J.; Vértes, A.; Hernández-Apaolaza, L. Influence of pH, iron source, and Fe/ligand ratio on iron speciation in lignosulfonate complexes studied using Mössbauer spectroscopy. Implications on their fertilizer properties. J. Agric. Food Chem. 2012, 60, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Xu, Q.; Cao, S.; Peng, P.; She, D. Controlled-release fertilizer with lignin used to trap urea/hydroxymethylurea/urea-formaldehyde polymers. BioRes 2018, 13, 1711–1728. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, Y.; Tang, J.; Chen, D.; Li, X.; Lin, Q.; Yin, G.; Zhang, M.; Hu, H. Potassium lignosulfonate as a washing agent for remediating lead and copper co-contaminated soils. Sci. Total Environ. 2019, 658, 836–842. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef]
- Rodríguez-Lucena, P.; Tomasi, N.; Pinton, R.; Hernández-Apaolaza, L.; Lucena, J.J.; Cesco, S. Evaluation of 59Fe-lignosulfonate complexes as Fe sources for plants. Plant Soil 2009, 325, 53–63. [Google Scholar] [CrossRef]
- Ta’negonbadi, B.; Noorzad, R. Stabilization of clayey soil using lignosulfonate. Transp. Geotech. 2017, 12, 45–55. [Google Scholar] [CrossRef]
- Gael, A.G.; Smirnova, L.F. Sand and Sandy Soils; GEOS: Moscow, Russia, 1999. [Google Scholar]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high growth temperatures. N. Phytol. 2007, 174, 367–380. [Google Scholar] [PubMed]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Cold tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol. 2009, 50, 203–215. [Google Scholar] [CrossRef]
- Atkin, O.K.; Turnbull, M.H.; Zaragoza-Castells, J.; Fyllas, N.M.; Lloyd, J.; Meir, P.; Griffin, K.L. Light inhibition of leaf respiration as soil fertility declines along a post-glacial chronosequence in New Zealand: An analysis using the Kok method. Plant Soil 2013, 367, 163–182. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F.; Usmanov, I.Y. Morphological and physiological characteristics of wheat cultivars differing in resistance and productivity under normal and stress conditions. Russ. J. Plant Physiol. 2000, 47, 534–539. [Google Scholar]
- Ikkonen, E.N.; Shibaeva, T.K.; Titov, A.F. Influence of Daily Short-Term Temperature Drops on Respiration to Photosynthesis Ratio in Chilling-Sensitive Plants. Russ. J. Plant Physiol. 2018, 65, 94–99. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F. Physiological aspects of photosynthesis–respiration interrelation. Russ. J. Plant Physiol. 2019, 66, 365–374. [Google Scholar] [CrossRef]
- Docquier, S.; Kevers, C.; Lambe, P.; Gaspar, T.; Dommes, J. Beneficial use of lignosulfonates in in vitro plant cultures: Stimulation of growth, of multiplication and of rooting. Plant Cell Tiss. Organ Cult. 2007, 90, 285–291. [Google Scholar] [CrossRef]
- Low, L.-Y.; Abdullah, J.O.; Wee, C.-Y.; Sekeli, R.; Tan, C.-K.; Loh, J.-Y.; Lai, K.-S. Effects of lignosulfonates on callus proliferation and shoot induction of recalcitrant indica rice. Sains. Malays 2019, 48, 7–13. [Google Scholar] [CrossRef]
- Abdullah, W.M.A.N.; Low, L.-Y.L.; Mumaiyizah, S.B.; Chai, Q.-Y.; Loh, J.Y.; Abdullah, J.O.; Koksong, L. Effect of lignosulphonates on Vanilla planifolia shoot multiplication, regeneration and metabolism. Acta Physiol. Plant. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Kok, A.D.-X.; Abdullah, W.M.A.N.W.; Tang, C.-N.; Low, L.-Y.; Yuswan, M.H.; Ong-Abdullah, J.; Tan, N.-P.; Lai, K.-S. Sodium lignosulfonate improves shoot growth of Oryza sativa via enhancement of photosynthetic activity and reduced accumulation of reactive oxygen species. Sci. Rep. 2021, 11, 13226. [Google Scholar]
- Stapanian, M.A.; Shea, D.W. Lignosulfonates: Effects on plant growth and survival and migration through the soil profile. Int. J. Environ. Stud. 1986, 27, 45–56. [Google Scholar] [CrossRef]
- Ikkonen, E.; Chazhengina, S.; Jurkevich, M. Photosynthetic Nutrient and Water Use Efficiency of Cucumis sativus under Contrasting Soil Nutrient and Lignosulfonate Levels. Plants 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Savy, D.; Cozzolino, V.; Vinci, G.; Nebbioso, A.; Piccolo, A. Water-soluble lignins from different bioenergy crops stimulate the early development of maize (Zea mays, L.). Molecules 2015, 20, 19958–19970. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3th ed; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Maksimov, V.F.; Stadnitskij, G.V. Introduction to the Specialty: Textbook for Universities; Chemistry: Saint Petersburg, Russia, 1988. [Google Scholar]
- Neiberte, B.; Jablonsky, A.; Shulga, G.; Verovkins, A.; Vitolina, S.; Brovkina, J. Comparative study of industrial lignosulfonates and some their properties. In Proceedings of the 12th International Scientific and Practical Conference, Rezekne, Latvia, June 20–22, 2019; Rezekne Academy of Technologies: Rezekne, Latvia, 2019; pp. 189–192. [Google Scholar]
- Ijaz, N.; Dai, F.; Rehman, Z. Paper and wood industry waste as a sustainable solution for environmental vulnerabilities of expansive soil: A novel approach. J. Environ. Manag. 2020, 262, 110285. [Google Scholar]
- Benedicto, A.; Hernández-Apaolaza, L.; Rivas, I.; Lucena, J.J. Determination of 67Zn distribution in navy bean (Phaseolus vulgaris L.) after foliar application of 67Zn-lignosulfonates using isotope pattern deconvolution. J. Agric. Food Chem. 2011, 59, 8829–8838. [Google Scholar]
- Islas-Valdez, S.; López-Rayo, S.; Hristov-Emilov, H.; Hernández-Apaolaza, L.; Lucena, J.J. Assessing metal–lignosulfonates as fertilizers using gel filtration chromatography and high-performance size exclusion chromatography. Int. J. Biol. Macromol. 2020, 142, 163–171. [Google Scholar] [CrossRef]
- Mortvedt, J.J.; Gilkes, R.J. Zinc fertilizers. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 33–44. [Google Scholar]
- Cieschi, M.T.; Benedicto, A.; Hernández-Apaolaza, L.; Lucena, J.J. EDTA Shuttle Effect vs. Lignosulfonate Direct Effect Providing Zn to Navy Bean Plants (Phaseolus vulgaris L. ‘Negro Polo’) in a Calcareous Soil. Front. Plant Sci. 2016, 7, 1767–1779. [Google Scholar]
- Ikkonen, E.N.; Grabelnykh, O.I.; Sherudilo, E.G.; Shibaeva, T.G. Salicylhydroxamic Acid-Resistant and Sensitive Components of Respiration in Chilling-Sensitive Plants Subjected to a Daily Short-Term Temperature Drop. Russ. J. Plant Physiol. 2020, 67, 60–67. [Google Scholar] [CrossRef]
- Titov, A.F.; Shibaeva, T.G.; Ikkonen, E.N.; Sherudilo, E.G. Plant Responses to a Daily Short-term Temperature Drop: Phenomenology and Mechanisms. Russ. J. Plant Physiol. 2020, 67, 1003–1017. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Amir, M.; Bano, H.; Chater, C.C.; Jellason, N.P. Clarity on frequently asked questions about drought measurements in plant physiology. Sci. Afr. 2020, 8, e00405. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 99, 87–92. [Google Scholar] [CrossRef]
- Campbell, C.; Atkinson, L.; Zaragaza-Castells, J.; Lundmark, M.; Atkin, O.; Hurry, V. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. N. Phytol. 2007, 176, 375–389. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Lu, Z.; Xu, F. Accumulation of ammonium and reactive oxygen mediated drought-induced rice growth inhibition by disturbed nitrogen metabolism and photosynthesis. Plant Soil 2018, 431, 107–117. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Tuzen, M.; Souza, A.S.; Korn, M.D.G.A.; Ferreira, S.L.C. Optimization of microwave assisted digestion procedure for the determination of zinc, copper and nickel in tea samples employing flame atomic absorption spectrometry. J. Hazard. Mater. 2007, 149, 264–268. [Google Scholar] [CrossRef]
- Sajwan, K.S.; Lindsay, W.L. Response of flooded rice to various sources of zinc. J. Agric. Sci. 1988, 111, 197–210. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovic, A.; Abadia, A.; Abadia, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 371–399. [Google Scholar]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Loveys, B.R.; Atkinson, L.J.; Sherlock, D.J.; Roberts, R.L.; Fitter, A.H.; Atkin, O.K. Thermal acclimation of leaf and root respiration: An investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 2003, 9, 895–910. [Google Scholar] [CrossRef]
- Garmash, E.V.; Golovko, T.K. CO2 gas exchange and growth in Rhaponticum carthamoides under the conditions of the middle taiga subzone of northeastern Europe. 1. Dependence of photosynthesis and respiration on environmental factors. Russ. J. Plant Physiol. 1997, 44, 737–745. [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurement. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosyn. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Aung, M.S.; Masuda, H. How Does Rice Defend Against Excess Iron?: Physiological and Molecular Mechanisms. Front. Plant Sci. 2020, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Sabinin, D.A. Physiological Basis of Plant Nutrition; Academy of Sciences of the USSR: Moscow, Russia, 1955. [Google Scholar]
- Rodríguez-Lucena, P.; Benedicto, A.; Lucena, J.J.; Rodríguez-Castrillo, J.A.; Moldovan, M.; García Alonso, J.I.; Hernandez-Apaolaza, L. Use of the stable isotope 57Fe to track the efficacy of the foliar application of lignosulfonate/Fe3+ complexes to correct iron deficiencies in cucumber plants. J. Sci. Food Agric. 2011, 91, 395–404. [Google Scholar] [PubMed]
- López-Rayo, S.; Correas, C.; Lucena, J.J. Novel chelating agents as manganese and zinc fertilisers: Characterisation, theoretical speciation and stability in solution. Chem. Speciat. Bioavailab. 2012, 24, 147–158. [Google Scholar] [CrossRef]
- Jurkevich, M.G.; Ikkonen, E.N. Lignosulfonate effect on loamy soil and cucumber plants. Waste Resour. 2020, 7, 1–9. [Google Scholar]
- Ahmad, N.; Hussain, S.; Ali, M.A.; Minhas, A.; Waheed, W.; Danish, S.; Fahad, S.; Ghafoor, U.; Baig, K.S.; Sultan, H. Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District. Horticulturae 2022, 8, 61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).