Multifarious Effects of Arsenic on Plants and Strategies for Mitigation
Abstract
:1. Introduction
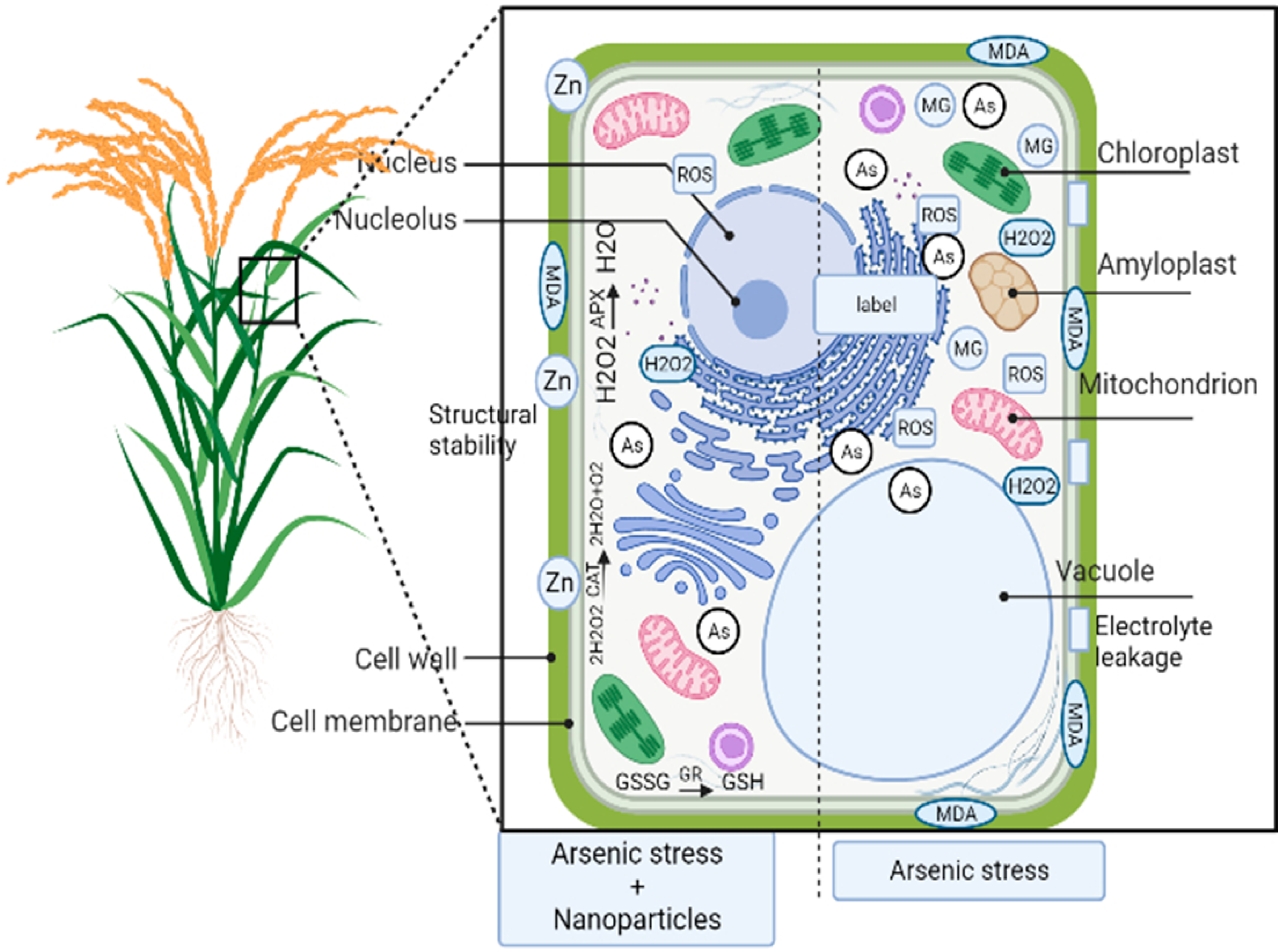
2. Effect of Arsenic on Plants
3. Arsenite and Arsenate Transporters Mediated Transport from Soil and Root to Various Plant Tissues

4. Phytoremediation and Rhizoremediation to Mitigate the Toxic Effects of Arsenic
5. Arsenic Accumulation Depends on Plant Genotype
6. Application of Nanotechnology to Counter Negative Effects of Arsenic on Plants
Plant Extracts Mitigate Arsenic Effects
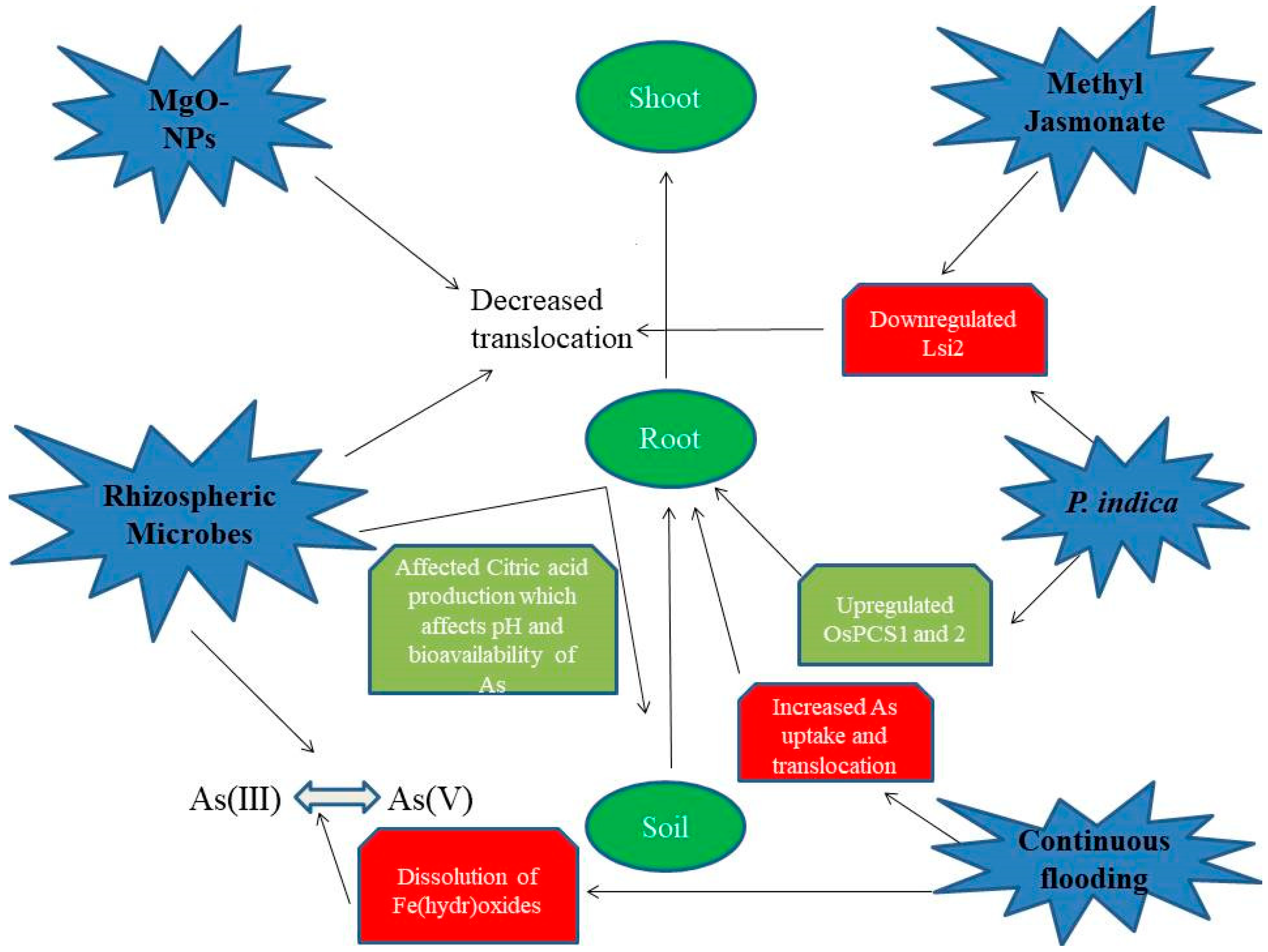
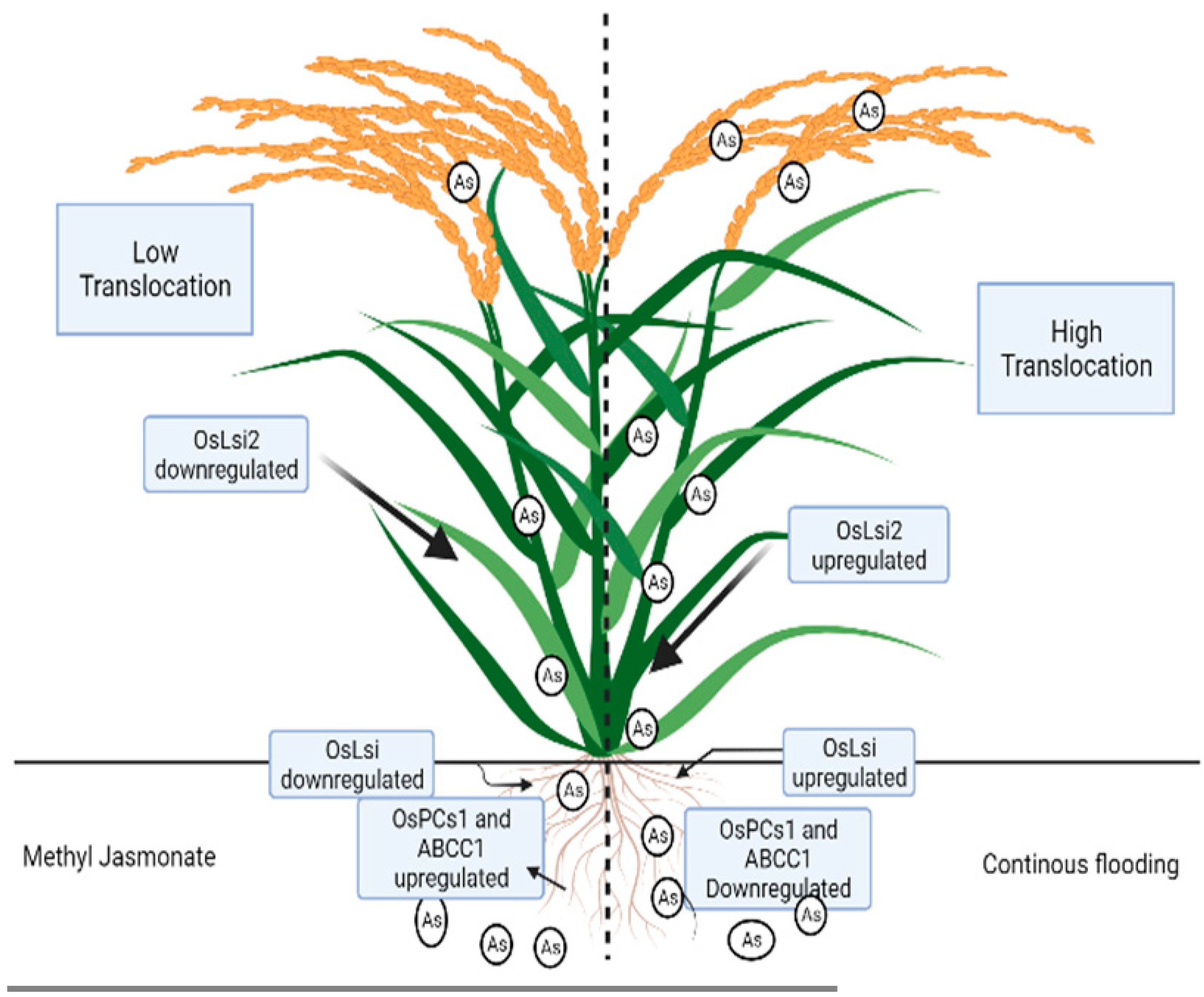
7. Interrelationship among Flooding, Nitric Oxide Production, Methyl Jasmonate, Iron, and Arsenic in Plants
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors-a review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Yan, G.; Chen, X.; Du, S.; Deng, Z.; Wang, L.; Chen, S. Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr. Genet. 2019, 65, 329–338. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, P.; Wang, K.; Yang, Z.; Wang, L. Relationship between ionomics and transcriptomics of rice plant in response to arsenite stress. Environ. Exp. Bot. 2021, 189, 104565. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Chen, T.; Su, Y.; Yuan, X. Influx and efflux of arsenic in cotton fields irrigated with arsenic-contaminated groundwater. Bioremediation J. 2018, 22, 103–111. [Google Scholar] [CrossRef]
- Cao, Z.; Pan, J.; Yang, Y.; Cao, Z.; Xu, P.; Chen, M.; Guan, M. Water management affects arsenic uptake and translocation by regulating arsenic bioavailability, transporter expression and thiol metabolism in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 206, 111208. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, D.S.; Tian, J.C.; He, F.L. Toxicity and accumulation of arsenic in wheat (Triticum aestivum L.) varieties of China. Phyton 2009, 78, 147–154. [Google Scholar]
- Toledo, M.C.; Lee, J.S.; Batista, B.L.; Olympio, K.P.K.; Nardocci, A.C. Exposure to inorganic arsenic in rice in Brazil: A human health risk assessment. Int. J. Environ. Res. Public Health 2022, 19, 16460. [Google Scholar] [CrossRef]
- Baris, D.; Waddell, R.; Beane Freeman, L.E.; Schwenn, M.; Colt, J.S.; Ayotte, J.D.; Ward, M.H.; Nuckols, J.; Schned, A.; Jackson, B.; et al. Elevated bladder cancer in Northern New England: The role of drinking water and arsenic. J. Natl. Cancer Inst. 2016, 108, djw099. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Ahmad, M.F.; Ahmad, I.; Ashfaq, F.; Wahab, S.; Alsayegh, A.A.; Kumar, S.; Hakeem, K.R. Arsenic exposure through dietary intake and associated health hazards in the Middle East. Nutrients 2022, 14, 2136. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller AL, W.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef]
- Mohd, S.; Shukla, J.; Kushwaha, A.S.; Mandrah, K.; Shankar, J.; Arjaria, N.; Saxena, P.N.; Narayan, R.; Roy, S.K.; Kumar, M. Endophytic fungi Piriformospora indica mediated protection of host from arsenic toxicity. Front. Microbiol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Shamim, M.Z.; Pandey, A. Effects of arsenic toxicity on morphological characters in blackgram (Vigna mungo L.) during early growth stage. Cell. Mol. Biol. 2017, 63, 38–43. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on hu mans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.M. Arsenic toxicity in male reproduction and development. Dev. Reprod. 2015, 19, 167. [Google Scholar] [CrossRef]
- Roy, N.K.; Murphy, A.; Costa, M. Arsenic methyltransferase and methylation of inorganic arsenic. Biomolecules 2020, 10, 1351. [Google Scholar] [CrossRef]
- Coryell, M.; McAlpine, M.; Pinkham, N.V.; McDermott, T.R.; Walk, S.T. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat. Commun. 2018, 9, 5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhi, A.; Yu, C.; Rahman, M.M.; Amin, M.N. Arsenic in the water and agricultural crop production system: Bangladesh perspectives. Environ. Sci. Pollut. Res. Int. 2022, 29, 51354–51366. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.J. Decreasing arsenic accumulation in rice by overexpressing OsNIP 1;1 and OsNIP 3;3 through disrupting arsenite radial transport in roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Bhardwaj, R. Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L . Ecotoxicol. Environ. Saf. 2015, 115, 119–125. [Google Scholar] [CrossRef]
- Zvobgo, G.; Lwalaba JL, W.; Sagonda, T.; Mapodzeke, J.M.; Muhammad, N.; Shamsi, I.H.; Zhang, G.P. Alleviation of arsenic toxicity by phosphate is associated with its regulation of detoxification, defense, and transport gene expression in barley. J. Integr. Agric. 2019, 18, 381–394. [Google Scholar] [CrossRef]
- Shabnam, N.; Kim, M.; Kim, H. Iron (III) oxide nanoparticles alleviate arsenic induced stunting in Vigna radiata . Ecotoxicol. Environ. Saf. 2019, 183, 109496. [Google Scholar] [CrossRef]
- Mushtaq, T.; Shah, A.A.; Akram, W.; Yasin, N.A. Synergistic ameliorative effect of iron oxide nanoparticles and Bacillus subtilis S4 against arsenic toxicity in Cucurbita moschata: Polyamines, antioxidants, and physiochemical studies. Int. J. Phytoremediation 2020, 22, 1408–1419. [Google Scholar] [CrossRef]
- Panaullah, G.M.; Alam, T.; Hossain, M.B.; Loeppert, R.H.; Lauren, J.G.; Meisner, C.A.; Ahmed, Z.U.; Duxbury, J.M. Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 2009, 317, 31–39. [Google Scholar] [CrossRef]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Saleem, M. Unrevealing arsenic and lead toxicity and antioxidant response in spinach: A human health perspective. Environ. Geochem. Health 2022, 44, 487–496. [Google Scholar]
- Várallyay, S.; Bódi, É.; Garousi, F.; Veres, S.; Kovács, B. Effect of arsenic on dry weight and relative chlorophyll content in greeningmaize and sunflower tissues. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 167–169. [Google Scholar] [CrossRef]
- Zemanová, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 130. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610, 1239–1250. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Chakrabarty, D.; Mallick, S.; Pandey, V.; et al. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front. Plant Sci. 2016, 6, 1272. [Google Scholar] [CrossRef]
- Singh, N.; Marwa, N.; Mishra, J.; Verma, P.C.; Rathaur, S.; Singh, N. Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol. Environ. Saf. 2016, 125, 25–34. [Google Scholar] [CrossRef]
- Andrade, H.M.; Oliveira, J.A.; Farnese, F.S.; Ribeiro, C.; Silva, A.A.; Campos, F.V.; Neto, J.L. Arsenic toxicity: Cell signalling and the attenuating effect of nitric oxide in Eichhornia crassipes . Biol. Plant. 2016, 60, 173–180. [Google Scholar] [CrossRef]
- Dixit, G.; Singh, A.P.; Kumar, A.; Mishra, S.; Dwivedi, S.; Kumar, S.; Trivedi, P.K.; Pandey, V.; Tripathi, R.D. Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem. 2016, 99, 86–96. [Google Scholar] [CrossRef]
- Mishra, S.; Alfeld, M.; Sobotka, R.; Andresen, E.; Falkenberg, G.; Küpper, H. Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: Subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J. Exp. Bot. 2016, 67, 4639–4646. [Google Scholar] [CrossRef]
- Kaur, S.; Chowhan, N.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R. β-Pinene alleviates arsenic (As)-induced oxidative stress by modulating enzymatic antioxidant activities in roots of Oryza sativa . Ecotoxicol. Environ. Saf. 2022, 229, 113080. [Google Scholar] [CrossRef]
- Pan, D.; Yi, J.; Li, F.; Li, X.; Liu, C.; Wu, W.; Tao, T. Dynamics of gene expression associated with arsenic uptake and transport in rice during the whole growth period. BMC Plant Biol. 2020, 20, 133. [Google Scholar] [CrossRef]
- Sun, D.; Feng, H.; Li, X.; Ai, H.; Sun, S.; Chen, Y.; Xu, G.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expression of new Pteris vittata phosphate transporter PvPht1; 4 reduces arsenic translocation from the roots to shoots in tobacco plants. Environ. Sci. Technol. 2019, 54, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Tafteh, M.; Roudbari, N.; Pishkar, L.; Zhang, W.; Wu, C. Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol. Environ. Saf. 2021, 209, 111793. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, P.; Hartley, S.E.; Travis, A.J.; Price, A.H.; Norton, G.J. Genotypic differences in shoot silicon concentration and the impact on grain arsenic concentration in rice. J. Plant Nutr. Soil Sci. 2019, 182, 265–276. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, C.Y.; Chen, J.X.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expressing arsenite antiporter PvACR3; 1 in rice (Oryza sativa L.) decreases inorganic arsenic content in rice grains. Environ. Sci. Technol. 2019, 53, 10062–10069. [Google Scholar] [CrossRef]
- Saitoh, Y.; Suga, M. Structure and function of a silicic acid channel Lsi1. Front. Plant Sci. 2022, 13, 982068. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ago, Y.; Mitani, N.; Li, R.Y.; Su, Y.H.; Yamaji, N.; McGrath, S.P.; Ma, J.F. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010, 186, 392–399. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Miller, A.J.; Zhao, F.J. The C-type ATP-binding cassette transporter OsABCC7 is involved in the root-to-shoot translocation of arsenic in rice. Plant Cell Physiol. 2019, 60, 1525–1535. [Google Scholar] [CrossRef]
- Hayashi, S.; Kuramata, M.; Abe, T.; Yamaguchi, N.; Takagi, H.; Tanikawa, H.; Iino, M.; Sugimoto, K.; Ishikawa, S. Deficiency in alcohol dehydrogenase 2 reduces arsenic in rice grains by suppressing silicate transporters. Plant Physiol. 2021, 186, 611–623. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP 1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef]
- Chen, J.X.; Cao, Y.; Yan, X.; Chen, Y.; Ma, L.Q. Novel PvACR3; 2 and PvACR3; 3 genes from arsenic-hyperaccumulator Pteris vittata and their roles in manipulating plant arsenic accumulation. J. Hazard. Mater. 2021, 415, 125647. [Google Scholar] [CrossRef]
- Młodzińska, E.; Zboińska, M. Phosphate uptake and allocation—A closer look at Arabidopsis thaliana L. and Oryza sativa L. Front. Plant Sci. 2016, 7, 1198. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2449–2484. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M.K. Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol. Biol. 2018, 98, 101–120. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous expression of Pteris vittata phosphate transporter PvPht1; 3 enhances arsenic translocation to and accumulation in tobacco shoots. Environ. Sci. Technol. 2019, 53, 10636–10644. [Google Scholar] [CrossRef]
- Luan, M.; Liu, J.; Liu, Y.; Han, X.; Sun, G.; Lan, W.; Luan, S. Vacuolar phosphate transporter 1 (VPT1) affects arsenate tolerance by regulating phosphate homeostasis in Arabidopsis . Plant Cell Physiol. 2018, 59, 1345–1352. [Google Scholar] [CrossRef]
- Mitra, S.; Subba, S. Alleviative effects of ferrous sulfate on arsenic toxicity in Lens culinaris Medik. by enhancing iron plaque formation on roots. Acta Physiol. Plant. 2022, 44, 126. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Webb, S.M.; Andrews, J.C.; Fendorf, S. Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environ. Sci. Technol. 2010, 44, 8108–8113. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zheng, F.; Yu, M.; Shabala, S.; Song, W.-Y. Comparative analysis of arsenic transport and tolerance mechanisms: Evolution from prokaryote to higher plants. Cells 2022, 11, 2741. [Google Scholar] [CrossRef]
- Popov, M.; Zemanová, V.; Sácký, J.; Pavlík, M.; Leonhardt, T.; Matoušek, T.; Kaňa, A.; Pavlíková, D.; Kotrba, P. Arsenic accumulation and speciation in two cultivars of Pteris cretica L. and characterization of arsenate reductase PcACR2 and arsenite transporter PcACR3 genes in the hyperaccumulating cv. Albo-lineata. Ecotoxicol. Environ. Saf. 2021, 216, 112196. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Nati. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Yu, M.; Martinoia, E.; Song, W.Y. Ideal cereals with lower arsenic and cadmium by accurately enhancing vacuolar sequestration capacity. Front. Genet. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Thounaojam, T.C.; Upadhyaya, H. Arsenic stress in rice (Oryza sativa) and its amelioration approaches. Plant Stress 2022, 4, 100076. [Google Scholar] [CrossRef]
- Alsafran, M.; Saleem, M.H.; Al Jabri, H.; Rizwan, M.; Usman, K. Principles and applicability of integrated remediation strategies for heavy metal removal/recovery from contaminated environments. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Li, K.; Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef]
- Adhikary, A.; Kumar, R.; Pandir, R.; Bhardwaj, P.; Wusirika, R.; Kumar, S. Pseudomonas citronellolis; a multi-metal resistant and potential plant growth promoter against arsenic (V) stress in chickpea. Plant Physiol. Biochem. 2019, 142, 179–192. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; El-Serehy, H.A.; Moustakas, M.; Ahmad, P. Magnesium oxide nanoparticles (MgO-NPs) alleviate arsenic toxicity in soybean by modulating photosynthetic function, nutrient uptake and antioxidant potential. Metals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Q.; Xue, S.; Pan, W.; Lou, L.; Li, D.; Hartley, W. Do aeration conditions affect arsenic and phosphate accumulation and phosphate transporter expression in rice (Oryza sativa L.)? Environ. Sci. Pollut. Res. 2018, 25, 43–51. [Google Scholar] [CrossRef]
- Diba, F.; Khan, M.Z.H.; Uddin, S.Z.; Istiaq, A.; Shuvo, M.S.R.; Ul Alam, A.R.; Hossain, M.A.; Sultana, M. Bioaccumulation and detoxification of trivalent arsenic by Achromobacter xylosoxidans BHW-15 and electrochemical detection of its transformation efficiency. Sci. Rep. 2021, 11, 21312. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas rhizobacteria of Avicennia marina of Indian Sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Yu, H.; Zhang, T.; Qiu, F. Controlled fabrication of functionalized nanoscale zero-valent iron/celluloses composite with silicon as protective layer for arsenic removal. Chem. Eng. Res. Des. 2019, 151, 242–251. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Hussaini, K.M.; Rizwan, M.; Ali, S.; Maqsood, A.; Li, B. Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ. Pollut. 2021, 288, 117785. [Google Scholar] [CrossRef]
- Gautam, A.; Pandey, A.K.; Dubey, R.S. Azadirachta indica and Ocimum sanctum leaf extracts alleviate arsenic toxicity by reducing arsenic uptake and improving antioxidant system in rice seedlings. Physiol. Mol. Biol. Plants 2020, 26, 63–81. [Google Scholar] [CrossRef]
- Mahajan, P.; Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. β-Pinene moderates Cr (VI) phytotoxicity by quenching reactive oxygen species and altering antioxidant machinery in maize. Environ. Sci. Pollut. Res. 2019, 26, 456–463. [Google Scholar] [CrossRef]
- Farias, G.J.; Frescura, D.V.; Boligon, A.A.; Trapp, C.K.; Andriolo, L.J.; Tedesco, B.S.; Bernardy, K.; Schwalbert, R.; Del Frari, K.B.; Carey, M.; et al. Chemical properties and protective effect of Rosmarinus officinalis: Mitigation of lipid peroxidation and DNA-damage from arsenic exposure. J. Appl. Bot. Food Qual. 2018, 91, 1–7. [Google Scholar]
- Mawia, A.M.; Hui, S.; Zhou, L.; Li, H.; Tabassum, J.; Lai, C.; Wang, J.; Shao, G.; Wei, X.; Tang, S.; et al. Inorganic arsenic toxicity and alleviation strategies in rice. J. Hazard. Materials 2021, 408, 124751. [Google Scholar] [CrossRef]
- Salavati, J.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol. Mol. Biol. Plants 2021, 27, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Ueda, Y.; Mukai, A.; Ochiai, K.; Matoh, T. Rice phytochelatin synthases OsPCS1 and OsPCS2 make different contributions to cadmium and arsenic tolerance. Plant Direct 2018, 2, e00034. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Pishkar, L.; Rood Cao, N.; Tavakoli, S.A.; Jahromi, E.M.; Wu, C. Nitrate reductase is needed for methyl jasmonate-mediated arsenic toxicity tolerance of rice by modulating the antioxidant defense system, glyoxalase system and arsenic sequestration mechanism. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Niknejad, Y.; Fallah, H.; Tari, D.B. Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep. 2020, 39, 1041–1060. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Narayan, S.; Shirke, P.A.; Chakrabarty, D. Exogenous application of methyl jasmonate alleviates arsenic toxicity by modulating its uptake and translocation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 201, 110735. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Majumdar, A.; Suresh Kumar, J.; Srivastava, S. Arsenic in rice agro-ecosystem: Solutions for safe and sustainable rice production. Front. Sustain. Food Syst. 2020, 4, 53. [Google Scholar] [CrossRef]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
| Plant | Change in Morphological and Physiological Parameters | Reference |
|---|---|---|
| Black Gram Vigna mungo | Increased activity of APX, SOD, and peroxidase (POD), decreased activity of Catalase (CAT), reduced photosynthetic pigment, and increased lipid peroxidation | [3] |
| Wheat Triticum aestivum | Decreased biomass of roots, stems and spikes, decreased number of spikes per plant, stunted root and stems, necrosis, and wilting of leaf margins | [9] |
| Soybean Glycine max | Decreased number of lateral roots, thickening and darkening of roots, necrotic and slimy root tips, darker and thicker stems, inhibited leaf development, affected biomass and root cell death, thin-walled parenchyma cells, reduced root cortex area, increased total peroxidase and superoxide dismutase (SOD) activity in As(III) treatment compared to As(V), and decreased chlorophyll a | [13] |
| Blackgram Vigna mungo | Root and shoot growth decreased, fresh root weight, fresh shoot weight, and fresh total biomass reduced, and shoot weight more affected than root weight | [17] |
| B. juncea | Decreased shoot length and number of leaves, increased activity of enzymes SOD, CAT, POD, APX, GR, and MDA content in a 30-day old plant, while only SOD and GR increased in 60 days old, protein content enhanced in 30-day old plant | [24] |
| Barley Hordeum vulgare | Decreased root and shoot dry weight, SOD and CAT activity increase | [25] |
| V. radiata | Length, root oxidizing capacity of seedling, and dry biomass decreased, ferric chelate reductase (FCR) activity increased, increased proline, H2O2, malondialdehyde (MDA), SOD, and CAT | [26] |
| C. moschata | Reduced phenolic compound and flavonoids, root length, reduced shoot length, root and shoot fresh weight, root and shoot dry weight. Reduced Chl a and Chl b, increased H2O2 and MDA content while increasing the activity of SOD, CAT, and APX | [27] |
| Rice | Decreased number of tillers, above-ground biomass, and grain yield | [28] |
| Rice cultivar Lalat Oryza sativa ssp. indica | Decreased germination with weight more affected than length, roots turned black, more accumulation in shoots when As(V) used, otherwise more in roots, increased lipid peroxidation, SOD, APX, and glutathione reductase (GR) activity | [29] |
| Spinach | Decreased plant biomass and chlorophyll-a and b content, increased oxidative stress through high H2O2, upregulated CAT, guaiacol peroxidase (GPX), APX, and SOD | [30] |
| Maize Zea mays | Decreased dry weight of roots and shoot, As(V) decreased chlorophyll content | [31] |
| Transporter | As(III) or As(V) | Location | Active or Passive | Plant | Reference |
|---|---|---|---|---|---|
| Lsi1 | As(III) | Distal side of plasma membrane | Passive | Rice | [23] |
| Lsi2 | As(III) | Proximal side of plasma membrane | Active | Rice | [23] |
| OsNIP1;1 | As(III) | Plasma membrane | Passive | Rice | [23] |
| OsNIP3;3 | As(III) | Plasma membrane | Passive | Rice | [23] |
| OsABCC1 | As(III) | Tonoplast of roots, stems, leaves, and husks of rice | Rice | [40] | |
| PvPht1;4 | As(V) | Plasma membrane (roots and fronds) | P. vittata | [41] | |
| PvACR3;1 | As(III) | Vacuolar membrane | P. vittata | [44] | |
| OsABCC7 | As(III) | Xylem parenchyma | Active | Rice | [47] |
| PvACR3;2 | As(III) | Plasma membrane | P. vittata | [50] | |
| PvACR3;3 | As(III) | Vacuolar membrane | P. vittata | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beniwal, R.; Yadav, R.; Ramakrishna, W. Multifarious Effects of Arsenic on Plants and Strategies for Mitigation. Agriculture 2023, 13, 401. https://doi.org/10.3390/agriculture13020401
Beniwal R, Yadav R, Ramakrishna W. Multifarious Effects of Arsenic on Plants and Strategies for Mitigation. Agriculture. 2023; 13(2):401. https://doi.org/10.3390/agriculture13020401
Chicago/Turabian StyleBeniwal, Rahul, Radheshyam Yadav, and Wusirika Ramakrishna. 2023. "Multifarious Effects of Arsenic on Plants and Strategies for Mitigation" Agriculture 13, no. 2: 401. https://doi.org/10.3390/agriculture13020401
APA StyleBeniwal, R., Yadav, R., & Ramakrishna, W. (2023). Multifarious Effects of Arsenic on Plants and Strategies for Mitigation. Agriculture, 13(2), 401. https://doi.org/10.3390/agriculture13020401







