Author Contributions
M.B.D.: investigation, visualization, data curation, writing—original draft. W.C.R.: visualization, formal analysis, software, methodology, writing—original draft. J.T.: supervision. D.S.: resources, methodology, supervision. J.E.S.: resources, methodology. D.A.S.: conceptualization, funding acquisition, supervision, writing—review and editing, project administration. M.V.K.: conceptualization, funding acquisition, supervision, writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Figure 1.
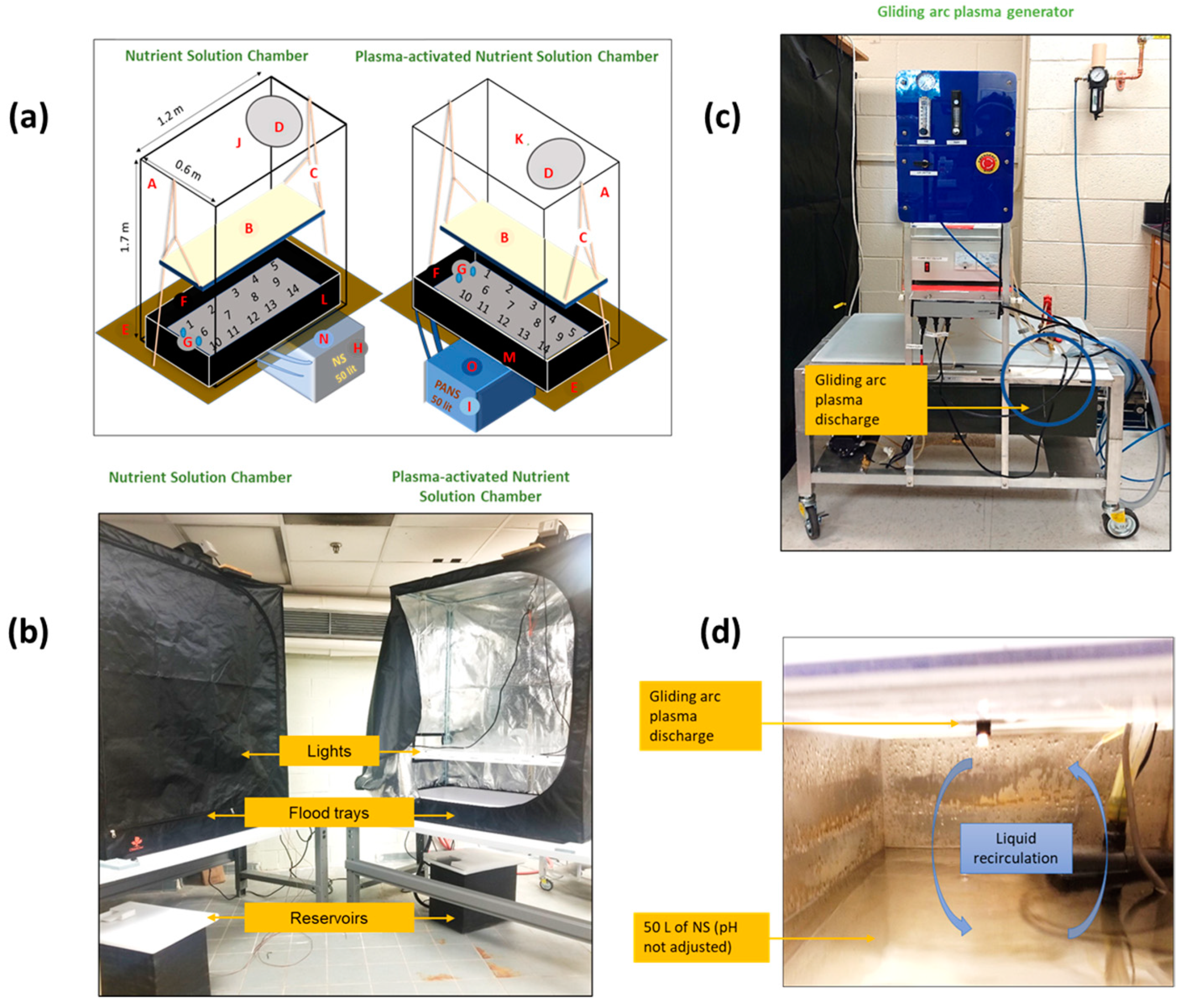
Experimental setup: (a) Diagram of growth chambers where A—enclosed chambers; B—white fluorescent lights; C—adjustable ropes for lights; D—fans; E—tables for chambers; F—flood tray showing placements of 14 basil plants; G—inlet and outlet pipes from the reservoir to the flood tray; H—NS reservoir; I—PANS reservoir; J, K—CO2 sensors; L, M—temperature and relative humidity sensors; N, O—temperature sensors. (b) Picture of enclosed chambers. (c) Picture of the gliding arc plasma generator. (d) Schematic of plasma treatment of NS by gliding arc plasma discharge inside the equipment.
Figure 1.
Experimental setup: (a) Diagram of growth chambers where A—enclosed chambers; B—white fluorescent lights; C—adjustable ropes for lights; D—fans; E—tables for chambers; F—flood tray showing placements of 14 basil plants; G—inlet and outlet pipes from the reservoir to the flood tray; H—NS reservoir; I—PANS reservoir; J, K—CO2 sensors; L, M—temperature and relative humidity sensors; N, O—temperature sensors. (b) Picture of enclosed chambers. (c) Picture of the gliding arc plasma generator. (d) Schematic of plasma treatment of NS by gliding arc plasma discharge inside the equipment.
Figure 2.
Schematic representation of experimental treatments based on the irrigation of PANS, where PANST1 indicates irrigation of basil seedlings with the same PANS throughout the complete growth cycle (Treatment 1), and PANST2 indicates irrigation of basil seedlings with newly prepared PANS at the beginning of each week (Treatment 2).
Figure 2.
Schematic representation of experimental treatments based on the irrigation of PANS, where PANST1 indicates irrigation of basil seedlings with the same PANS throughout the complete growth cycle (Treatment 1), and PANST2 indicates irrigation of basil seedlings with newly prepared PANS at the beginning of each week (Treatment 2).
Figure 3.
Schematic of the apparatus for puncture test and calculation of elasticity of basil leaves, where ω is the maximum deflection at the center, ν is the Poisson’s ratio for a plant leaf, po is pressure the applied by the probe, c is the radius of the probe, E is the Young’s modulus, t is the thickness of the leaf, a is the radius of the leaf area exposed.
Figure 3.
Schematic of the apparatus for puncture test and calculation of elasticity of basil leaves, where ω is the maximum deflection at the center, ν is the Poisson’s ratio for a plant leaf, po is pressure the applied by the probe, c is the radius of the probe, E is the Young’s modulus, t is the thickness of the leaf, a is the radius of the leaf area exposed.
Figure 4.
pH, EC, and ORP monitoring over 21 days of Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2) testing basil plant growth, showing (a) pH of NST1 and PANST1 for Treatment 1 (pH adjustment to approximately 5.8 on days 4, 7, 11, 15, and 19), (b) EC of NST1 and PANST1 for Treatment 1, (c) oxidation-reduction potential (ORP) over the harvest period of 21 days of NST1 and PANST1, (d) pH of NST2 and PANST2 for Treatment 2 over (pH adjustment to approximately 5.8 on days 4, 7, 11, 15, 18, and 20), (e) EC of NST2 and PANST2 for Treatment 2, and (f) ORP over the harvest period of 21 days of NST2 and PANST2. Note: Data points are presented as averages (n = 9) with error bars indicating standard deviation. Data not sharing the same letters in (c,f) are significantly different from each other at the p < 0.05 level.
Figure 4.
pH, EC, and ORP monitoring over 21 days of Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2) testing basil plant growth, showing (a) pH of NST1 and PANST1 for Treatment 1 (pH adjustment to approximately 5.8 on days 4, 7, 11, 15, and 19), (b) EC of NST1 and PANST1 for Treatment 1, (c) oxidation-reduction potential (ORP) over the harvest period of 21 days of NST1 and PANST1, (d) pH of NST2 and PANST2 for Treatment 2 over (pH adjustment to approximately 5.8 on days 4, 7, 11, 15, 18, and 20), (e) EC of NST2 and PANST2 for Treatment 2, and (f) ORP over the harvest period of 21 days of NST2 and PANST2. Note: Data points are presented as averages (n = 9) with error bars indicating standard deviation. Data not sharing the same letters in (c,f) are significantly different from each other at the p < 0.05 level.
Figure 5.
Algae analysis of Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2) at the end of every week. Note: Error bars indicate standard deviation. Data not sharing the same letters on the specific week sampled are significantly different from each other (Steel–Dwass test, p < 0.05). The algae counts were compared on the same week, such that the subscript of each letter indicates the week for which the comparison was made (i.e., on week one NST1, PANST1, NST2, and PANST2 were compared as all-pairs by Steel–Dwass method only for week 1), and they were not compared across multiple weeks.
Figure 5.
Algae analysis of Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2) at the end of every week. Note: Error bars indicate standard deviation. Data not sharing the same letters on the specific week sampled are significantly different from each other (Steel–Dwass test, p < 0.05). The algae counts were compared on the same week, such that the subscript of each letter indicates the week for which the comparison was made (i.e., on week one NST1, PANST1, NST2, and PANST2 were compared as all-pairs by Steel–Dwass method only for week 1), and they were not compared across multiple weeks.
Figure 6.
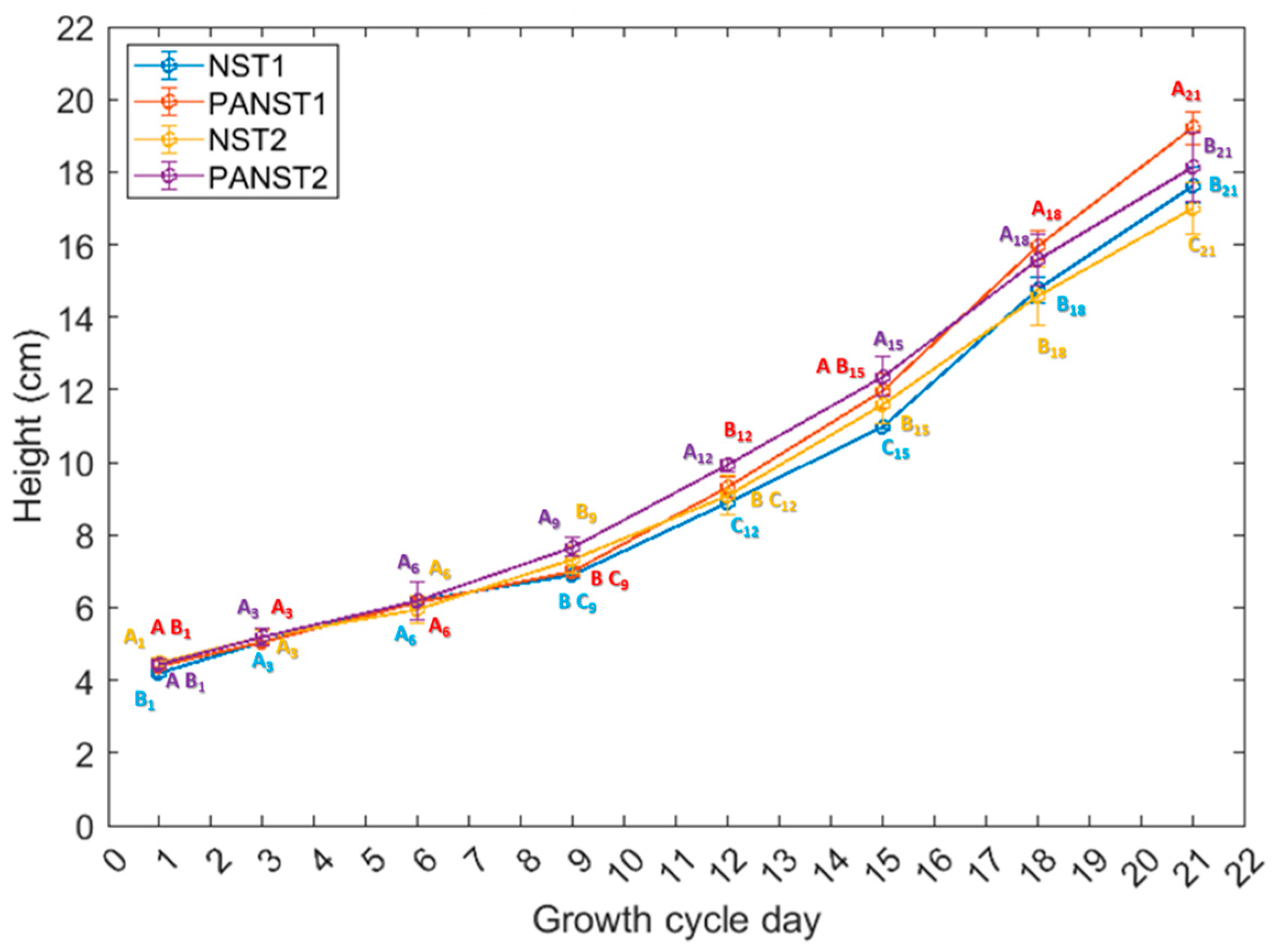
Plant height analysis of control (NST1 and NST2) and two plasma treatments (PANST1 and PANST2) over 21 days. Note: Error bars indicate standard deviation. Data not sharing the same letters on the specific growth cycle day are significantly different from each other (Steel–Dwass test, p < 0.05). The height of sweet basil plants was compared on the same growth cycle day, such that the subscript of each letter indicates the growth cycle day for which the comparison was made (i.e., on day one NST1, PANST1, NST2, and PANST2 were compared as all-pairs by Steel–Dwass method only for day 1), and they were not compared across multiple growth cycle days.
Figure 6.
Plant height analysis of control (NST1 and NST2) and two plasma treatments (PANST1 and PANST2) over 21 days. Note: Error bars indicate standard deviation. Data not sharing the same letters on the specific growth cycle day are significantly different from each other (Steel–Dwass test, p < 0.05). The height of sweet basil plants was compared on the same growth cycle day, such that the subscript of each letter indicates the growth cycle day for which the comparison was made (i.e., on day one NST1, PANST1, NST2, and PANST2 were compared as all-pairs by Steel–Dwass method only for day 1), and they were not compared across multiple growth cycle days.
Figure 7.
Colorimetric analysis of sweet basil leaves grown in Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Error bars indicate standard deviation. Data not sharing the same upper case letters for the specific color value (L*, a* or b*) are significantly different from each other (Steel–Dwass test, p < 0.05).
Figure 7.
Colorimetric analysis of sweet basil leaves grown in Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Error bars indicate standard deviation. Data not sharing the same upper case letters for the specific color value (L*, a* or b*) are significantly different from each other (Steel–Dwass test, p < 0.05).
Table 1.
Macronutrient concentrations of NS and PANS on day 1.
Table 1.
Macronutrient concentrations of NS and PANS on day 1.
| Macro-Nutrient | Total N (ppm) | NH4-N (ppm) | NO3-N (ppm) | P (ppm) | K (ppm) | Ca (ppm) | Mg (ppm) | S (ppm) |
|---|
| Optimum value | 150–200 | - | - | 50 | 200 | 150 | 80 | 60 |
| NS | 159 ± 2 | 9.4 ± 0.01 | 149.5 ± 1.5 | 49.1 ± 12.5 | 176 ± 38 | 142 ± 17 | 49.6 ± 4.9 | 77.4 ± 7.8 |
| PANS | 170.5 ± 2.5 | 9.9 ± 0.07 | 160.5 ± 2.5 | 44.7 ± 6.8 | 191 ± 43 | 147.5 ± 21.5 | 51 ± 3.6 | 78.8 ± 7 |
| Micronutrient | Mn (ppm) | Zn (ppm) | Cu (ppm) | B (ppm) | Na (ppm) | Cl (ppm) | Fe (ppm) |
| Optimum value | 0.8 | 0.3 | 0.2 | 0.7 | - | - | 2.8 |
| NST | 0.38 ± 0.03 | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.46 ± 0.04 | 26.3 ± 2.8 | 28.2 ± 14.8 | 2.4 ± 0.2 |
| PANST | 0.4 ± 0.03 | 0.2 ± 0.03 | 0.2 ± 0.04 | 0.4 ± 0.04 | 26.4 ± 2.6 | 28.6 ± 2.6 | 2.5 ± 0.2 |
Table 2.
Nitrate–nitrogen (NO3-N) and nitrite–nitrogen (NO2-N) concentration on day 1 for Treatment 1 for nutrient solution (NST1) and plasma-activated nutrient solution (PANST1), and for Treatment 2 for nutrient solution (NST2) and plasma-activated nutrient solution (PANST2) measured weekly (days 1, 8, and 15 of growth cycle). Note: Data points are presented as averages (n = 9) with error bars indicating standard deviation.
Table 2.
Nitrate–nitrogen (NO3-N) and nitrite–nitrogen (NO2-N) concentration on day 1 for Treatment 1 for nutrient solution (NST1) and plasma-activated nutrient solution (PANST1), and for Treatment 2 for nutrient solution (NST2) and plasma-activated nutrient solution (PANST2) measured weekly (days 1, 8, and 15 of growth cycle). Note: Data points are presented as averages (n = 9) with error bars indicating standard deviation.
| Treatment | Reservoir | Day | Reactive Nitrogen Species |
|---|
| NO3-N (ppm) | NO2-N (ppm) |
|---|
| 1 | NST1 | 1 | 151.9 ± 3.3 | 9.6 ± 1.6 |
| 1 | PANST1 | 1 | 191.9 ± 3.1 | 18.8 ± 1.9 |
| 2 | NST2 | 1 | 151 ± 1.2 | 9.04 ± 1.2 |
| 2 | PANST2 | 1 | 189.8 ± 1.7 | 18.6 ± 0.9 |
| 2 | NST2 | 8 | 153.1 ± 2.4 | 9.1 ± 0.8 |
| 2 | PANST2 | 8 | 191.6 ± 1.6 | 18.7 ± 1.1 |
| 2 | NST2 | 15 | 152.2 ± 1.3 | 8.7 ± 1.2 |
| 2 | PANST2 | 15 | 192.2 ± 1.5 | 18.4 ± 0.8 |
Table 3.
Basil plants’ growth parameters in terms of fresh and dry weight, moisture, number of branches and nodes, root length, leaf length, leaf width, and leaf index (LI) by Treatment 1 or Treatment 2. Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test for fresh weight, number of branches, root length, and number of nodes; all other parameters were analyzed with Tukey’s HSD test, p < 0.05).
Table 3.
Basil plants’ growth parameters in terms of fresh and dry weight, moisture, number of branches and nodes, root length, leaf length, leaf width, and leaf index (LI) by Treatment 1 or Treatment 2. Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test for fresh weight, number of branches, root length, and number of nodes; all other parameters were analyzed with Tukey’s HSD test, p < 0.05).
| Parameters | Treatment 1 | Treatment 2 |
|---|
| NST1 | PANST1 | NST2 | PANST2 |
|---|
| Fresh weight (g) | 35.0 ± 1.1 c | 40.4 ± 1.4 a | 31.2 ± 3.5 b | 37.7 ± 2.4 a,c |
| Dry weight (g) | 3.0 ± 0.3 b,c | 3.8 ± 0.3 a | 2.6 ± 0.4 c | 3.4 ± 0.4 a,b |
| Moisture (%) | 91.5 ± 0.7 a | 90.5 ± 0.8 a | 91.5 ± 0.8 a | 90.8 ± 0.8 a |
| Number of branches | 9.4 ± 1.1 a | 9.2 ± 1.2 a | 8.1 ± 0.7 b | 8.7 ± 0.4 a,b |
| Number of nodes | 3.5 ± 0.4 a | 3.6 ± 0.4 a | 3.1 ± 0.2 a | 3.2 ± 0.2 a |
| Root length (mm) | 15.2 ± 0.8 a | 13.6 ± 0.7 b | 15.4 ± 0.9 a | 13.9 ± 0.9 b |
| Length (mm) | 9.5 ± 0.8 a | 10.0 ± 0.7 a | 9.3 ± 0.9 a | 10.0 ± 0.7 a |
| Width (mm) | 7.3 ± 0.9 b | 7.6 ± 0.8 b | 7.9 ± 0.6 a,b | 8.3 ± 0.5 a |
Table 4.
Peak rupture force, leaf toughness, and Young’s modulus of basil leaves on day 21 grown in Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test, p < 0.05).
Table 4.
Peak rupture force, leaf toughness, and Young’s modulus of basil leaves on day 21 grown in Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test, p < 0.05).
| Parameter | Treatment 1 | Treatment 2 |
|---|
| NST1 | PANST1 | NST2 | PANST2 |
|---|
| Peak rupture force (N) | 0.8 ± 0.1 a | 0.8 ± 0.2 a | 0.8 ± 0.3 a | 0.8 ± 0.2 a |
| Toughness (N.mm) | 0.6 ± 0.2 a | 0.6 ± 0.3 a | 0.6 ± 0.3 a | 0.6 ± 0.2 a |
| Young’s modulus (N/mm2) | (3.9 ± 8.7) × 109 a | (4 ± 0.8) × 109 a | (5.7 ± 1.0) × 109 a | (3.9 ± 0.8) × 109 a |
Table 5.
Relative peak area of selected essential oils commonly present in basil leaves’ aroma profile, measured on basil leaves on day 21 for Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test, p < 0.05).
Table 5.
Relative peak area of selected essential oils commonly present in basil leaves’ aroma profile, measured on basil leaves on day 21 for Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2). Note: Any two means within a row not sharing the same letter are significantly different from each other (Steel–Dwass test, p < 0.05).
| Aroma Compound | Treatment 1 | Treatment 2 |
|---|
| NST1 | PANST1 | NST2 | PANST2 |
|---|
| Eucalyptol (%) | 10.7 ± 4.00 a | 10.7 ± 2.01 a | 9.4 ± 2.3 a | 8.8 ± 0.9 a |
| Eugenol (%) | 0.4 ± 0.3 a | 0.6 ± 0.3 a | 0.1 ± 0.1 a | 0.1 ± 0.03 a |
| Linalool (%) | 55.5 ± 6.9 c | 67.7 ± 3.5 b | 78.5 ± 4.03 a | 80.01 ± 2.5 a |
| Methyl eugenol (%) | 0.8 ± 0.5 a,b | 1.2 ± 0.6 a | 0.5 ± 0.1 b | 0.8 ± 0.3 a,b |
Table 6.
Concentration of nutrients necessary for plant growth, measured on basil leaves on day 21 for Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2).
Table 6.
Concentration of nutrients necessary for plant growth, measured on basil leaves on day 21 for Treatment 1 (NST1 and PANST1) and Treatment 2 (NST2 and PANST2).
| Nutrient | Sufficiency Range | Treatment 1 | Treatment 2 |
|---|
| NST1 | PANST1 | NST2 | PANST2 |
|---|
| N (%) | 4–6 | 6.2 ± 0.2 | 6.4 ± 0.1 | 5.9 ± 0.5 | 6 ± 0.2 |
| P (%) | 0.62–1 | 0.8 ± 0.1 | 0.8 | 0.6 ± 0.04 | 0.6 ± 0.02 |
| K (%) | 1.55–2.05 | 6.8 ± 0.04 | 6.6 ± 0.2 | 5.8 ± 0.3 | 5.7 ± 0.3 |
| Ca (%) | 1.25–2 | 2.5 ± 0.07 | 2.5 ± 0.1 | 2.5 ± 0.04 | 2.4 ± 0.1 |
| Mg (%) | 0.6–1 | 0.5 ± 0.02 | 0.5 ± 0.02 | 0.5 ± 0.02 | 0.5 ± 0.03 |
| S (%) | 0.2–0.6 | 0.4 ± 0.04 | 0.5 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.04 |
| Mn (ppm) | 30–150 | 38 ± 7.6 | 36.9 ± 5.8 | 43.4 ± 0.8 | 39.4 ± 3.1 |
| Zn (ppm) | 30–70 | 39 ± 5.0 | 38.8 ± 0.3 | 37.4 ± 10 | 36.3 ± 14.2 |
| Cu (ppm) | 5–10 | 11.6 ± 1.5 | 12.8 ± 0.8 | 10.9 ± 2 | 11.6 ± 3.2 |
| B (ppm) | 25–60 | 48.1 ± 1.3 | 54.3 ± 5.5 | 40.8 ± 6 | 41.5 ± 3.2 |
| Fe (ppm) | 75–200 | 109.5 ± 8.5 | 57.7 ± 56.2 | 101.9 ± 6.6 | 95.4 ± 6.7 |
Table 7.
Total energy consumption during the 21 day growth period.
Table 7.
Total energy consumption during the 21 day growth period.
| | Treatment 1 | Treatment 2 |
|---|
| Equipment | Time (h) | Energy (kWh) | Time (h) | Energy (kWh) |
|---|
| Lights | 336 | 72.2 | 336 | 72.2 |
| Plasma generator | 2.1 | 1 | 6.3 | 3 |
| Pump | 22.4 | 1.2 | 22.4 | 1.2 |
| Fan | 504 | 0.5 | 504 | 0.5 |
| Total energy consumption | 74.9 | 76.9 |