Application of Cytokinin under Modified Atmosphere (MA) Delays Yellowing and Prolongs the Vase Life of Davallia solida (G. Forst.) Sw. Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Cytokinin Pulsing Treatments
2.2.1. Effect of CKs Pulsing on Delaying Leaf Yellowing and Extending the Vase Life of D. Solida Leaves
2.2.2. Simulations of the D. solida Leaves Vase Life after CKs Pulsing Treatment and Storage in a Modified Atmosphere Packaging (MAP)
2.2.3. Optimal Pre-storage Conditions of D. solida on Activities of Chlorophyll-Degrading Enzymes
2.3. Measurement of Fresh Weight and Water Uptake
2.4. Measurement of Yellowing and Vase Life of Leaves
2.5. Measurement of Chlorophyll Content
2.6. Measurement of Activities of Chlorophyll-Degrading Enzymes
- Preparation of acetone powder [47]
- Preparation of chlorophyll a
- Preparation of chlorophyllin a
- Preparation of pheophytin a
- Analysis of chlorophyll degrading enzyme activities
- Chlorophyllase activity (Chlase)
- Chlorophyll-degrading peroxidaseactivity (Chl-POX)
- Mg-dechelatase activity (MD)
- Pheophytinese activity (PPH)
2.7. Experimental Design and Statistics Analysis
3. Results
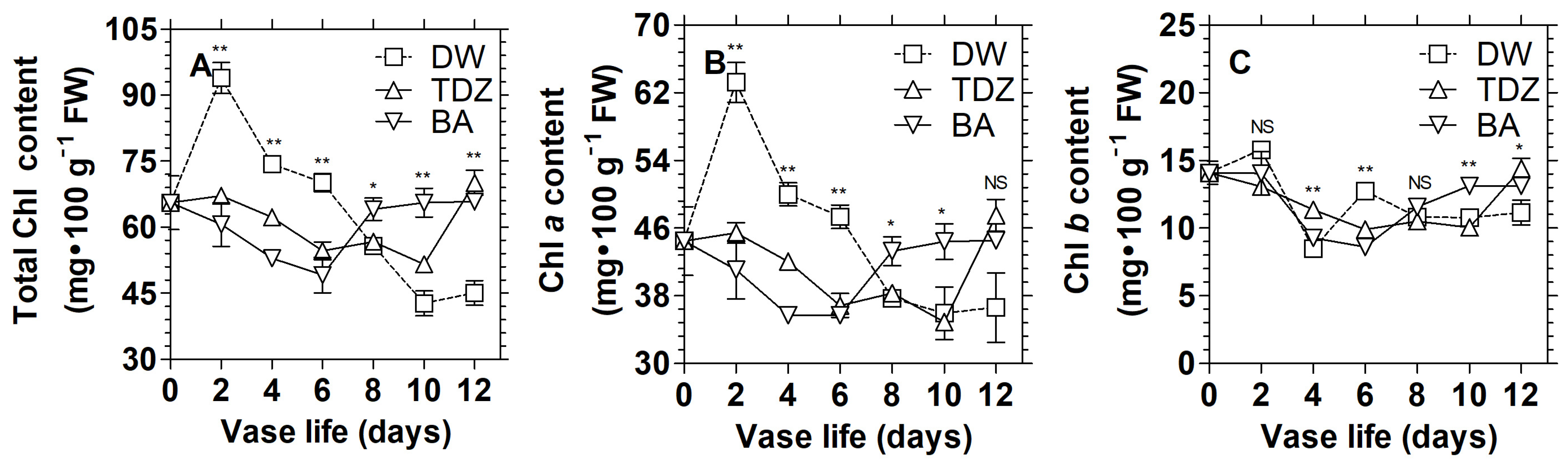
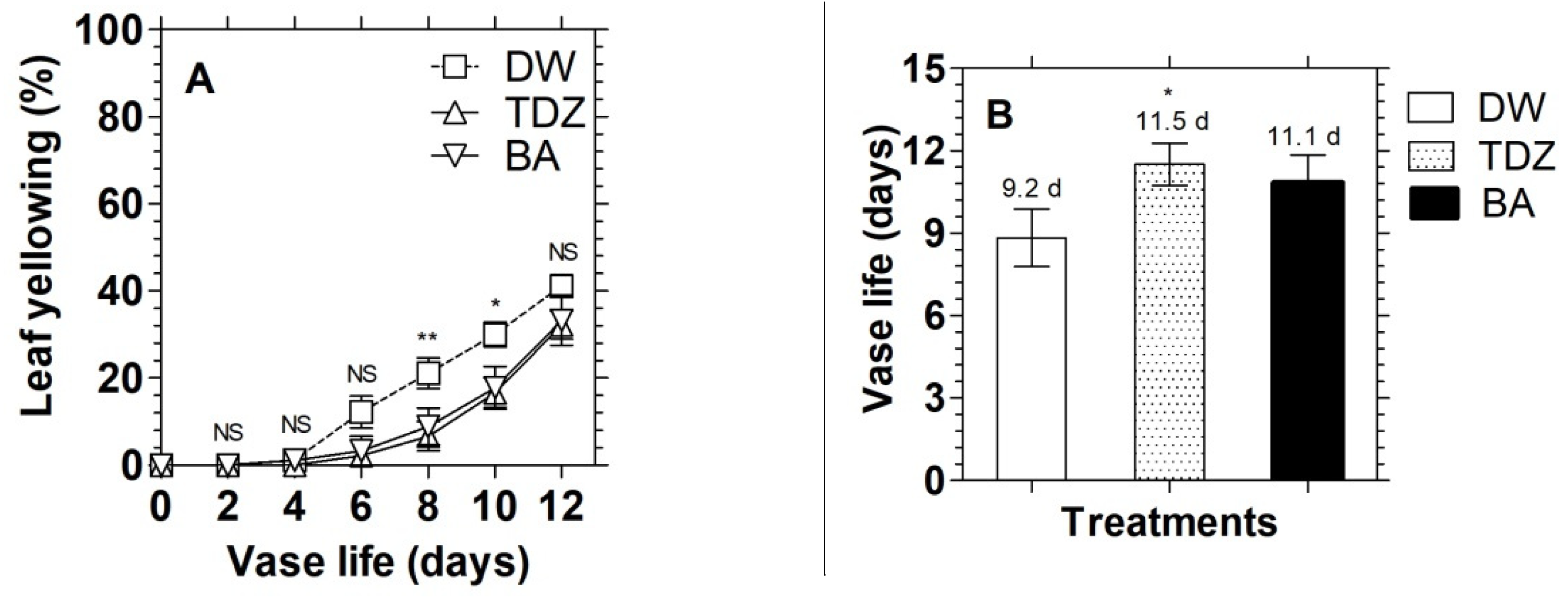
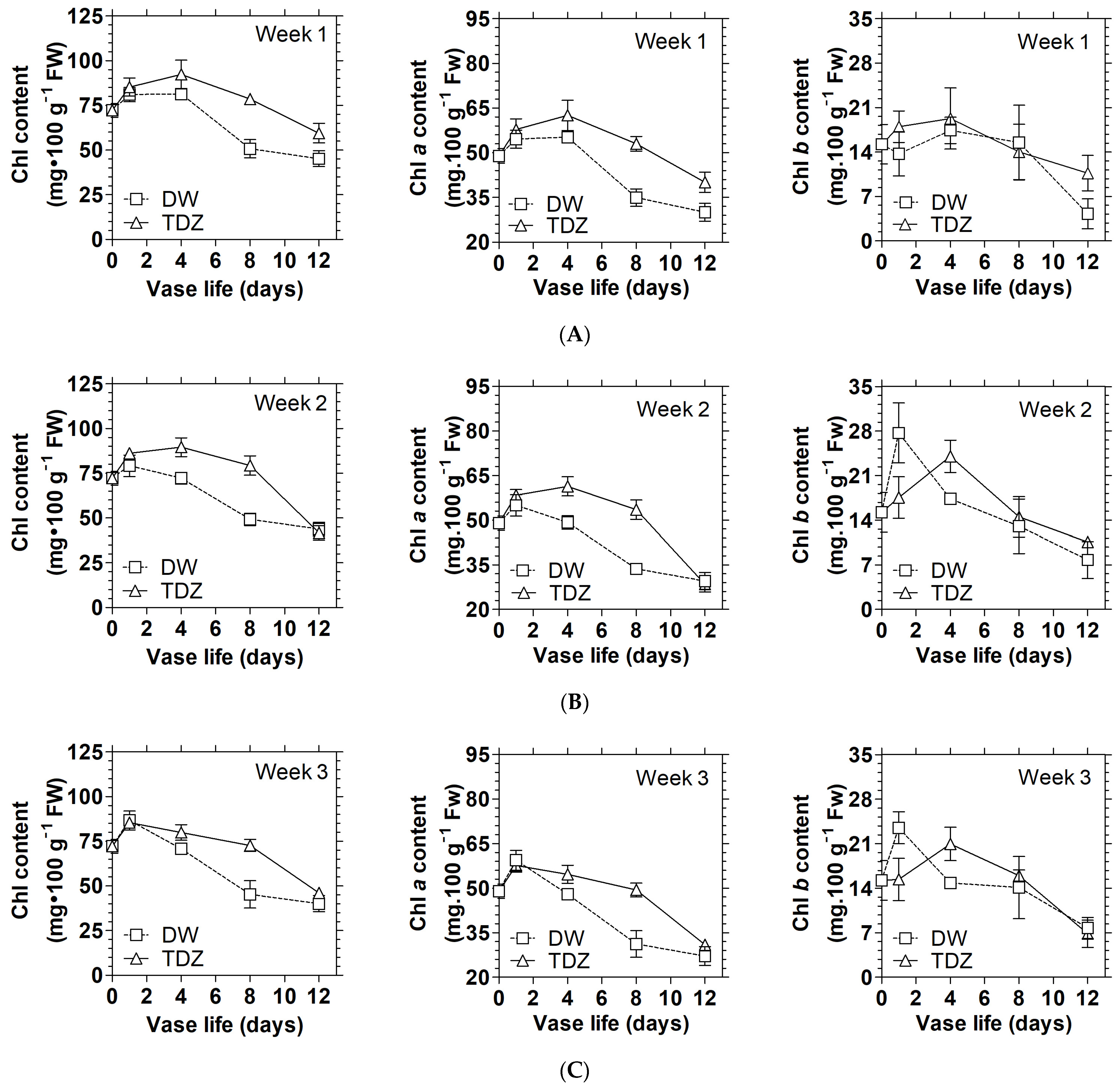
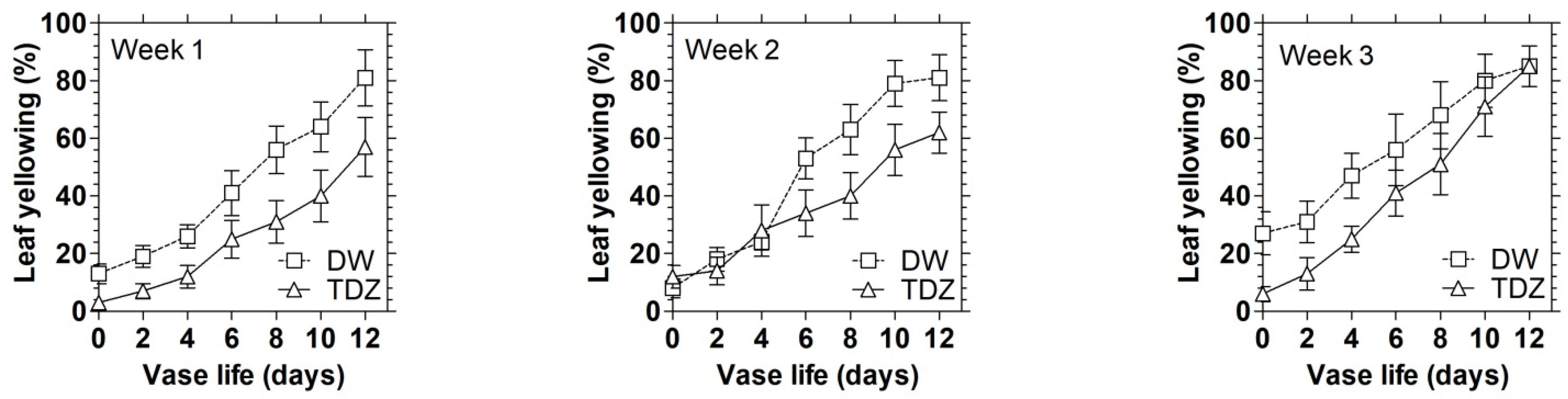
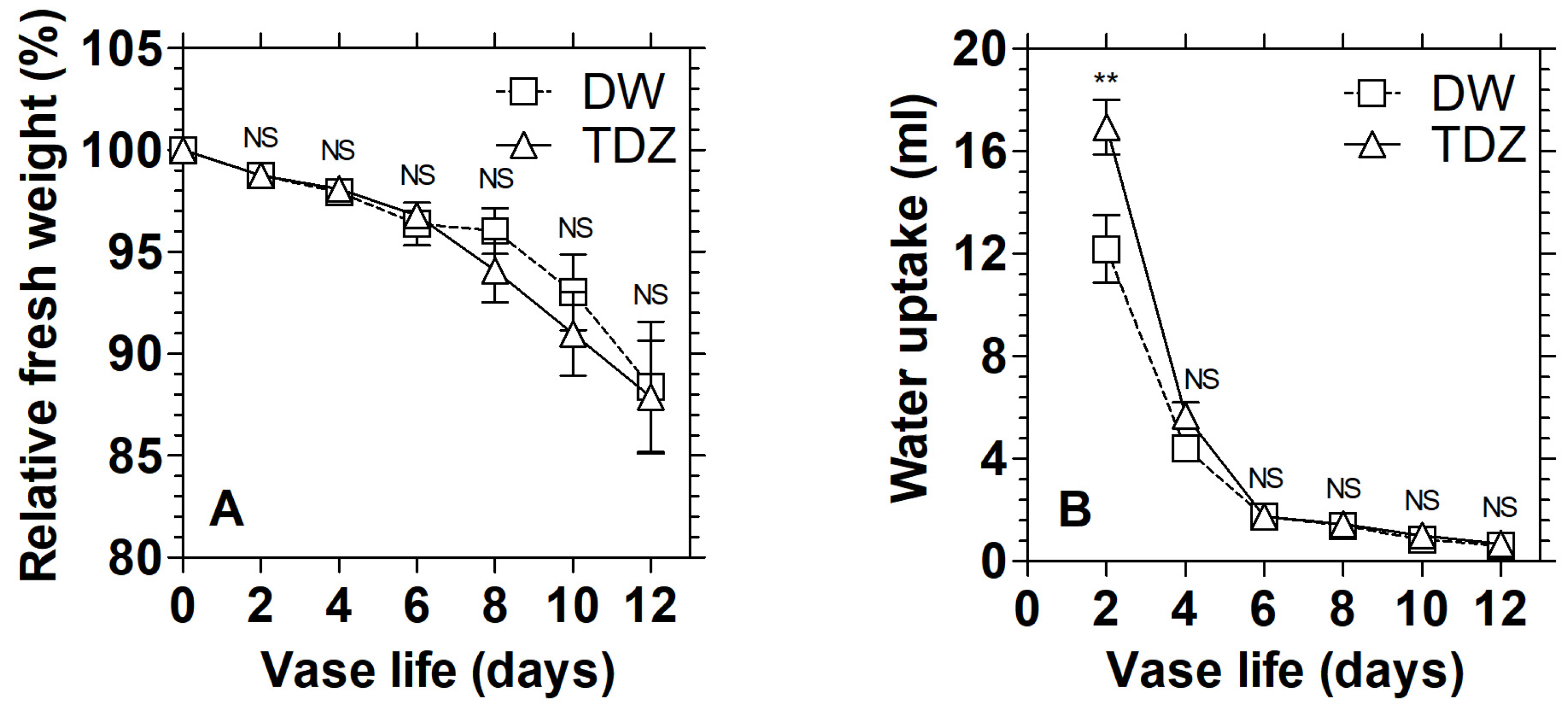
3.1. Effects of Cytokinins on Chlorophyll Content and Display Quality of D. solida
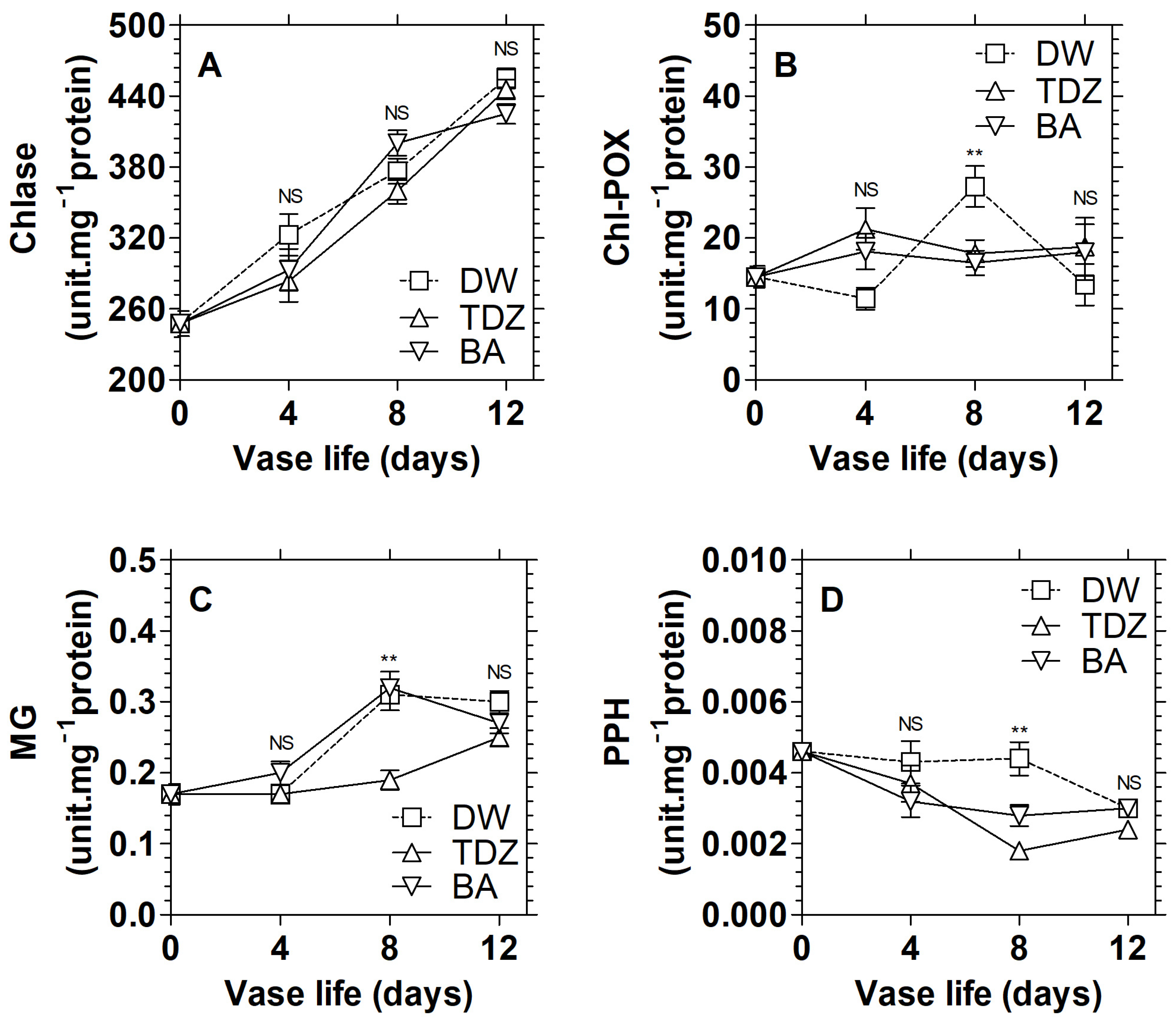
3.2. Effects of Cytokinins on the Activities of Chlorophyll Catabolism Enzymes in D. solida
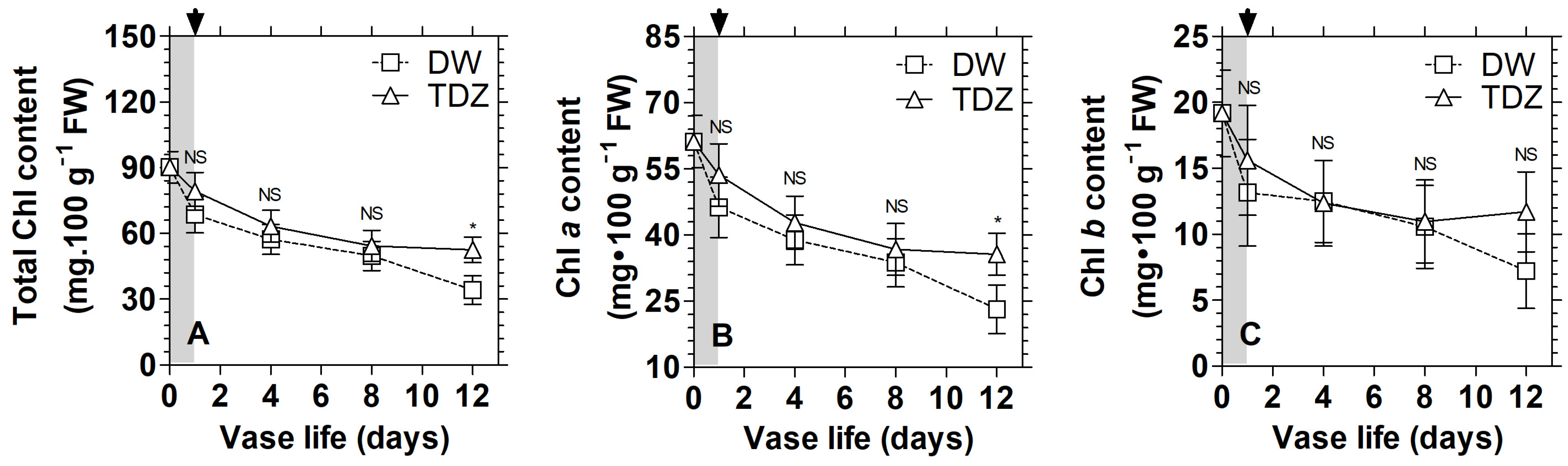
3.3. Effects of TDZ under MAP on the Display Quality of D. solida Leaves after Storage
3.4. Effects of TDZ under MAP on the Display Quality of D. solida Leaves after Storage
3.5. Effects of TDZ under MAP on the Activities of Chlorophyll Catabolism Enzymes in D. solida Leaves after Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacifici, S.; Burchi, G.; Del Carlo, A.; Ferrante, A. Effect of storage temperature and duration on vase life of cut Ruscus racemosus L. foliage. Acta Hortic. 2012, 970, 69–74. [Google Scholar] [CrossRef]
- Stamp, R.H.; Conover, C.A. Cut foliage production in Florida. Hort. Sci. 1986, 21, 178–339. [Google Scholar] [CrossRef]
- Stamp, R.H. Vase life characteristics of fern. Acta Hortic. 2007, 755, 155–162. [Google Scholar] [CrossRef]
- Stamps, R.H. Effects of postharvest dip treatments on leatherleaf fern (Rumohra adiantiformis) frond vase life. Acta Hortic. 2001, 543, 299–303. [Google Scholar] [CrossRef]
- Tatmala, N.; Kaewsuksaeng, S.; Kanlayanarat, S.; Buanong, M. Effect of thidiazuron holding treatments on delaying the senescence of Davallia ferns. Acta Hortic. 2012, 937, 463–466. [Google Scholar] [CrossRef]
- Kowwilaisaeng, P.; Teerarak, M. Biochemical changes during frond senescence of Rabbit’s Foot Fern (Davallia sp.) postharvest. Thai Sci. Technol. J. 2022, 30, 85–96. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R. Growth regulators and post-harvest longevity of Florists’ Greens. Agriculture 2022, 12, 1375. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V. The molecular biology of leaf senescence. J. Exp. Bot. 1997, 48, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Noode’n, L.D. Whole plant senescence. In Senescence and Aging in Plants; Noode’n, L.D., Thomas, H., Bortlik, K., Rentsch, D., Schellenberg, M., Matile, P., Leopold, A.C., Eds.; Academic, Catabolism of Chlorophyll In Vivo: Significance of Polar Press Inc.: Cambridge, MA, USA, 1989; pp. 391–439. [Google Scholar]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Matile, P. Biochemistry of Indian summer: Physiology of autumnal leaf coloration. Exp. Gerontol. 2000, 35, 145–158. [Google Scholar] [CrossRef]
- Wüthrich, K.L.; Bovet, L.; Hunziker, P.E.; Donnison, I.S.; Hörtensteiner, S. Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J. 2000, 21, 189–198. [Google Scholar] [CrossRef]
- Pruzinská, A.; Anders, I.; Aubry, S.; Schenk, N.; Tapernoux-Lüthi, E.; Müller, T.; Kräutler, B.; Hörtensteiner, S. In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 2007, 19, 369–387. [Google Scholar] [CrossRef] [Green Version]
- Pružinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Hirashima, M.; Satoh, S.; Tanaka, A. The Arabidopsis accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: Inhibition of the pheophorbide a oxygenase activity does not lead to the stay-green phenotype in Arabidopsis. Plant Cell Physiol. 2003, 44, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Greenberg, J.T. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 2006, 18, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Christ, B.; Schelbert, S.; Aubry, S.; Süssenbacher, I.; Müller, T.; Kräutler, B.; Hörtensteiner, S. MES16, a member of the methylesterase protein family, specifically demethylates fluorescent chlorophyll catabolites during chlorophyll breakdown in Arabidopsis. Plant Physiol. 2012, 158, 628–641. [Google Scholar] [CrossRef] [Green Version]
- Christ, B.; Süssenbacher, I.; Moser, S.; Bichsel, N.; Egert, A.; Müller, T.; Kräutler, B.; Hörtensteiner, S. Cytochrome P450 CYP89A9 is involved in the formation of major chlorophyll catabolites during leaf senescence in Arabidopsis. Plant Cell 2013, 25, 1868–1880. [Google Scholar] [CrossRef] [Green Version]
- Hauenstein, M.; Christ, B.; Das, A.; Aubry, S.; Hörtensteiner, S. A role for TIC55 as a hydroxylase of phyllobilins, the products of chlorophyll breakdown during plant senescence. Plant Cell 2016, 28, 2510–2527. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, N.; Funamoto, Y.; Shigyo, M. Peroxidase-mediated chlorophyll degradation in horticultural crops. Phytochem. Rev. 2004, 3, 221–228. [Google Scholar] [CrossRef]
- Aiamla-or, S.; Kaewsuksaeng, S.; Marayoshi, S.; Yamauchi, N. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem. 2010, 120, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaewsuksaeng, S. Chlorophyll degradation in horticultural crops. Walailak J. Sci. Technol. 2011, 8, 9–19. [Google Scholar]
- Teerarak, M.; Laosinwattana, C. Essential oil from ginger as a novel agent in delaying senescence of cut fronds of the fern (Davallia solida (G. Forst.) Sw.). Postharvest Biol. Technol. 2019, 156, 110927. [Google Scholar] [CrossRef]
- Tjosvold, S.A.; Wu, M.J.; Reid, M.S. Reduction of postproduction quality loss in potted miniature roses. Hort. Sci. 1994, 29, 293–294. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S. Role of sugar in the vase solution on postharvest flower and leaf quality of Oriental lily ‘Stargazer’. Hort. Sci. 2003, 38, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Skutnik, E.; Lukaszewska, A.; Tyborowska, K. Retarding senescence of cut leaves of Hosta plantaginea by growth regulators. Ann. Wars. Agric. Univ. SGGW Hortic. 1999, 20, 3–8. [Google Scholar]
- Asami, T.; Nakagawa, Y. Preface to the Special Issue: Brief review of plant hormones and their utilization in agriculture. J. Pestic. Sci. 2018, 43, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Argyros, R.D.; Mathews, D.E.; Chiang, Y.H.; Palmer, C.M.; Thibault, D.M.; Etheridge, N.; Argyros, D.A.; Mason, M.G.; Kieber, J.J.; Schaller, G.E. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 2008, 20, 2102–2116. [Google Scholar] [CrossRef] [Green Version]
- Cortleven, A.; Schmulling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef] [Green Version]
- Cortleven, A.; Marg, I.; Yamburenko, M.V.; Schlicke, H.; Hill, K.; Grimm, B.; Schaller, G.E.; Schmülling, T. Cytokinin regulates the etioplast-chloroplast transition through the two-component signaling system and activation of chloroplast-related genes. Plant Physiol. 2016, 172, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Janowska, B.; Andrzejak, R.; Jakubowska, P.; Antkowiak, A.; Nawrot, D.; Krzaczkowska, A. The effect of growth regulators on the post-harvest longevity of leaves of the Alchemilla mollis (Bauser) Rothm. leaf longevity. Folia Hort. 2016, 28, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Janowska, B.; Grabowska, R.; Ratajczak, E. Post-harvest longevity of leaves of the Sea lavender (Limonium latifolium (Sm.) Kuntze) after application of growth regulators. Hort. Sci. 2013, 40, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Janowska, B.; Schroeter-Zakrzewska, A. Effect of gibberellic acid, benzyladenine and 8-hydroxyquinoline sulphate on post-harvest leaf longevity of Arum italicum Mill. Zesz. Probl. Post. Nauk Roln. 2008, 525, 181–187. [Google Scholar]
- Skutnik, E.; Rabiza-Świder, J. Effect of pulsing with growth regulators on senescence of the detached cold-stored leaves of Zantedeschia aethiopica Spr. and Hosta ‘Undulata Erromena’. Acta Sci. Pol. Hort. Cultus. 2005, 4, 101–110. [Google Scholar]
- Mok, M.C.; Mok, D.W.S.; Armstrong, D.J.; Shudo, K.; Isogai, Y.; Okamoto, T. Cytokinin activity of N-phenyl-N′-1,2,3 thidiazol-5-ylurea (thidiazuron). Phytochem. Rev. 1982, 21, 1509–1511. [Google Scholar] [CrossRef]
- Suttle, J.C. Cytokinin-induced ethylene biosynthesis in non senescing cotton leaves. Plant Physiol. 1986, 82, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Genkov, T.; Ivanova, I. Effect of cytokinin-active phenylurea derivatives on shoot multiplication, peroxidase and superoxide dismutase activities of in vitro cultured carnation. Bulg. J. Plant Physiol. 1995, 21, 73. [Google Scholar]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Ferrante, A.; Hunter, D.A.; Wesley, P.H.; Reid, M.S. Thidiazuron—A potent inhibitor of leaf senescence in Alstroemeria. Postharvest Biol. Technol. 2002, 25, 333–338. [Google Scholar] [CrossRef]
- Ferrante, A.; Tognoni, F.; Mensuali-Sodi, A.; Serra, G. Treatment with thidiazuron for preventing leaf yellowing in cut tulips and chrysanthemum. Acta Hortic. 2003, 624, 357–363. [Google Scholar] [CrossRef]
- Sankhla, N.; Mackay, W.A.; Davis, T.D. Reduction of flower abscission and leaf senescence in cut phlox inflorescence by thidiazuron. Acta Hortic. 2003, 628, 837–841. [Google Scholar] [CrossRef]
- Sankhla, N.; Mackay, W.A.; Davis, T.D. Effect of thidiazuron on senescence of flowers in cut inflorescences of Lupinus densiflorus Benth. Acta Hortic. 2005, 669, 239–243. [Google Scholar] [CrossRef]
- Jiang, C.-Z.; Wu, L.; Macnish, A.J.; King, A.; Yi, M.; Reid, M.S. Thidiazuron, a non-metabolized cytokinin, shows promise in extending the life of potted plants. Acta Hortic. 2008, 847, 59–65. [Google Scholar] [CrossRef]
- Bryan, J.A.; Seiler, J.R. Accelerating fraser fir seedling growth with benzylaminopurine sprays. Hort. Sci. 1991, 26, 389–390. [Google Scholar] [CrossRef] [Green Version]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Arkus, K.A.J.; Cahoon, E.B.; Jez, J.M. Mechanistic analytic of wheat chlorophyllase. Arch. Biochem. Biophys. 2005, 438, 146–155. [Google Scholar] [CrossRef]
- Costa, M.L.; Civello, P.M.; Chaves, A.R.; Martinez, G.A. Effect of ethephon and 6-benzylaminopurine on chlorophyll degrading enzymes and a peroxidase-linked chlorophyll bleaching during postharvest senescence of broccoli (Brassica oleracea L.) at 20oC. Postharvest Biol. Technol. 2005, 35, 191–199. [Google Scholar] [CrossRef]
- Perkins, H.J.; Roberts, D.W. Purification of chlorophylls, pheophytins and pheophobides for specific activity determinations. Biochim. Biophys. Acta 1962, 58, 486–498. [Google Scholar] [CrossRef]
- Suzuki, T.; Shioi, Y. Re-examination of Mg-dechelation reaction in the degradation of chlorophylls using chlorophyllin a as a substrate. Photosynth. Res. 2002, 74, 217–223. [Google Scholar] [CrossRef]
- Bradford, M.M. A dye binding assay for protein. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hörtensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 2007, 19, 1007–1022. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, N.; Harada, K.; Watada, A.E. In vitro chlorophyll degradation in stored broccoli (Brassica oleracea L var. italica Plen) florets. Postharvest Biol. Technol. 1997, 12, 239–245. [Google Scholar] [CrossRef]
- Costa, M.L.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. Characterization of Mg-dechelatase activity obtained from Fragaria × ananassa fruit. Plant Physiol. Biochem. 2002, 40, 111–118. [Google Scholar] [CrossRef]
- Ginsburg, S.; Matile, P. Identification of catabolites of chlorophyll-porphyrin in senescent rape cotyledons. Plant Physiol. 1993, 102, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Dupont, J.; Siegenthaler, P.-A. A parallel study of pigment bleaching and cytochrome breakdown during aging of thylakoid membranes. Plant Cell Physiol. 1986, 27, 473–484. [Google Scholar]
- Merzlyak, M.N.; Hendry, G.A.F. Free radical metabolism, pigment degradation and lipid peroxidation in leaves during senescence. Proc. R. Soc. Edinb B Biol. Sci. 1994, 102, 459–471. [Google Scholar] [CrossRef]
- Breusegem, F.V.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Taverner, E.; Letham, D.S.; Wang, J.; Cornish, E.; Willcocks, D.A. Influence of ethylene on cytokinin metabolism in relation to Petunia corolla senescence. Phytochem. Rev. 1999, 51, 341–347. [Google Scholar] [CrossRef]
- Talla, S.K.; Panigrahy, M.; Kappara, S.; Nirosha, P.; Neelamraju, S.; Ramanan, R. Cytokinin delays dark-induced senescence in rice by maintaining the chlorophyll cycle and photosynthetic complexes. J. Exp. Bot. 2016, 67, 1839–1851. [Google Scholar] [CrossRef] [Green Version]
- McCabe, M.S.; Garratt, L.C.; Schepers, F.; Jordi, W.J.; Stoopen, G.M.; Davelaar, E.; van Rhijn, J.H.A.; Power, J.B.; Davey, M.R. Effects of P(SAG12)-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol. 2001, 127, 505–516. [Google Scholar] [CrossRef]
- Ori, N.; Juarez, M.T.; Jackson, D.; Yamaguchi, J.; Banowetz, G.M.; Hake, S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence activated promoter. Plant Cell 1999, 11, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Swartzberg, D.; Dai, N.; Gan, S.; Amasino, R.; Granot, D. Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants. Plant Biol. 2006, 8, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Karanov, E.N.; Iliev, L.K.; Georgiev, G.T.; Tsolova, M.; Alexieva, V.; Puneva, I. Physiology and application of phenylurea cytokinins. In Progress in Plant Growth Regulation; Karssen, C.M., van Loon, L.C., Vreugdenhil, D., Eds.; Kluwer Academic Publichers: Dordrecht, The Netherlands, 1992; pp. 841–850. [Google Scholar]
- Mutui, T.M.; Mibus, H.; Serek, M. Influence of thidiazuron, ethylene, abscisic acid and dark storage on the expression levels of ethylene receptors (ETR) and ACC synthase (ACS) genes in Pelargonium. Plant Growth Regul. 2007, 53, 87–96. [Google Scholar] [CrossRef]
- Ferrante, A.; Trivellini, A.; Mensuali-Sodi, A. Interaction of 1-methylcyclopropene and thidiazuron on cut stock flowers vase life. Open Hort. J. 2012, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef] [Green Version]
- Minguez-Mosquera, M.I.; Gallardo-Guerrero, L. Role of chlorophyllase in chlorophyll metabolism in olives cv. Grodal. Ann. Phytochem. 1996, 41, 691–697. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watada, A.E. Regulated chlorophyll degradation in spinach leaves during storage. Phytol Amer. Soc. Hortic. Sci. 1991, 116, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Gandul-rajas, B.; Roca, M.; Minguez-mosquera, M.I. Chlorophyll and carotenoid degradation mediated by thylakoid-associated peroxidative activity in olives (Olea europaea) cv. Hojiblanca. J. Plant Physiol. 2004, 161, 499–507. [Google Scholar] [CrossRef]
- Owens, T.G.; Falkowski, P.G. Enzymatic degradation of chlorophyll a by marine phytoplankton in vitro. Phytochem. Rev. 1982, 21, 979–984. [Google Scholar] [CrossRef]
- Morita, R.; Sato, Y.; Masuda, Y.; Nishimura, M.; Kusaba, M. Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009, 59, 940–952. [Google Scholar] [CrossRef]
- Capelle, S.C.; Mok, D.W.S.; Kirchner, S.C.; Mok, M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of N6-(Δ2-isopentenyl)[8-14C] adenosine in callus tissues of Phaseolus lunatus L. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Stamps, R.H.; Chase, A.R. Foliar applications of benomyl and mancozeb do not affect leatherleaf fern carbon assimilation, transpiration, light compensation point or vase life. Proc. Fla. State Hort. Soc. 1988, 100, 362–364. [Google Scholar]
- Singh, P.; Singh, K.; Kumar, R. Study on refrigerate storage of Nephrolepis fronds. J. Fruit Orn. Plant Res. 2003, 11, 121–126. [Google Scholar]
- Kraepiel, Y.; Miginiac, E. Photomorphogenesis and phytohormones. Plant Cell Env. 1997, 20, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Flores, S.; Tobin, E.M. Benzyladenine modulation of the expression of two genes for nuclear encoded chloroplast proteins in Lemna gibba: Apparent posttranscriptional regulation. Planta 1986, 168, 340–349. [Google Scholar] [CrossRef]
- Banowetz, G.M. Cultivars of hexaploid wheat of contrasting stature and chlorophyll retention differ in cytokinin content and responsiveness. Ann. Bot. 1997, 79, 185–190. [Google Scholar] [CrossRef] [Green Version]


| Treatments | Vase Life (Days) 1/ | |
|---|---|---|
| Solution | Storage Period (Weeks) | |
| DW | 5.2 b | |
| TDZ | 8.0 a | |
| F-test | ** | |
| 1 | 7.9 a | |
| 2 | 6.7 ab | |
| 3 | 5.2 b | |
| F-test | * | |
| DW | 1 | 6.2 bc |
| 2 | 5.8 bc | |
| 3 | 3.6 c | |
| TDZ | 1 | 9.6 a |
| 2 | 7.6 ab | |
| 3 | 6.8 abc | |
| F-test | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngamkham, P.; Srilaong, V.; Wongs-Aree, C.; Buanong, M. Application of Cytokinin under Modified Atmosphere (MA) Delays Yellowing and Prolongs the Vase Life of Davallia solida (G. Forst.) Sw. Leaves. Agriculture 2023, 13, 463. https://doi.org/10.3390/agriculture13020463
Ngamkham P, Srilaong V, Wongs-Aree C, Buanong M. Application of Cytokinin under Modified Atmosphere (MA) Delays Yellowing and Prolongs the Vase Life of Davallia solida (G. Forst.) Sw. Leaves. Agriculture. 2023; 13(2):463. https://doi.org/10.3390/agriculture13020463
Chicago/Turabian StyleNgamkham, Pattaraporn, Varit Srilaong, Chalermchai Wongs-Aree, and Mantana Buanong. 2023. "Application of Cytokinin under Modified Atmosphere (MA) Delays Yellowing and Prolongs the Vase Life of Davallia solida (G. Forst.) Sw. Leaves" Agriculture 13, no. 2: 463. https://doi.org/10.3390/agriculture13020463
APA StyleNgamkham, P., Srilaong, V., Wongs-Aree, C., & Buanong, M. (2023). Application of Cytokinin under Modified Atmosphere (MA) Delays Yellowing and Prolongs the Vase Life of Davallia solida (G. Forst.) Sw. Leaves. Agriculture, 13(2), 463. https://doi.org/10.3390/agriculture13020463







