Properties of Nano-Amendments and Their Effect on Some Soil Properties and Root-Knot Nematode and Yield Attributes of Tomato Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nano-Biochar (nB)
2.2. Synthesis of Green Nanobiochar (GnB)
2.3. Synthesis of Magnetic Nanobiochar (MnB)
2.4. Extraction of Nematode Eggs
2.5. Pot Experiment
2.6. Plant Growth Measurements
2.7. Soil Samples and Green Nanobiochar Analysis
2.8. Spectroscopic Analysis
2.9. Nematode Parameters
2.10. Statistical Analysis
3. Results
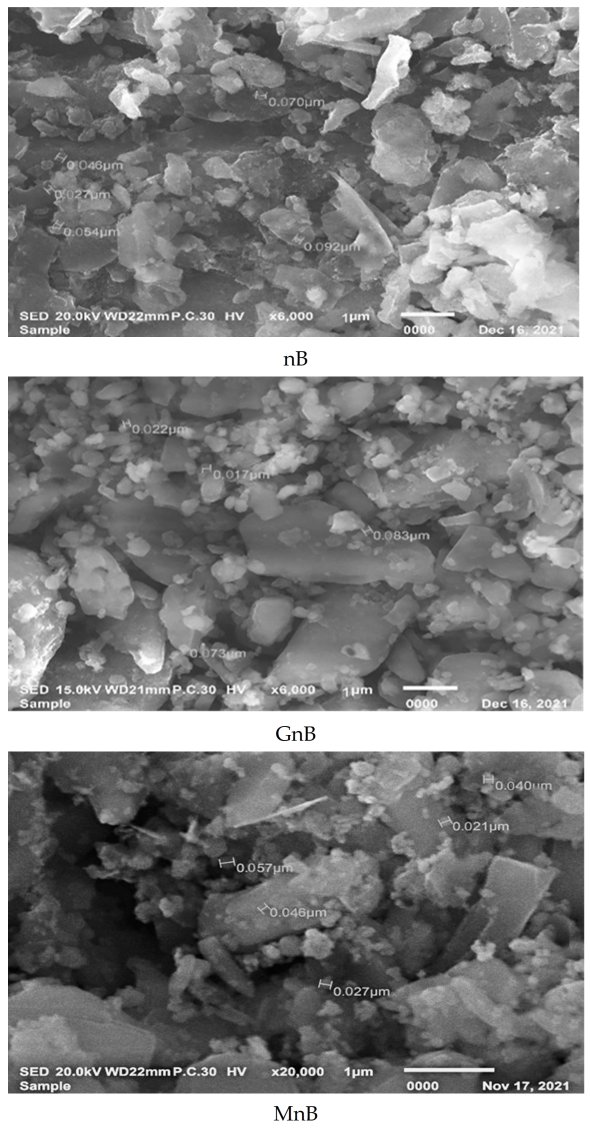
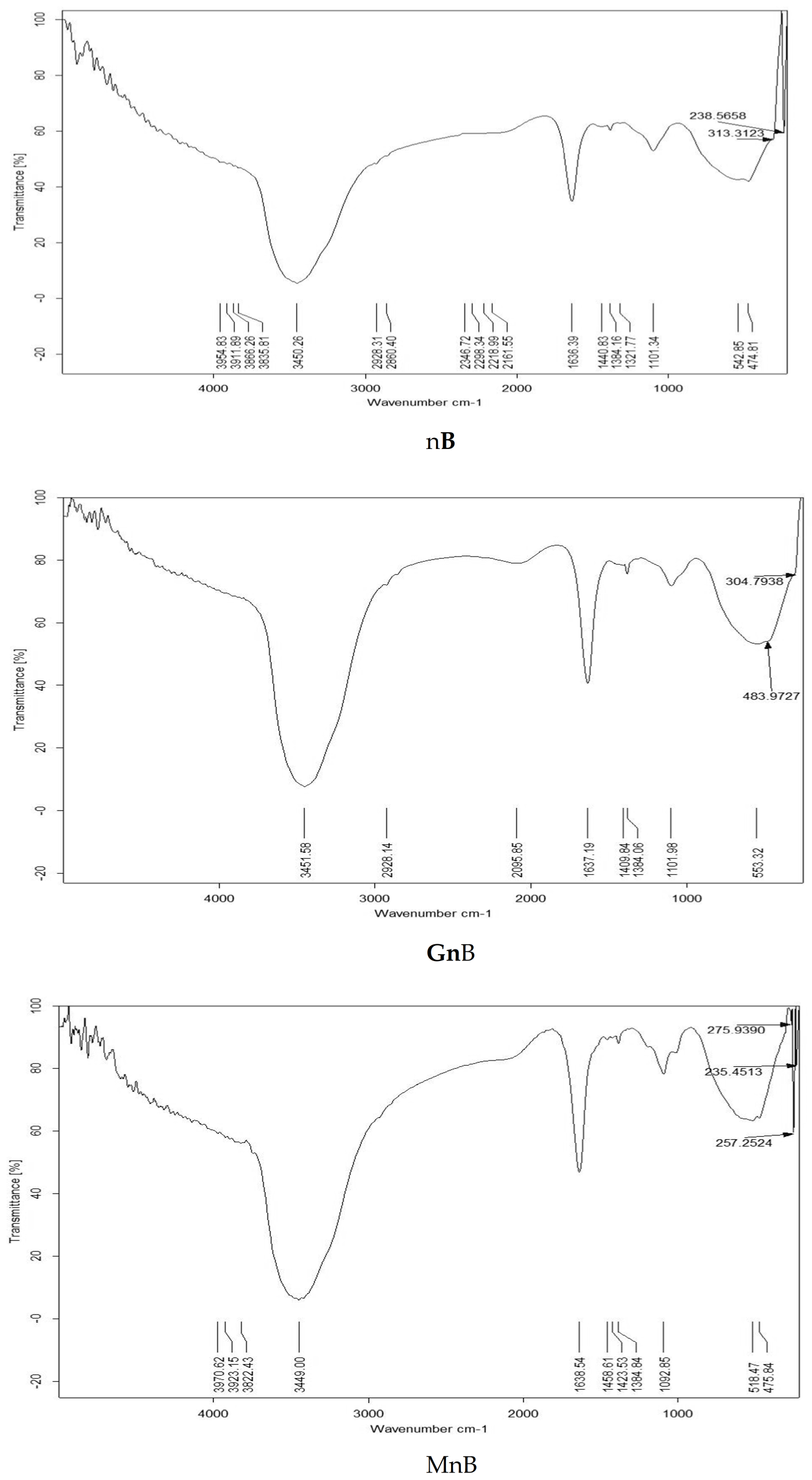
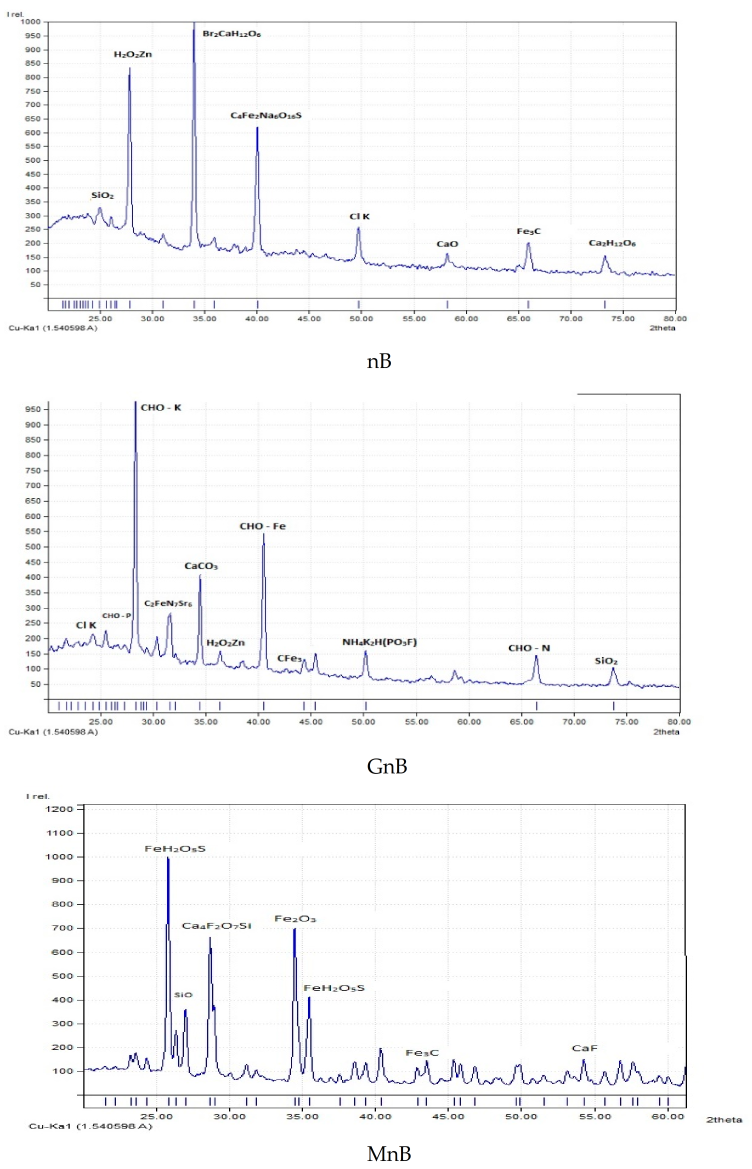
3.1. Properties of the Investigated Soil Amendments
3.2. Impact of Nano-Amendments on Soil pH, OM, and MBC
3.3. Effect of Nano-Amendments on Root-Knot Nematodes
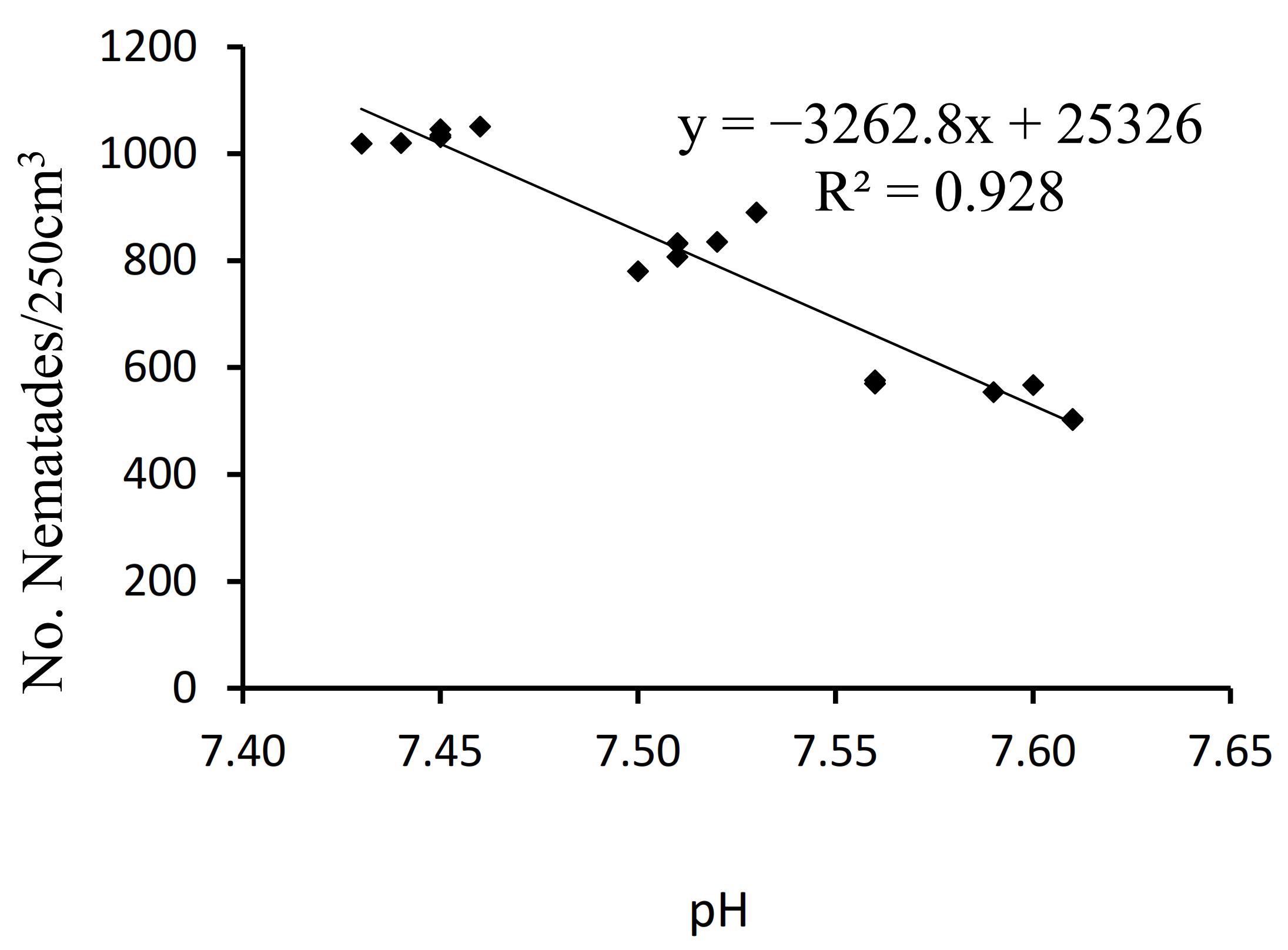
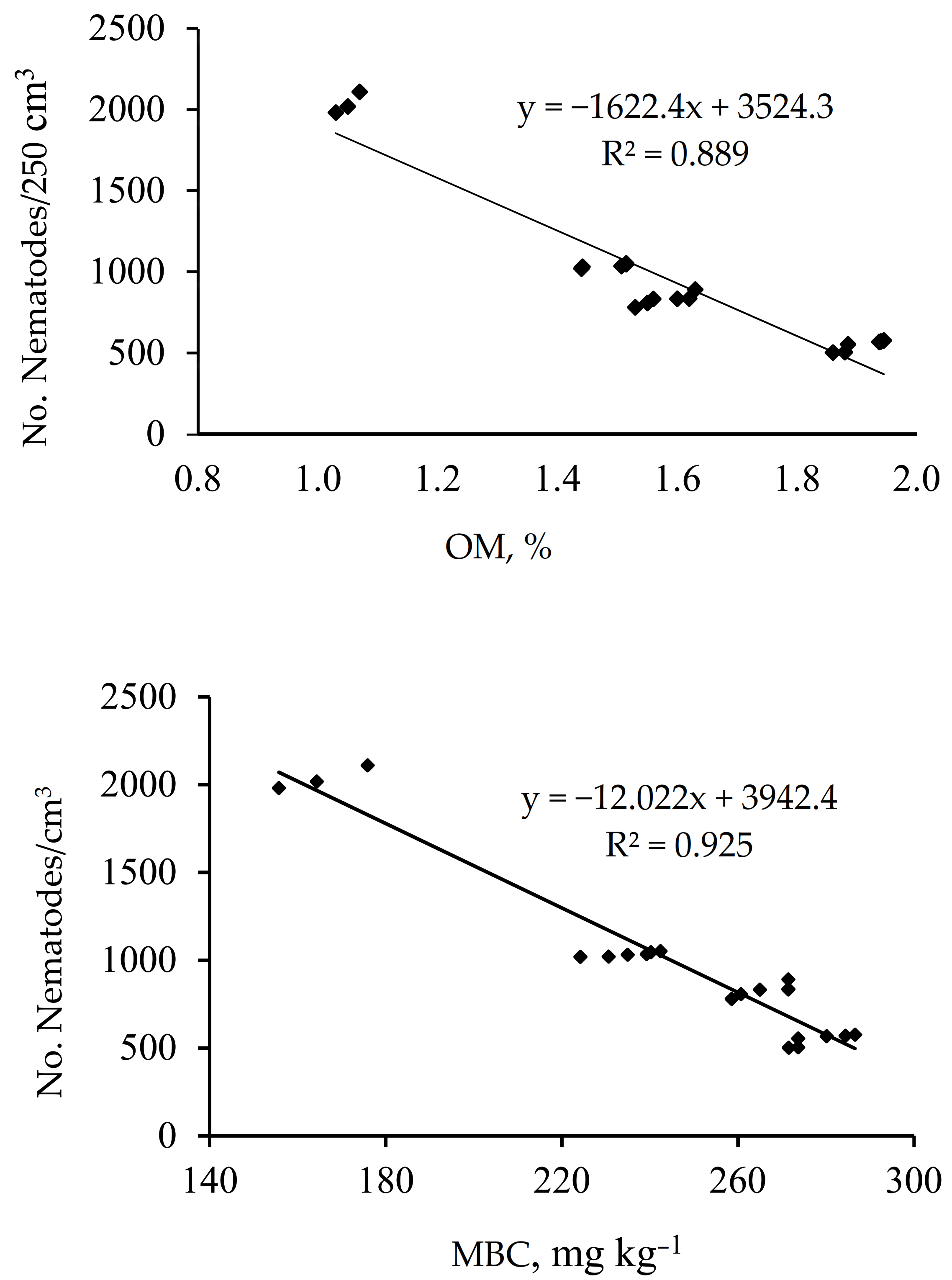
3.4. Correlation between Number of Nematodes per 250 cm3 and Soil pH, OM, and MBC
3.5. Impact of Nano-Amendments on Tomato Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandel, P.; Shahi, S.K. Microbes mediated synthesis of metal nanoparticles: Current status and future prospects. Int. J. Nanomater. Biostruct 2016, 6, 1–24. [Google Scholar]
- Veeramanikandan, V.; Madhu, G.; Pavithra, V.; Jaianand, K.; Balaji, P. Green synthesis, characterization of iron oxide nanoparticles using Leucas aspera leaf extract and evaluation of antibacterial and antioxidant studies. Int. J. Agri. Innov. Res. 2017, 6, 242–250. [Google Scholar]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotech. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Nazir, A. Green synthesis of metal nanoparticles and their applications in different fields: A review. Z. Für Phys. Chemie. 2019, 233, 1325–1349. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for plant disease management. Sci Direct Curr Opin Env. Sci Health. 2018, 66, 66–70. [Google Scholar] [CrossRef]
- Gkanatsiou, C.; Ntalli, N.; Dendrinou-Samara, U.; Menkissoglu-Spiroudi, C. Essential Metal-Based Nanoparticles (Copper/Iron NPs) as potent nematicidal agents against Meloidogyne spp. J. Nanotechnol. Res. 2019, 1, 44–58. [Google Scholar]
- Osei, K.; Addico, R.; Nafeo, A.; Edu-Kwarteng, A.; Agyemang, A.; Danso, Y.; Sackey-Asante, J. Effect of some organic waste extracts on hatching of Meloidogyne incognita eggs. Afr. J. Agri. Res. 2011, 6, 2255–2259. [Google Scholar]
- Izuogu, N.; Oyedunmade, E. Pathogenicity and Control of the Root-Knot Nematode, Meloidogyne incognita on Fluted Pumpkin, Telfairia occidentalis. J. Agri. Res. Develo. 2009, 7. [Google Scholar] [CrossRef]
- Annon. Annual progress report: All India Network Program on Gastrointestinal Parasites. CSWRI Avikanagar. 2004, 45, 473–483. [Google Scholar]
- Poswal, M.; Akpa, A. Current trends in the use of traditional and organic methods for the control of crop pests and diseases in Nigeria. Int. J. Pest Manage. 1991, 37, 329–333. [Google Scholar] [CrossRef]
- Pakeerathun, K.; Mikutan, G.; Tharsshan, N. Effects of different animal manure on Meloidogyne incognita on tomato IDOSI publication. World J. Agri. Sci. 2009, 5, 432–435. [Google Scholar]
- El-Sherif, A.; Gad, S.; Saadoon, S. Eco-friendly management of Meloidogyne incognita infecting eggplant under greenhouse conditions. Asian J. Nematol. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Arshad, U.; Naveed, M.; Javed, N.; Gogi, M.D.; Ali, M.A. Biochar application from different feedstocks enhances plant growth and resistance against Meloidogyne incognita in tomato. Intern J Agri Biol. 2020, 24, 961–968. [Google Scholar] [CrossRef]
- Liu, T.; Mao, P.; Shi, L.; Wang, Z.; Wang, X.; He, X.; Tao, L.; Liu, Z.; Zhou, L.; Shao, Y. Contrasting effects of nitrogen deposition and increased precipitation on soil nematode communities in a temperate forest. Soil Biol. Biochem. 2020, 148, 107869. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.; Tian, J.; Cai, K. Modified Biochars and Their Effects on Soil Quality: A Review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef]
- Joseph, S.; Kammann, C.I.; Shepherd, J.G.; Conte, P.; Schmidt, H.-P.; Hagemann, N.; Rich, A.M.; Marjo, C.E.; Allen, J.; Munroe, P. Microstructural and associated chemical changes during the composting of a high temperature biochar: Mechanisms for nitrate, phosphate and other nutrient retention and release. Sci. Total Environ. 2018, 618, 1210–1223. [Google Scholar] [CrossRef]
- Perez, N.D.; Kassim, Y.; Ringler, C.; Thomas, T.S.; ElDidi, H. Climate Change Adaptation Strategies for Egypt’s Agricultural Sector: A ‘Suite of Technologies’ Approach; International Food Policy Research Institute: Washington, DC, USA, 2021; Volume 18.
- Fao, G.D.; Jiang, J.-C. Theoretical investigation of CO2 conversion on corrugated g-C3N4 Surface decorated by single-atom of Fe, Co, and Pd. Mol. Catal. 2022, 526, 112402. [Google Scholar] [CrossRef]
- Anwar, R.; Fatima, T.; Mattoo, A.K. Tomatoes: A model crop of solanaceous plants. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Abu El Enain, S.M.; El-Rasoul, A.; Shaban, M.; Mohamed, M. Soil taxonomy and evaluation of some newly reclaimed areas adjacent to the Nile Delta Rims, EGYPT. Fayoum. J. Agri. Res. Devel. 2010, 24, 78–88. [Google Scholar] [CrossRef]
- Frolova, L.; Kharytonov, M. Synthesis of magnetic biochar for efficient removal of Cr (III) cations from the aqueous medium. Advn. Mater. Sci. Eng. 2019, 2019, 2187132. [Google Scholar] [CrossRef]
- Elbadri, G.; Lee, D.W.; Park, J.C.; Choo, H.Y. Nematicidal efficacy of herbal powders on Meloidogyne incognita (Tylenchida: Meloidogynidae) on potted watermelon. J. Asia-Pac. Entom. 2009, 12, 37–39. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, X.; Henry, L. The Effect of nano-biochar on soil, water, and nutrient loss of a sloping land with different vegetation covers on Loess plateau of China. App. Ecol. Envirn. Res. 2020, 18, 2845–2861. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil. 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.; Keeney, D.R. Methods of Soil Analysis: Chemical and Microbiological Properties; Amen Society Agronomy: Madison, WI, USA, 1982; Volume 2. [Google Scholar]
- Nelson, D.a.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar] [CrossRef]
- Graber, E.R.; Singh, B.; Hanley, K.; Lehmann, J. Determination of cation exchange capacity in biochar. Biochar: A Guide Anal. Methods 2017, 11, 74–84. [Google Scholar]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. App. Microbio. Biotech. 2006, 69, 485–492. [Google Scholar] [CrossRef]
- Bridge, J.; Page, S. Estimation of root-knot nematode infestation levels on roots using a rating chart. Int. J. Pest Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Taylor, A.; Sasser, J. Biology, Identification and Control of Root-Knot Nematodes; North Carolina State University Graphics: Raleigh, NC, USA, 1978; Volume 111. [Google Scholar]
- Southey, J.F. Laboratory Methods for Work with Plant and Soil Nematodes; HMSO: London, UK, 1970. [Google Scholar]
- Kasim, N.; Mustari, K.; Iswari, I.; Padjung, R.; Widiayani, N. Effect of the application of chicken manure compost tea on the growth of certified cocoa (Theobroma cacao L.) seedlings. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 042050. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Aisida, S.O.; Madubuonu, N.; Alnasir, M.H.; Ahmad, I.; Botha, S.; Maaza, M.; Ezema, F.I. Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. App. Nanosci. 2020, 10, 305–315. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Gharayebi, Y.; Shameli, K.; Nadi, B. Fabrication and characterization of SiO2/(3-aminopropyl) triethoxysilane-coated magnetite nanoparticles for lead (II) removal from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ibrahim, M.; Khader, A. Phosphorus fertilisation and biochar impacts on soil fertility and wheat (Triticum aestivum) productivity under semiarid conditions. Crop Pasture Sci. 2021, 74, CP21095. [Google Scholar] [CrossRef]
- Brendecke, J.W.; Axelson, R.D.; Pepper, I.L. Soil microbial activity as an indicator of soil fertility: Long-term effects of municipal sewage sludge on an arid soil. Soil Biol. Biochem. 1993, 25, 751–758. [Google Scholar] [CrossRef]
- Heinze, S.; Hemkemeyer, M.; Schwalb, S.A.; Khan, K.S.; Joergensen, R.G.; Wichern, F. Microbial Biomass Sulphur—An Important Yet Understudied Pool in Soil. Agron. 2021, 11, 1606. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719. [Google Scholar] [CrossRef]
- Ling, W.; Ren, L.; Gao, Y.; Zhu, X.; Sun, B. Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil. Soil Biol. Biochem. 2009, 41, 2187–2195. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q.; Emerson, E.; Pulliam, R. Suppressing plant parasitic nematodes and arthropod pests with vermicompost teas. Biocy 2007, 48, 38–39. [Google Scholar]
- El-Mougy, N.S.; Abdel-Kader, M.; Lashin, S.; AA, M. Fungicides alternatives as plant resistance inducers against foliar diseases incidence of some vegetables grown under plastic houses conditions. Environment 2013, 3, 71–81. [Google Scholar]
- Tolba, H.I.; Morsy, E.M.; El-Badawy, E. Impact of microbial inoculation on maize (Zea mays) productivity under different levels of potassium fertilization. Egypt J. Biotech. 2010, 35, 172–184. [Google Scholar]
- Ibrahim, F.; Quainoo, A.K.; Kankam, F. Effect of shea nut shell biochar on root knot nematodes (Meloidogyne spp.) of tomato (Solanum lycopersicum L.). Annu. Res. Rev. Biol. 2019, 30, 1–7. Available online: http://hdl.handle.net/123456789/2391 (accessed on 10 January 2023). [CrossRef]
- Wang, Y.; Wei, Y.; Sun, J. Biochar application promotes growth parameters of soybean and reduces the growth difference. Comm. Soil Sci. Plant Analy. 2016, 47, 1493–1502. [Google Scholar] [CrossRef]
- Ansari, M.I.; Masood, F.; Malik, A. Bacterial biosorption: A technique for remediation of heavy metals. Microbes Microb. Technol. 2011, 283–319. [Google Scholar] [CrossRef]
- Ahmed, N. Rice Straw and Peanut Residues Biochars as Eco-Friendly Approaches for Controlling Root-Knot Nematode, Meloidogyne incognita Infecting Eggplant. Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2021, 13, 91–102. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J Essent. OilRes. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Thoden, T.C.; Korthals, G.W.; Termorshuizen, A.J. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology 2011, 13, 133–153. [Google Scholar] [CrossRef]
- Marimón-Bolívar, W.; Tejeda-Benítez, L.; Herrera, A.P. Removal of mercury (II) from water using magnetic nanoparticles coated with amino organic ligands and yam peel biomass. Env. Nanotechn Monit Manage. 2018, 10, 486–493. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sed. 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [Green Version]
- Ali, H. The Effect of Biochar and Compost Application in Remediating the Contaminated Soil with Some Heavy Metals and on Canola Plant Growth; Tanta University: Tanta, Egypt, 2018. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agri Food Chemi. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A. Root–knot nematodes (Meloidogyne incognita) suppression through pre-colonized arbuscular mycorrhiza (Glomus intraradices) in tomato-PT3. Sci. Agri. 2015, 12, 52–57. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Env. Sci. Poll Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Elsawy, H.; El-shahawy, A.; Ibrahim, M.; El-Halim, A.E.-H.A.; Talha, N.; Sedky, A.; Alfwuaires, M.; Alabbad, H.; Almeri, N.; Mahmoud, E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agri. 2022, 12, 2096. [Google Scholar] [CrossRef]

| Amendments | pH | EC, dSm−1 | OC, % | N, % | P, % | K, % | CEC, cmolc kg−1 |
|---|---|---|---|---|---|---|---|
| Soil | 7.79 | 1.65 | 0.62 | 30 | 5.89 | 368 | 29.03 |
| CT | 8.01 | 4.08 | 24.60 | 0.42 | 0.36 | 0.49 | - |
| nB | 8.45 a | 2.45 a | 56.90 a | 2.70 c | 3.51 b | 5.01 b | 31.30 b |
| GnB | 7.58 b | 2.29 b | 46.62 b | 4.02 a | 4.61 a | 5.74 a | 32.41 a |
| MnB | 6.69 c | 2.24 c | 45.70 c | 3.08 c | 3.47 c | 5.03 b | 29.60 c |
| Treatments | pH | OM % | MBC mg kg−1 |
|---|---|---|---|
| C | 7.79 a | 1.05 g | 165 f |
| nB3 | 7.51 c | 1.55 d | 271 b |
| nB6 | 7.52 c | 1.62 c | 272 b |
| GnB3 | 7.60 b | 1.88 b | 261 c |
| GnB6 | 7.57 b | 1.94 a | 283 a |
| MnB3 | 7.45 d | 1.44 f | 229 e |
| MnB6 | 7.44 d | 1.55 e | 240 d |
| F-test | ** | ** | ** |
| LSD (0.01) | 5.48 | 3.02 | 11.51 |
| LSD (0.05) | 3.95 | 2.18 | 8.29 |
| Treatments | Root Gall | Egg Mass | Number of Nematodes per 250 cm3 Soil |
|---|---|---|---|
| C | 71.67 a | 79.33 a | 2036 a |
| nB3 | 24.67 c | 18.57 c | 852.31 c |
| nB6 | 23.00 c | 17.61 c | 806 c |
| GnB3 | 18.00 d | 11.00 d | 571 d |
| GnB6 | 16.00 d | 9.00 d | 520 d |
| MnB3 | 33.34 b | 28.76 b | 1023 b |
| MnB6 | 35.67 b | 27.00 b | 1044 b |
| F-test | ** | ** | ** |
| LSD (0.01) | 2.41 | 5.39 | 55.62 |
| LSD (0.05) | 3.50 | 7.48 | 77.20 |
| Treatments | Root Length, cm | Number of Leaves | Number of Flowers | Number of Laterals | Plant Height, cm | Fresh Weight, g | Dry Weight, g |
|---|---|---|---|---|---|---|---|
| C | 19.35 a | 6 d | 2 f | 12 f | 28.3 d | 60.23d | 12.40 d |
| nB3 | 26.07 b | 11 b | 7 c | 46.20 c | 54.04b | 83.52b | 19.28 c |
| nB6 | 26.08 b | 11 b | 7 c | 46.00 c | 52.61b | 87.81 a | 21.70 bc |
| GnB3 | 26.45 a | 11 b | 8.53 b | 49.43 b | 56.36a | 81.25 b | 21.84 b |
| GnB6 | 26.64 a | 12 a | 10 a | 57.13 a | 60.03 a | 88.82 a | 24.65 a |
| MnB3 | 25.63 d | 9 c | 4 e | 31.00 e | 46.07c | 70.91c | 13.25d |
| MnB6 | 25.31 c | 9 c | 5 d | 34.00 d | 44.37c | 70.93c | 13.60 d |
| F-test | ** | ** | ** | ** | ** | ** | ** |
| LSD (0.05) | 0.27 | 0.38 | 0.50 | 1.87 | 1.20 | 2.54 | 2.56 |
| LSD (0.01) | 0.37 | 0.50 | 0.75 | 2.59 | 1.67 | 3.55 | 3.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khader, A.; Ibrahim, M.; Alkhathami, F.; Elsawy, H.; El-Kader, N.A.; Shaker, E.; Sedky, A.; Mahmoud, E. Properties of Nano-Amendments and Their Effect on Some Soil Properties and Root-Knot Nematode and Yield Attributes of Tomato Plant. Agriculture 2023, 13, 366. https://doi.org/10.3390/agriculture13020366
Khader A, Ibrahim M, Alkhathami F, Elsawy H, El-Kader NA, Shaker E, Sedky A, Mahmoud E. Properties of Nano-Amendments and Their Effect on Some Soil Properties and Root-Knot Nematode and Yield Attributes of Tomato Plant. Agriculture. 2023; 13(2):366. https://doi.org/10.3390/agriculture13020366
Chicago/Turabian StyleKhader, Asmaa, Mahmoud Ibrahim, Fahad Alkhathami, Hany Elsawy, Nasser Abd El-Kader, Eman Shaker, Azza Sedky, and Esawy Mahmoud. 2023. "Properties of Nano-Amendments and Their Effect on Some Soil Properties and Root-Knot Nematode and Yield Attributes of Tomato Plant" Agriculture 13, no. 2: 366. https://doi.org/10.3390/agriculture13020366





