Abstract
Intermediate wheatgrass (Thinopyrum intermedium; IWG) is a perennial grass under development as a grain and forage crop. Although IWG is known for its ability to take up nitrate and improve water quality, seasonal nitrogen (N) demand and uptake by IWG is not well known. We measured IWG shoot, root, and grain production, tissue N concentration, and soil mineral N at multiple plant growth stages in 1- and 2-year-old IWG stands fertilized with various rates of N: (1) 80 kg N ha−1 applied at spring regrowth (spring), (2) 40–40 kg N ha−1 applied at spring regrowth and anthesis (split), and (3) an unfertilized control. We also calculated nitrogen use efficiency and biomass N yield. Soil mineral N, N-mineralization rates, and plant N concentration increased with fertilization, and lodging increased with spring fertilization, while root physiological N use efficiency (PNUE) declined with fertilization. Seasonally, shoot and root N concentration declined at physiological maturity, while shoot PNUE was highest at maturity, suggesting either that surplus N was allocated to grain or that more biomass was being produced per unit N taken up. In the 1-year-old stand, during fall regrowth, soil mineral N levels were among the lowest; however, the total soil N was highest compared with other sampling times, suggesting a large influx of organic N between physiological maturity and fall regrowth. Based on our results, IWG is well suited to use nitrogen inputs and avoid excess N leaching into groundwater, but it is also clear that IWG has strong seasonal N allocation patterns that should be taken into consideration with N recommendations and best practices.
1. Introduction
The ability to manage the nitrogen (N) balance of cropping systems in a way that meets crop N demand efficiently is critical for optimizing production without N losses to the environment. N losses to the environment not only cause eutrophication and environmental degradation but are also related to human health and equity crises worldwide [1,2]. Perennial cropping systems such as intermediate wheatgrass (IWG; Thinopyrum intermedium (Host) Barkworth and D.R. Dewey) can substantially improve environmental quality [3,4]. IWG is a cool-season grass used as a perennial grain crop, with potential as a dual-use grain and forage crop, that appears effective at taking up N, preventing off-target N transport to soils or water, and consequently can decrease nitrate leaching to groundwater by ten times compared to annual crops, under a variety of circumstances [5,6,7].
As a plant species and crop, IWG not only takes up significant amounts of N, but appears to be efficient at using N [6,8]. N fertilizer rates to maximize IWG grain yields are currently less than half the rates typically applied to Maize in the upper Midwest [6]. IWG’s extensive fibrous root system is likely the reason for this high N use efficiency [9], as is the case for other perennial grass cropping systems [10]. IWG has 12–16 times the root N content as annual wheat [11], with estimates of 2 to over 3.5 Mg ha−1 in 2-year-old stands [12,13]. This potential for a perennial crop to provide economic value through grain and forage production, while improving the important ecosystem service of reduced nitrate leaching, could be a pathway toward needed improvements in the environmental quality of agricultural lands.
The currently recommended best N fertilization practice for IWG is ~89 kg ha−1 per year as a single spring application [14]; however, second-year stands are likely N limited, even within or after the first growing season [9,14,15]. Increasing N deficiency with stand age has also been proposed as a cause for the IWG grain yield declines that occur after 2–3 years after establishment [16], limiting the economic viability of this crop. The amount of N removed from the grain and vegetative growth harvested each summer has been estimated at 40–75 kg ha−1 per year [17]. Even though the recommended rate is above this removal estimate, N is needed to support the 2 to 3.5 Mg ha−1 of root biomass, some of which dies and regrows annually. Questions remain around when N is needed most to support the growth of various plant tissues. For example, excess N fertilizer applied in spring can lead to disproportionally high increases in stem and leaf biomass, which can increase the risk of lodging, or the aboveground biomass falling over before grain maturity [14]. A first step to improving IWG crop management could then be to better understand the within-season N uptake, N allocation, and translocation of N across plant tissues over the growing season and as stands age, and how this may translate to both the most appropriate amount and timing of N.
Perennial grasses translocate N across plant organs seasonally to conserve nutrients. In agronomic systems, these efforts are usually focused on N content and uptake in vegetative aboveground biomass [18,19]; however, translocation occurs across all plant organs and is different among species. For example, some perennial grasses assimilate N into roots the fall then translocate it into aboveground tissues the subsequent spring [20], while others assimilate N throughout the growing season and then translocate that N to roots for storage over the winter. Nitrogen allocated to aboveground vegetative tissues may be maximized and highly efficient, especially during the earlier stages of plant development when light is a limiting resources [21]. At the same time, it is known that at certain times of year, in early spring and fall, IWG N content is relatively high, lending to its value as a dual-use grain and forage crop [22,23]. This suggests lower efficiency or biomass production per unit of N uptake or per unit of N added during those stages of vegetative growth. Seed N demand and allocation of N toward grain during physiological maturity can be important as it represents a large portion of N use and nutritional content in any grass crop; uniquely it is a primary economic output in IWG as one of the world’s first commercially viable perennial grain crops. Root N content is a key trait of IWG’s N use efficiency [9]; however, the seasonal dynamics in relation to soil’s available N pools have not been largely unexplored.
Synchronously matching crop N demand with enough, but not excess, available N through the growing season is critical for efficient N use [24]. The N allocation and demand of IWG at different growth stages could lead to different responses to N inputs over time in different plant organs. Adjusting N timing based on seasonal N need could be a strategy for improved N use. Instead of a single spring fertilizer application, the split application of fertilizer could increase seed yield, forage yield, and harvest index in cool-season grasses by allowing a greater percentage of N to be taken up by plants as they need it at key growth stages in the growing season [25,26,27,28,29]. A better understanding of the effects of splitting N fertilization in IWG on grain yield, forage yield, and soil N dynamics might allow researchers and producers to manage the crop more effectively.
The rates and timing of N fertilization happens within the context of the pool of the soil’s mineral N, which is continually being released through mineralization processes. N mineralization depends on the quantity of organic N in the soil, the soil temperature, and the soil moisture over the course of the growing season. The amount of available N, as well as the rate at which N is being mineralized into available N pools, adds to the N fertilizer as the total pool of N available for plant uptake, contributing to IWG’s fertility over time [15]. A wholistic perspective of IWG N use, seasonal reallocation, and available soil N across different strategies of fertilization will advance our knowledge of best practices for this new perennial grain crop. An assessment of the N balance of IWG grain production systems, N use efficiency, and N uptake in the context of N inputs and soil N availability would be informative for producing profitable grain and forage yield [30] while also preventing N pollution to the environment.
Our objective was to understand the effect of N fertilization timing on soil mineral N concentration and mineralization rates, plant N content and allocation over time, and plant N use efficiency in 1- and 2-year-old IWG stands. We used three N fertilizer treatments (control, spring N application, and spring/summer split application) and sampled roots, shoots, grain, and soil at four times within a growing season when IWG was at agronomically important growth stages. Our hypotheses were as follows: We predicted that spring fertilization would lead to a higher nitrogen input efficiency (NIE; the difference in biomass yield in fertilized versus unfertilized plots, per N input) of shoots versus grain or roots because of spring and summer shoot investment to compete for light resources. We expected that because the split-fertilization treatment provided N at two times, the latter of which was during peak plant growth and flowering, it would lead to higher soil mineral N over the growing season, as well as higher shoot and grain N content, grain NIE, and higher grain yields. We predicted that the physiological N use efficiency (PNUE; or biomass yield per biomass nitrogen) would be highest when N was most limiting [per 9] during spring and fall regrowth and in unfertilized controls. Lastly, we predicted that the older 2-year-old stand would be more N efficient and conservative in nutrient use than a newly establishing stand.
2. Materials and Methods
2.1. Experimental Design and Location
Research plots were located at the Rosemount Research and Outreach Center (44°41′05″ N 93°04′13″ W). IWG variety TLI Cycle 5 was sown in 15 cm rows in two nearby fields at a seeding rate of 13 kg pure live seed ha−1. The 1-year-old stand was established in September of 2017 and the 2-year-old stand was established in September of 2016. The 2-year-old stand was fertilized at 80 kg N ha−1 in the establishment year (year prior to this study). In both fields, the soil was a Tallula silt loam (Coarse–silty, mixed, superactive, mesic Typic Hapludoll) and the previous crop was soybean (Glycine max L.). In the 2018 growing season, there were no climactic abnormalities that would have affected IWG production (Table S1).
A randomized complete block design with four replicates (one replicate randomly assigned to each of the four blocks within each field) was used to implement treatments in both the 1-year and 2-year-old stands. Plots sizes were 3.05 m by 4.57 m in the 1-year-old stand and 1.83 m by 6.1 m in the 2-year-old stand. The three fertilization treatments were (1) 80 kg N ha−1 urea in early May (spring); (2) 40 kg N ha−1 urea in early May plus 40 kg N ha−1 urea in mid-June (split); and (3) no fertilization (control). Fertilization took place on 4 May 2018 (2-year-old stand) and 7 May 2018 (1-year-old stand). The mid-June fertilizer application occurred on 12 June 2018 for the 2-year-old stand and 13 June 2018 for the 1-year-old stand. Fertilization took place immediately after plant sampling and before soil sampling and insertion of N-mineralization incubation cores. The exception was of the 2-year-old stand at spring regrowth, which was fertilized two days after plant sampling. Soil was sampled after fertilization so that the immediate effect of fertilization on soil N pools would be known, whereas immediate effects were not anticipated for plants. Plant and soil response variables (described below) were measured four times during the growing season to quantify N dynamics in IWG stands as they progressed through different growth stages (Table 1). Separate sample locations for all four timepoints were assigned randomly and marked within each plot at the beginning of the growing season. The growth stage index of IWG plants was determined by following the procedures of Moore and Moser [31]. Briefly, 1.0–1.9 is the tillering stage, in which leaves appear. Anthesis occurs at stage 3.7, and grain harvest occurs at stage 3.9, coinciding with plant physiological maturity. We measured response variables at the start of the growing season (spring regrowth), at anthesis (when flowering was evident in 50% of plants), after physiological maturity (maturity), and in autumn during fall regrowth.

Table 1.
Stage index, sampling date, day of year (DOY), and growing degree days (GDDs) accumulated to sampling date for four sampling times in 1-year-old and 2-year-old intermediate wheatgrass.
2.2. Plant Biomass, Grain Yield, and Biomass Nitrogen Yield
Aboveground IWG tissues were collected on dates that corresponded to key growth stages (see Table 1) by manually sampling three 33 cm by 15 cm quadrats (0.1394 m2 total sampled area) centered on the row. Placement of quadrats was assigned randomly within each plot and pre-determined for all sample timepoints to ensure representative sampling and to avoid interference with other data collection. Plants were harvested at the soil surface and cleaned. Tissue was dried at 35 °C until constant mass and recorded. At the physiological maturity sample date, grain was removed from dry plants after collection using a laboratory thresher (Wintersteiger LD-50) and weighed. Biomass yield (kg ha−1) was then calculated by dividing the mass by the total sampled area (0.1485 m2 = 0.000001485 ha). Since the quadrat width was the same as the planted width between rows, no correction was made for interrow space in the biomass per area calculations.
Root biomass was sampled using a hydraulic soil probe (Giddings Machine Company, Inc.; Colorado, USA) to the beginning of the gravel layer, approximately 90 cm deep in the soil. Two cores, each 5 cm in diameter, were randomly taken from each plot. Cores were partitioned into 0–15 cm, 15–30 cm, 30–45 cm, and 45–60 cm. Root cores were stored at −20 °C until analysis. Root biomass was separated from soil via a hydropneumatic elutriation system [32], which forced water through soil being held by a series of mesh screens until only biomass remained. Samples were cleaned by hand to remove aboveground tissue, rhizomes, and sand. Roots were then dried at 35 °C until a constant weight. Dry weights were recorded for each sample. Root biomass was then combined across depths for total N analysis. Roots collected at anthesis were not processed due to resource constraints. Dry mass data were then normalized by sampled area and reported as kg ha−1.
Plant N concentration and biomass N yield were determined for available aboveground biomass, grain, and root samples. Each sample was ground after drying and passed through a 1 mm sieve prior to determination of total percent N concentration on a combustion analyzer (Elementar Americas Inc., Ronkonkoma, NY, USA). Tissue N concentrations were then multiplied by biomass dry-matter yield to report tissue N as kg N ha−1. No aboveground percent of N data was available for aboveground tissues at spring regrowth for the 2-year-old stand, so tissue N concentration from anthesis, the closest possible sampling date, was used to calculate tissue N yield as kg N ha−1.
2.3. Lodging, and Plant Heights
Lodging and plant heights were measured at physiological maturity (late August, Table 1). Lodging was observed on a whole-plot basis by multiplying the percent of stems that were lodged by the degree to which they were lodged on a 0–100% scale. Percentages were then converted to a 10-point scale, where 10% = 1 and 90% = 9. The same individual determined each observed rating. Plant height was measured as the distance from the soil to the tip of the inflorescence on 5 randomly selected plants per plot. The 5 scores were then averaged to give one final value per plot.
2.4. Soil Total N and N Mineralization
Soil sampling took place after fertilization at the spring regrowth and anthesis timepoints, and after plant tissue sampling at the physiological maturity and fall regrowth samplings (see Table 1). At each timepoint, bulk soil samples for total N analysis were taken using the same hydrolytic probe method described for root sampling. Soil samples were homogenized and dried at 35 °C until constant mass, then analyzed for total C and N analysis via dry combustion (Brookside Laboratories, New Bremen, OH, USA). Percent of total N is reported on a per mass basis (mg N kg soil−1)
To collect soil for mineral N and in situ net N-mineralization analysis, sharpened 5 cm diameter by 17 cm PVC cores were used [33]. In situ cores had four evenly spaced 0.5 cm holes drilled in the top to allow oxygen exchange. In each plot, at each timepoint, two cores were installed 15 cm away from one another to a depth of 15 cm. Core placement was predetermined to ensure representative sampling and avoid interference with other data collection. One core was immediately removed, and the soil was placed in a cooler before transporting for mineral N analysis. The remaining core was left in situ, capped, and then extracted at the next phenological plant sampling point (37–77 days) for mineral N analysis. The difference between the mineral N in the in situ cores after incubation and the mineral N in the core removed at the initial time, divided by days of in situ incubation, was calculated as the net N mineralization between sampling timepoints (spring regrowth to anthesis, anthesis to maturity, and maturity to fall regrowth) reported as mg kg soil−1 day−1 [33]. An additional soil sample was taken at fall regrowth for the purpose of having mineral N from that timepoint to compare with N-mineralization rates. Fertilization always took place before capping in situ cores.
Once removed from the field, soil for mineral N analysis was stored at 4 °C and N was extracted within 7 days. First, 4.0 g +/− 0.05 g of wet soil was placed in an acid-washed or sterile 50 mL centrifuge tube. Then, 20 mL 2M KCl was added to each tube. Tubes were shaken for 1 h at 200 rpm, then centrifuged for 5 min at speed of 2500 rpm. Aliquot was then filtered through pre-rinsed Whatman #42 filter paper into an acid-washed 50 mL beaker before being stored in a scintillation vial at −20 °C.
Soil NH4+-N was determined using a well plate assay modified from Sinsabaugh, Reynolds, and Long [34]. In brief, 100 μL pre-prepared standards and soil extracts were transferred into a 96-well plate. Then, 40 μL ammonia salicylate was added to each well, converting ammonia to ammonium. Exactly 3 min after beginning to add the ammonia salicylate to the well plate, 40 μL ammonia cyanurate was added to the well plate. Once ammonia cyanurate was added to each well, the plate was incubated in the dark for 20 min. After incubation, the plate was read at 630 nm using a BioTek Synergy HT microplate spectrophotometer (BioTek, Winooski, VT, USA).
Soil NO3−-N was determined using a well plate assay modified from Doane and Horwath [35]. In brief, 100 μL pre-prepared standards and soil extracts were transferred into a 96-well plate. Then, 1 mL vanadium cocktail solution was added to each tube. Tubes were covered and incubated overnight. Between 15 and 15.5 h later, 200 μL was transferred from each tube into the well of a 96-well plate. The plate was then read at 540 nm using a BioTek Synergy HT microplate spectrophotometer (BioTek, Winooski, VT, USA). Standards were used to convert absorbance values to a concentration of N reported as mg ammonium or nitrate N kg−1.
2.5. Plant Nitrogen Use Efficiency
We calculated N use efficiency from both physiological [36] and biomass productivity [10] perspectives (Equations (1) and (2)):
We also measured N uptake to understand the seasonal perspective of IWG’s N use (Equation (3)):
where BY = dry biomass yield (regardless of treatment, kg ha−1), BNY = biomass N yield (kg N ha−1), BYtreatment = dry-matter biomass yield of fertilized plots, and BYcontrol = dry-matter biomass yield of unfertilized plots. N added = the N fertilization addition at the time of sampling (kg ha−1), which was 40 kg ha−1 at the anthesis timepoint and 80 kg ha−1 at maturity and fall regrowth. The variable timing of N-mineralization rate incubations did not allow for determining the N input from the soil at each timepoint. N uptake was calculated using total BNY (IWG + roots + grain). PNUE was calculated for shoots at all timepoints, grain at maturity, and roots at maturity and fall regrowth. NIE was calculated for shoots at anthesis, maturity, and fall regrowth, for grain at maturity, and for roots at maturity and fall regrowth. Fall regrowth is an optimal time to harvest forage for IWG dual-use management [23].
2.6. Statistical Analysis and Calculations
All analysis of variance was conducted in JMP pro (16.0, SAS Institute Inc., Cary, NC, USA). N fertilization treatment and sample timing were considered fixed effects, and block was considered a random effect. When analyzing total soil N, average sampling depth was considered a continuous variable. For NUE calculations, only shoot data available at more dates were analyzed with the main mixed-effects model described above. Additional statistics were performed on PNUE and NUE data with tissue type (grain, roots, shoots) as a fixed effect to determine differences in N use across IWG tissues. Data were analyzed separately for each stand age since the 2-year-old stand was subjected to different weather during its first year of growth. Post hoc analysis was conducted using Tukey’s HSD. Interquartile range analysis was used to determine and remove outliers with values outside three times the interquartile range of each variable prior to analysis.
3. Results
3.1. Above- and Belowground Biomass, Grain Yield, and Lodging
Grain yields averaged 386 +/− 51 kg ha−1 in the 1-year-old stand and 536 +/− 137 kg ha−1 in the 2-year-old stand. Fertilization treatment did not affect above- or belowground biomass in either stand age (Table 2). As expected, shoot biomass, root biomass, and whole-plant biomass (including grain at maturity) varied by sampling time in both stand ages, where shoot and total biomass was greatest at maturity and lowest at spring regrowth (Table 3). Root biomass was highest at fall regrowth in the 1-year-old stand, whereas in the 2-year-old stand, root biomass was greatest at maturity (Table 4). Although there was no fertilizer treatment effect on grain yield or plant height in the 1-year-old stand, lodging scores were nearly two times greater in the spring fertilization treatment than in the control treatment (Table 2), with averages of 5.5 +/− 0.45 and 2.7 +/− 0.45, respectively. In the 2-year-old stand, grain yields and lodging scores were similar among treatments.

Table 2.
Results of analysis of variance to test for significant nitrogen-addition treatment, sampling time, and interaction effects on plant variables.

Table 3.
Seasonal N yield, nitrogen concentration, and biomass.
3.2. Plant N Concentrations and Biomass N Yield
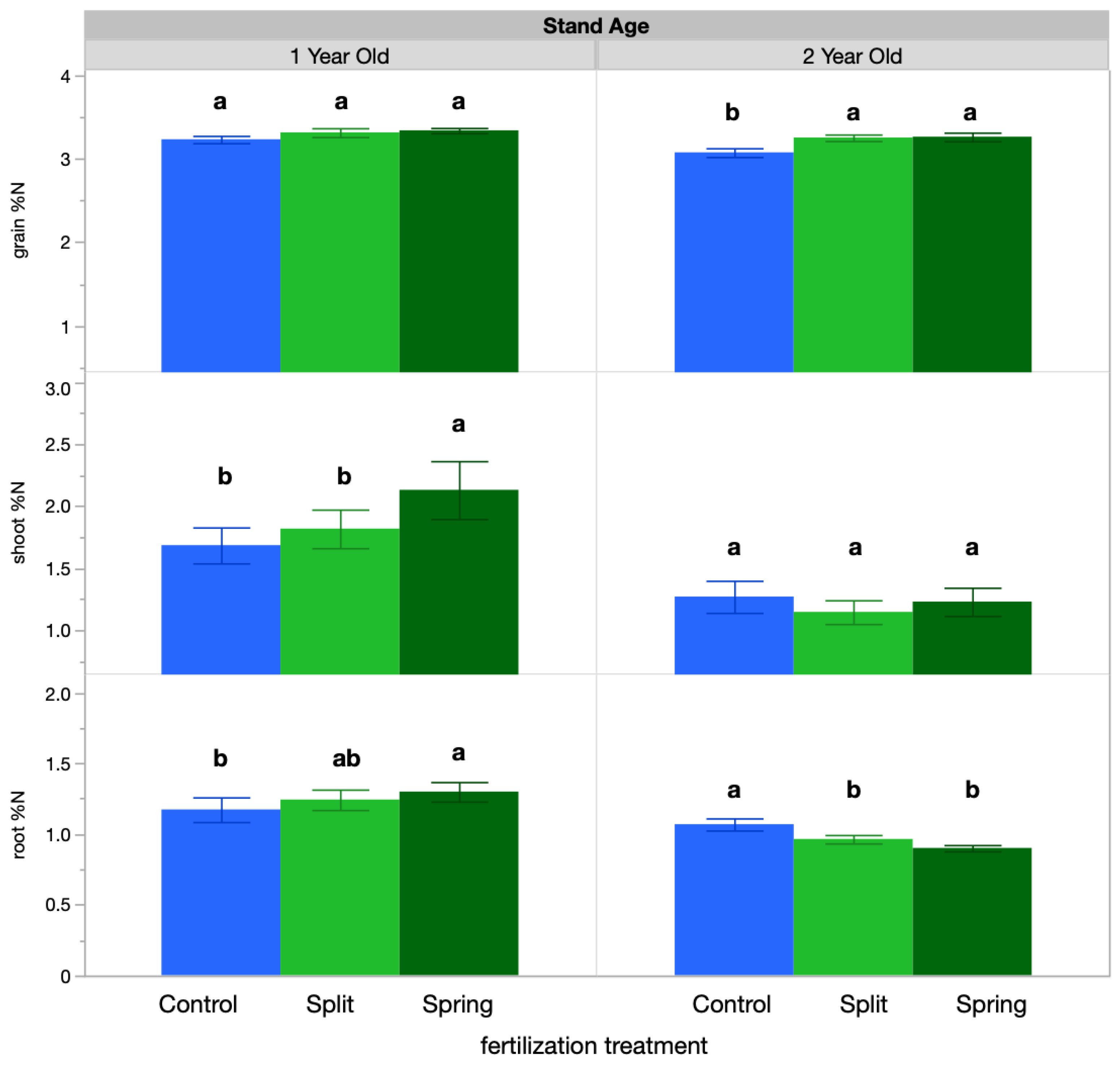
IWG shoot tissue concentration averaged 1.54% N, root tissue concentration averaged 1.1% N, and grain ranged from 3.24% N. IWG tissue N concentration responded significantly to both fertilization treatment and sampling time (Table 2). Regarding fertilization treatment, the 1-year-old stand IWG shoot tissue had a higher N concentration in the spring fertilization treatment (2.1%) than in the split treatment or the control (1.8% and 1.68%, respectively; Figure 1). Additionally, in the 1-year-old stand, root tissue had greater N concentration with spring fertilization (1.29%) than in the split treatment or control (1.24% and 1.17%, respectively), with the exception of the control at the spring regrowth sampling (1.5%; Figure 1). In the 2-year-old stand, however, root tissue N concentration was highest in the control (1.06%), followed by the split and then spring fertilization treatments (0.96% and 0.90%, respectively). Grain N concentration also varied by treatment in the 2-year-old stand, with both the spring and split-fertilization treatments having higher N (3.3%) than the control (3.0%). Over the growing season, tissue N concentrations were highest at spring regrowth and anthesis in the 1-year-old stand for both roots and shoots (Table 3); however, in the 2-year-old stand, root N concentration was highest at spring regrowth, but shoot N concentration was highest at anthesis and fall regrowth (Table 3).
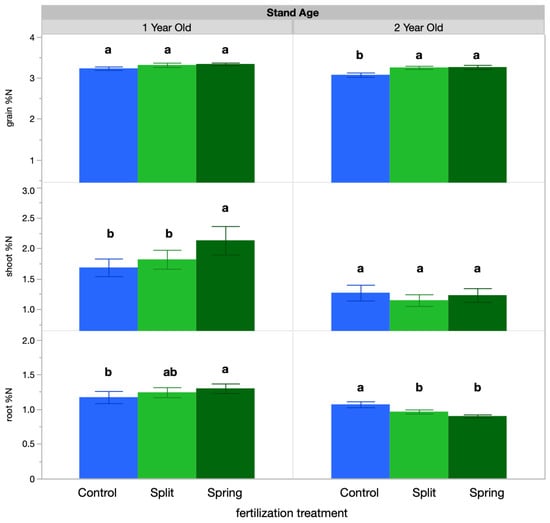
Figure 1.
Tissue percent N results by treatment. Percent N values for each treatment were averaged across sample timepoints. Within each stand age, bars with the same or no letter are statistically similar, as per Tukey’s HSD (p = 0.05).
Nitrogen fertilization effects on tissue N concentration did not lead to changes in the biomass N yield of plant components, calculated as the product of N concentration and biomass yield of each component (Table 2). Biomass N yield did vary over sampling time. In the 1-year-old stand, shoot N yield was highest at anthesis and similar to fall regrowth, while shoot N yield was intermediate at maturity and lowest at spring regrowth (Table 3). Root N yield did not vary over time in the 1-year-old stand. In the 2-year-old stand, shoot, root, and total N yield were highest at maturity and lowest at spring regrowth (Table 3).
3.3. Plant Nitrogen Use Efficiency and Net Uptake
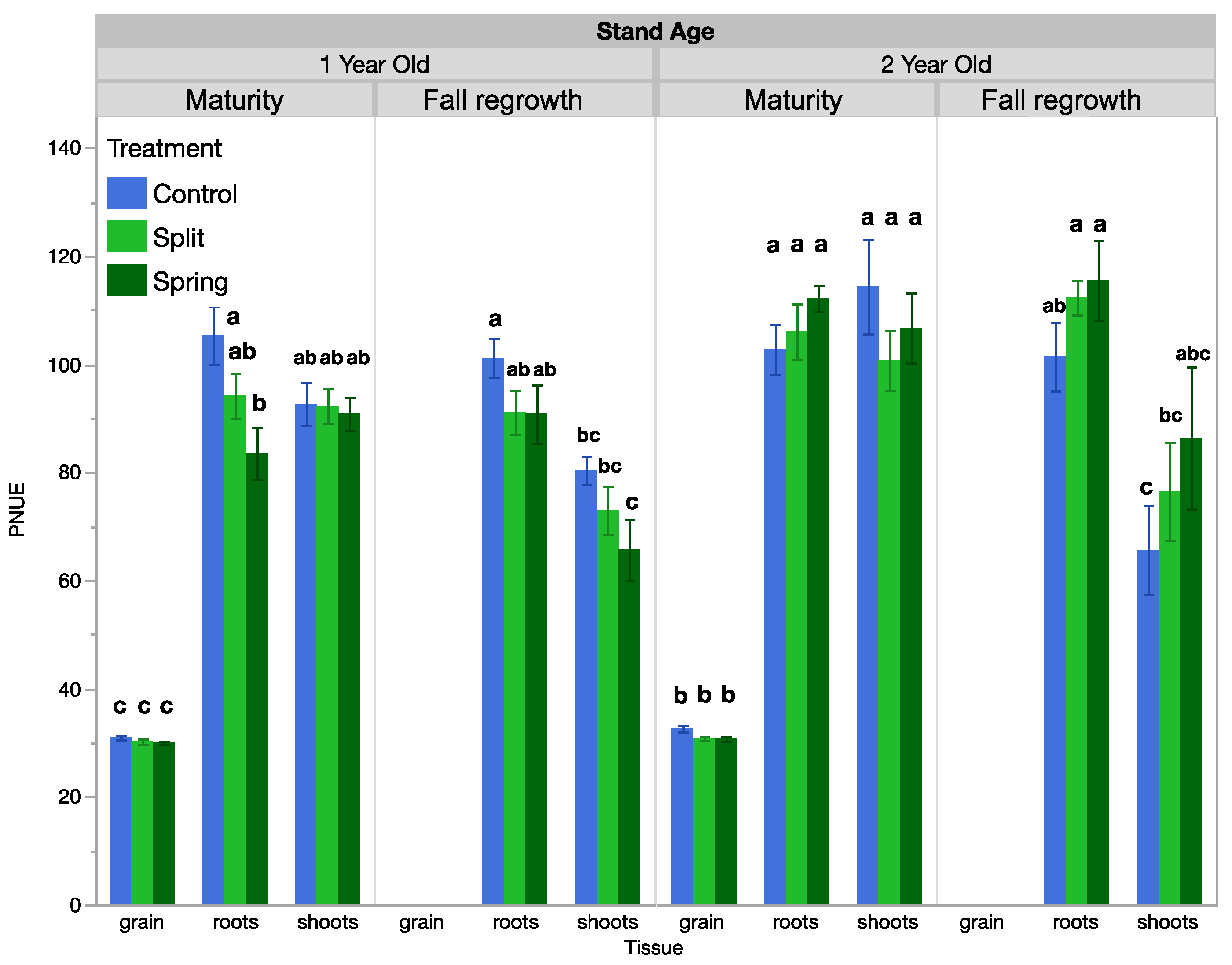
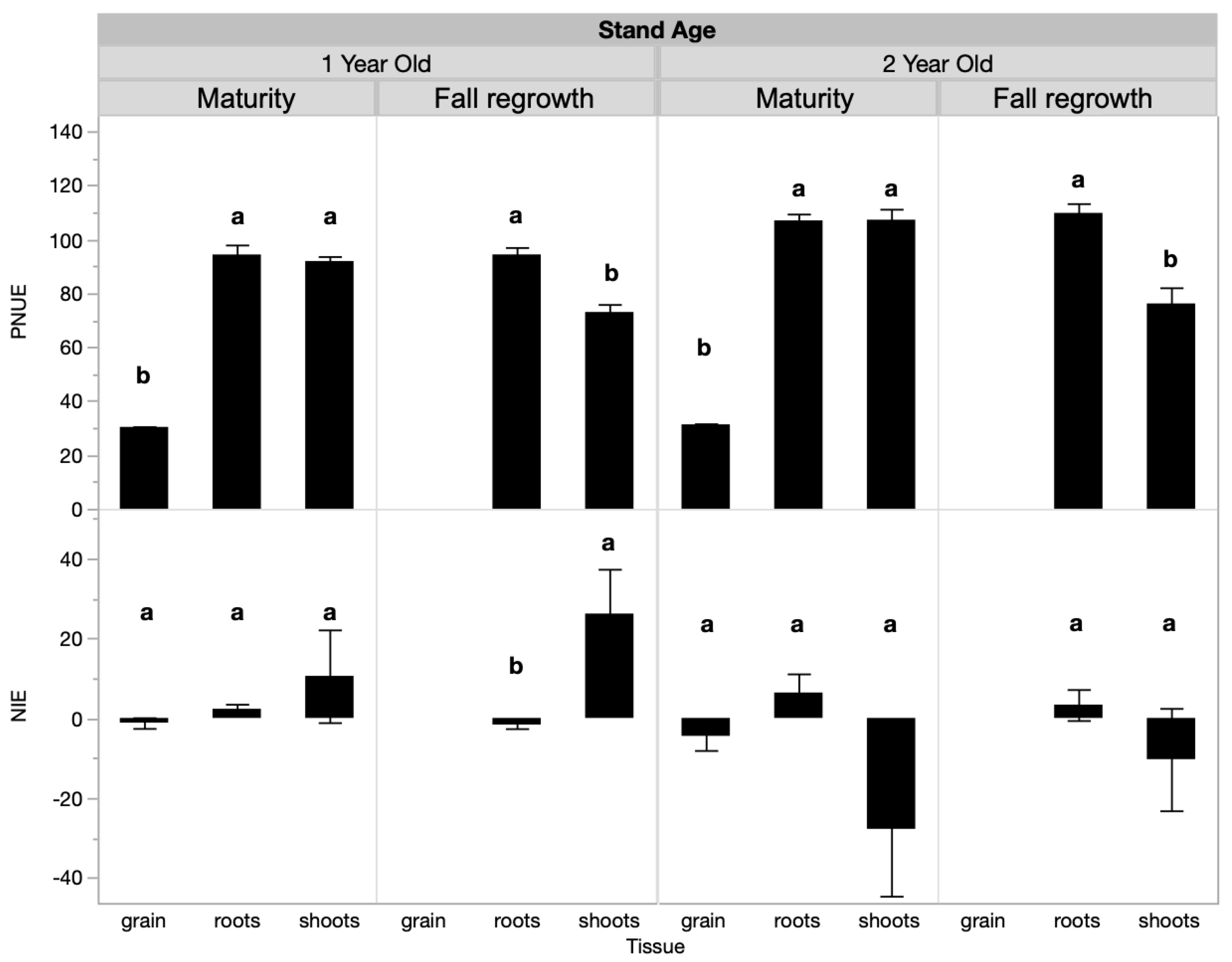
Shoot PNUE, but not NIE, changed over time and was influenced by fertilization treatments when analyzed across the growing season (Table 2, Table 3 and Table S2). Seasonally, in the 1-year-old stand, shoot PNUE at maturity was approximately 2.2 times that of the spring regrowth or anthesis sample dates and was 1.7 times higher than at fall regrowth (Table 3). In the 2-year-old stand, shoot PNUE was approximately 0.25 times higher at maturity than at the other sample dates (Table 3). Regarding treatments for PNUE, there was a significant interactive effect between treatment and tissue type at maturity in the 1-year-old stand. At maturity, root PNUE was higher in the control than in the spring fertilized treatment, while shoot and grain PNUE were not responsive to treatment (Figure 2, Table 4). In the 1-year-old stand at fall regrowth, root and shoot PNUE trended higher in the control treatment, but this was not significant (Figure 2). In the 2-year-old stand, no tissue type was responsive to treatment at either timepoint. There was an insignificant trend at fall regrowth, where shoot and root PNUE were lower in the control than in the spring-fertilized treatment (Figure 2). Both the PNUE and NIE calculations for nitrogen use efficiency differed by plant tissue type (Table 4). NIE differed by tissue type only at the fall regrowth sampling of the 1-year-old stand, where shoot NIE was positive and higher than root NIE. We often observed negative or near-zero NIE values, which is a result of small or negative differences between unfertilized and fertilized plots and reflects the lack of treatment effect we observed on biomass yields. PNUE at maturity was similar in roots and shoots, which both had significantly higher PNUE than grain in both stand ages (Figure 3). At fall regrowth in both stand ages, root PNUE was higher than that of shoots (Figure 3). The net N uptake from spring regrowth to maturity did not vary by treatment (p > 0.05) but averaged 66 kg N ha−1 in 1-year-old IWG and 96 kg N ha−1 in 2-year-old IWG.
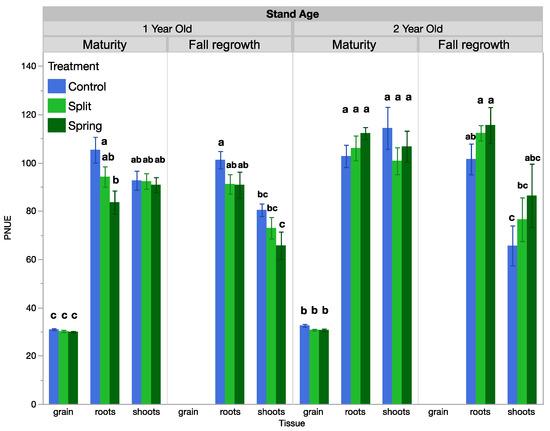
Figure 2.
PNUE averaged by treatment in 1-year-old and 2-year-old IWG stands. Within each stand age, bars with the same or no letter are statistically similar, as per Tukey’s HSD (p = 0.05).
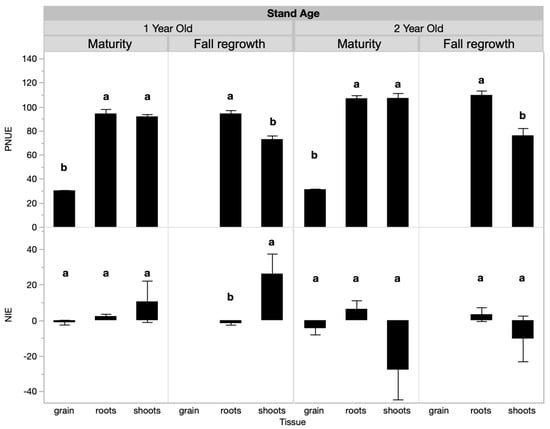
Figure 3.
PNUE and NIE by tissue type and sample timing in 1-year-old and 2-year-old IWG stands. Within each stand age, bars with the same or no letter are statistically similar as per Tukey’s HSD (p = 0.05).

Table 4.
Results of analysis of variance to test for significant nitrogen-addition treatment and tissue-type effects on Physiological Nitrogen use Efficiency (PNUE) and Nitrogen Input Efficiency (NIE).
Table 4.
Results of analysis of variance to test for significant nitrogen-addition treatment and tissue-type effects on Physiological Nitrogen use Efficiency (PNUE) and Nitrogen Input Efficiency (NIE).
| PNUE | NIE | |||
|---|---|---|---|---|
| Factor | Maturity | Fall Regrowth | Maturity | Fall Regrowth |
| 1-year-old stand | ||||
| Treatment | 0.0317 | 0.0012 | 0.1128 | 0.2064 |
| Tissue | <0.0001 | <0.0001 | 0.6069 | 0.0323 |
| Tissue X Treatment | 0.0454 | 0.8987 | 0.4669 | 0.7286 |
| 2-year-old stand | ||||
| Treatment | 0.3723 | 0.0023 | 0.7694 | 0.6111 |
| Tissue | <0.0001 | <0.0001 | 0.0844 | 0.5735 |
| Tissue X Treatment | 0.4734 | 0.9658 | 0.9197 | 0.9423 |
Statistics were based on a randomized block design with 4 replicates of each treatment per block. Because stand ages were not able to be included in the randomization, and because grain was only present at maturity, statistics were performed separately for each stand age and for each timepoint. Bold numbers are significant at p < 0.05.
3.4. Soil% Nitrogen
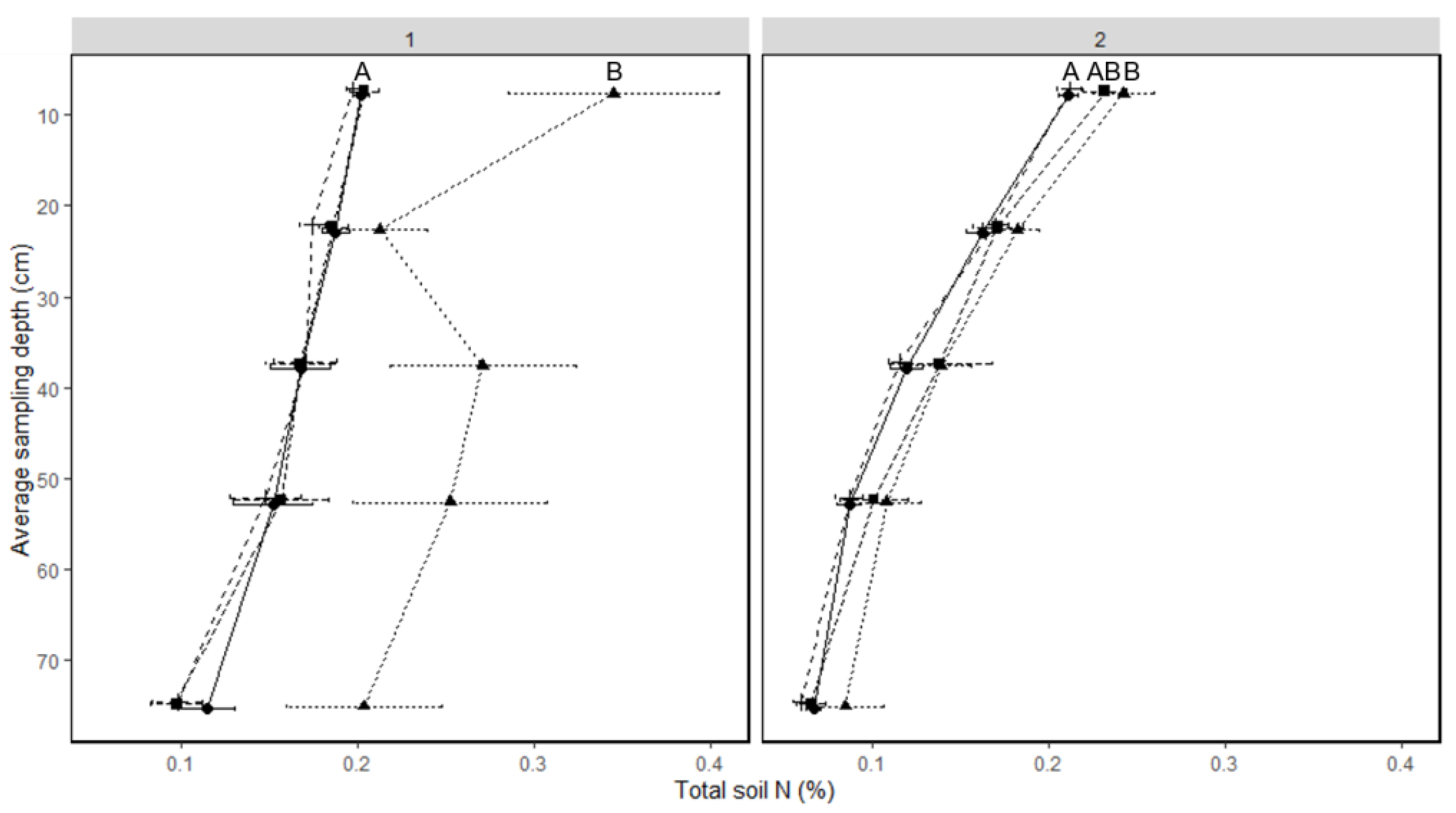
N fertilization treatment did not affect total soil N, but there were significant differences across sampling times (Table 5), depth (1-year-old stand p < 0.001, 2-year-old stand p < 0.001), and in the 2-year-old stand, an interaction of fertilization treatment with sampling time. In both fields, more soil N was observed at fall regrowth than earlier in the season (Figure 4). This effect was most pronounced in the 1-year-old field, when the soil N at fall regrowth was approximately twice as much as all other timepoints at most depths (Figure 4).

Table 5.
Results of analysis of variance to test for significant nitrogen-addition treatment, sampling time, and interaction effects on soil mineral N, mineralized N, and N-mineralization rates in 1-year-old and 2-year-old intermediate wheatgrass stands. Totals are a sum of NO₃−-N (0–15) and soil NH₄⁺-N, respectively. Statistics were based on a randomized block design with 4 replicates of each treatment per block. Because stand ages were not able to be included in the randomization, statistics were performed separately for each stand age.
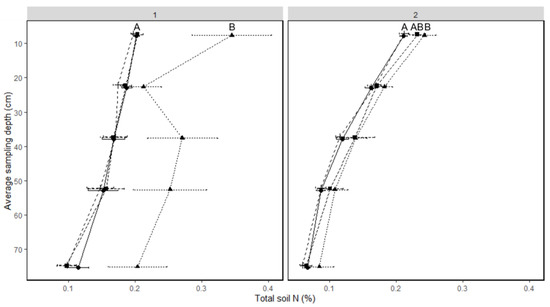
Figure 4.
Soil total percent nitrogen by depth and sampling time in 1- and 2-year-old intermediate wheatgrass: 1 = 1-year-old field; 2 = 2-year-old IWG stand. Within a stand, soil N lines with the same or no upper-case letter are statistically similar as per Tukey’s HSD (p = 0.05). Sampling depth indicated on Y axis is the center of the depth increment sampled. Sampling times with the same uppercase letter are statistically similar as per Tukey’s HSD (p = 0.05). Crosses with long dashed line = spring regrowth, circles with solid line = anthesis, squares with short dashed line = maturity, triangles with dotted line = fall regrowth.
3.5. Soil Mineral Nitrogen
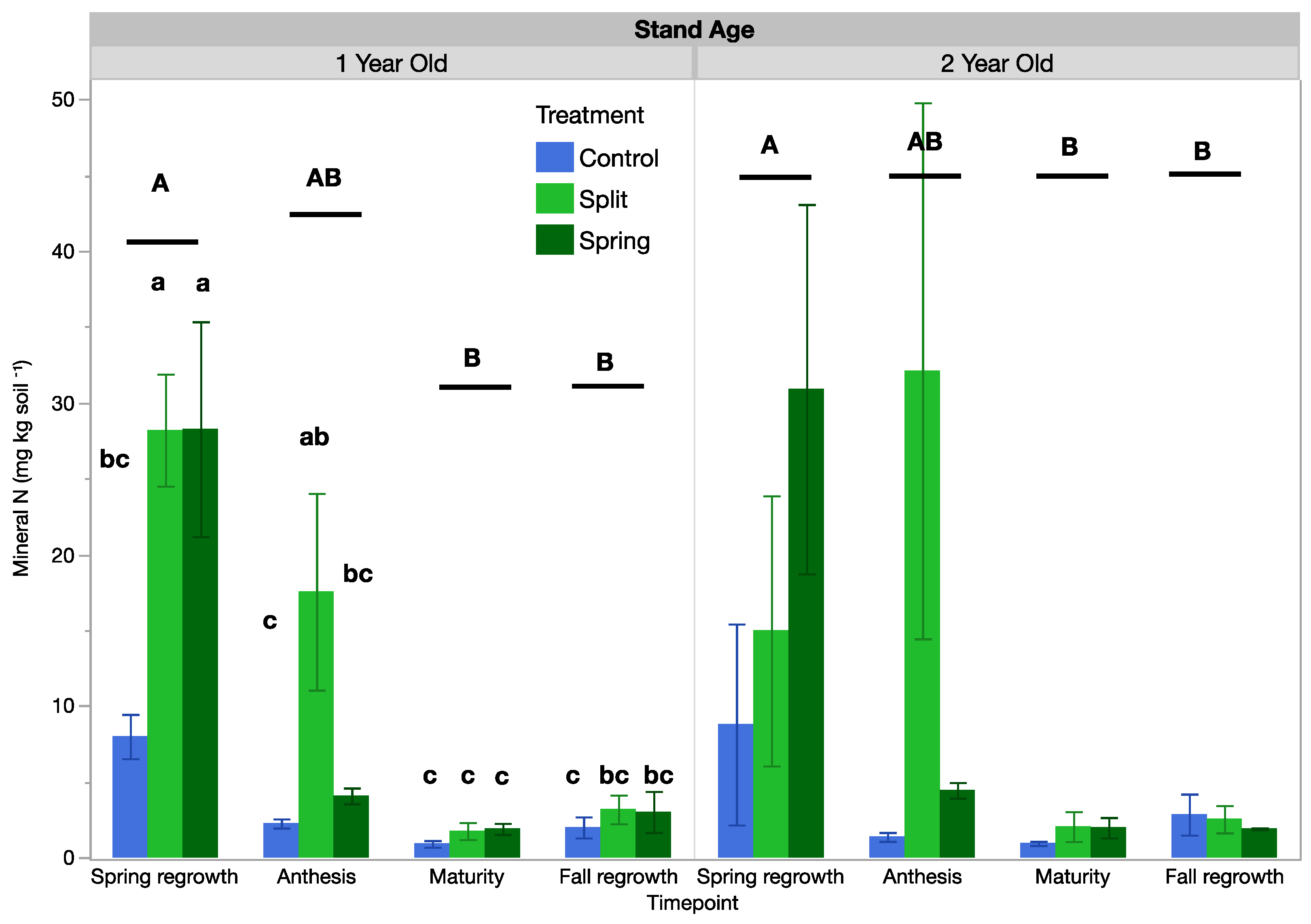
A full analysis of mineral NO3−-N, NH4+-N, nitrification, and ammonification is reported in the Supplementary Materials. Here, mineral N, mineralized N, and N mineralization rates refer to the sum of ammonium and nitrate since the trends of all three (nitrate, ammonium, and the total sum) were similar (Table 5 and Table S3). Mineral N was influenced by treatment, sample date, and their interaction in the 1-year-old stand, but it was only influenced by sample date in the 2-year-old stand. In the 1-year-old stand, the control had lower mineral N than fertilized treatments at spring regrowth, which had the highest mineral N across the growing season (Figure 5). At anthesis, the split treatment had three-times-higher mineral N, with spring N and the control not being statistically different, and at maturity and fall regrowth, no mean mineral N was different across the control or fertilization treatment plots. In the 2-year-old stand, the only statistically significant difference was that mineral N was higher at the spring regrowth date (overall averages of 18.2 mg kg−1 at spring regrowth compared with 12.6 mg kg−1 at anthesis, 1.66 mg kg−1 at maturity, and 2.89 mg kg−1 at fall regrowth).
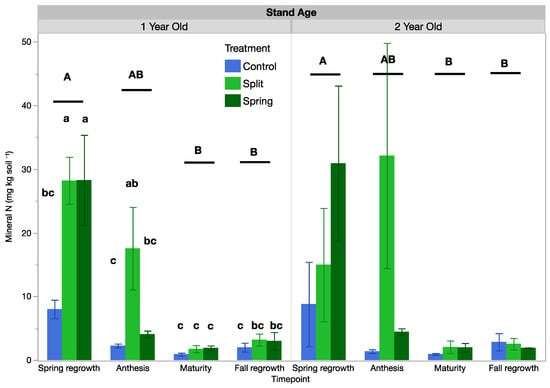
Figure 5.
Soil mineral N in 1-year-old and 2-year-old intermediate wheatgrass fields across sampling times and nitrogen fertilization treatments. Within a field, bars with the same or no lowercase letter are statistically similar in terms of treatment effects as per Tukey’s HSD (p = 0.05). Control = 0 kg N ha−1), split = 40–40 kg N ha−1, and spring = 80 kg N ha−1. Within a field, sets of bars with the same uppercase letter did not differ between sampling times as per Tukey’s HSD (p = 0.05).
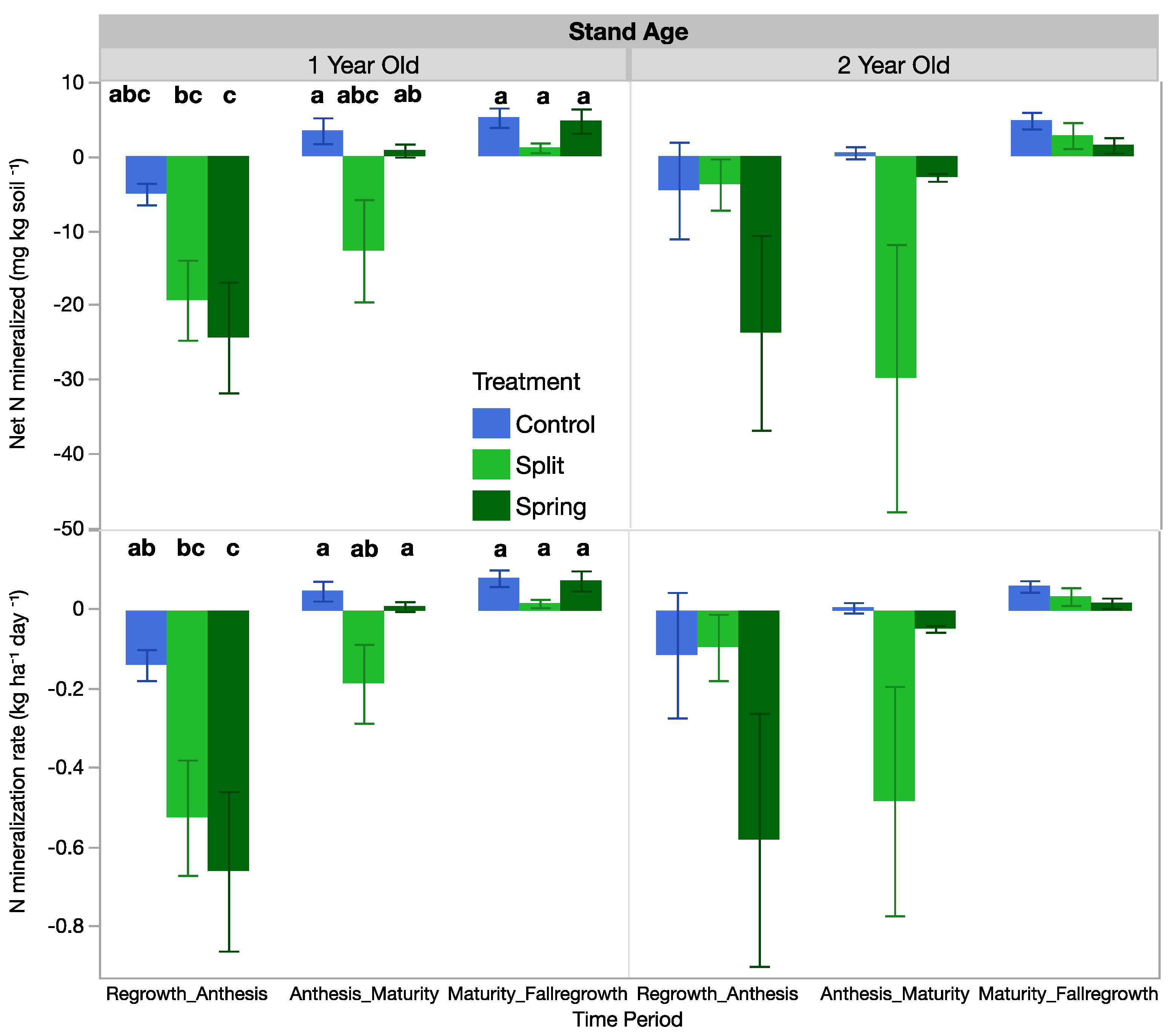
3.6. Net Mineralized Nitrogen and Mineralization Rates
Fertilization treatment influenced the net mineralized N and the mineralization rate in the 1-year-old stand, but those effects were not significant in the 2-year-old stand (Table 5, Figure 6). In the 1-year-old stand at the first sampling period, N-mineralization rates and the net mineralized N were negative, with the control having only slightly negative rates and the fertilized treatments having more negative rates (Figure 6). The same trend was present in the 2-year-old stand, but it was not significant (Figure 6). The negative values suggest that N was being immobilized in all fertilized plots. At the second sampling period from anthesis to maturity, only the split-fertilization treatment had negative mineralization rates, but no treatments were significantly different. In the fall sampling from maturity to fall regrowth, mineralization rates were positive, meaning that N was being mineralized in the system (Figure 6).
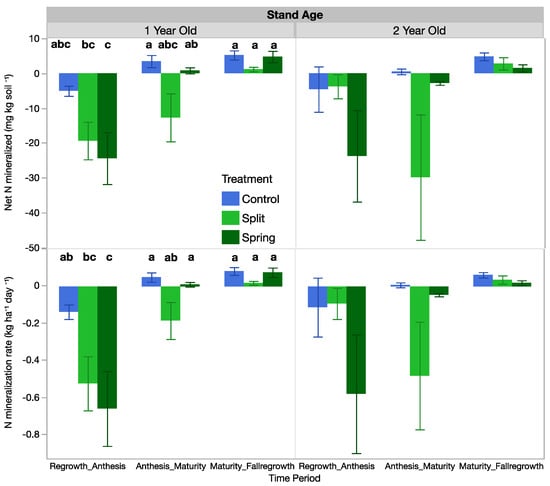
Figure 6.
Net mineralized N and the N-mineralization rate of 1-year-old and 2-year-old intermediate wheatgrass fields across sampling times and nitrogen fertilization. Within treatments in each field, bars with the same or no letter are statistically similar as per Tukey’s HSD (p = 0.05). Control = 0 kg N ha−1), split = 40–40 kg N ha−1, and spring = 80 kg N ha−1.
4. Discussion
IWG is a new crop that has demonstrated potential for producing grain with fewer N fertilizer inputs and reducing N losses in managed agroecosystems. Here, we sought to understand the uptake and efficiency of N use in IWG systems in various N fertilizer regimes and in relation to changes in soil N pools. Although fertilization and the timing of N inputs changed the amount of lodging, IWG tissue percent of N, PNUE, and soil total and mineralized N, this did not translate into changes in biomass yield, grain yield, or plant biomass N yield. We did find that changes in mineral and mineralized N, plant biomass N yield, and both the PNUE and NIE calculations for nitrogen use efficiency all varied over the course of the growing season, and this could be helpful for managing IWG in the future. We found that although soil-mineralization rates increased through the growing season, the total mineral N stayed low. This was paired with lower plant tissue N concentrations but higher total plant N content and higher PNUE, suggesting that producing more biomass per unit N is more likely at maturity and fall regrowth, as well as in soils with relatively high N availability. We discuss these results in more detail below.
In the 1-year-old stand, shoot and root tissue had the highest N concentration in the spring fertilization treatment compared with the control, with trends of higher root N concentration with split fertilization. PNUE was actually lower with any fertilizer input in the 1-year-old stand, but root PNUE was higher with fertilization in the 2-year-old stand. The lack of biomass and biomass N yield response to N fertilization implies that at these rates of N addition, IWG is responsive but not to the extent of altering plant content. This lack of responsiveness to 89 kg ha−1 spring-applied N in 1- or 2-year-old IWG stands is in contrast with current best practices and results at some sites [8,14,24], but in agreement with others [9], suggesting that the growing environment plays a strong role in the N demand of IWG [14,15]. The net uptake from spring regrowth to harvest was slightly less than the known annual removal rates [17]. This is in line with current fertilizer recommendations of 61 to 96 kg N m−2, assuming that little to no off-target N transport occurred [6,14]. Because the unfertilized control did not have different net uptake values than the split and spring fertilization treatments, IWG was likely capable of meeting its 1st- and 2nd-year N demand from native soil N alone in these study conditions. Further evidence for this idea that native soil N could supply 1- and 2-year IWG growth was that our total mineral N values in unfertilized controls were between 0.9 and ~10 mg kg soil −1, substantially higher than other studies hypothesizing that native soil N can fulfill perennial grass N demand [37].
The major focus of nitrogen fertility trials in IWG or any crop is invariably for optimizing yields, and for this dual-use crop, the focus was on grain yields. IWG grain yields were within the range of reports for the variety used in this study [14]. As expected, grain had higher N concentrations and lower PNUE, but we did not observe differences in NIE between grain and other tissues. Because we did not observe an increase in shoot, root, or grain biomass with fertilization, and the NIE calculation relies on the difference per unit N, it is logical that we saw no changes in NIE. There was also a decline in IWG shoot tissue N concentration from anthesis to maturity in both stands, along with a decline in root N concentration from spring regrowth to maturity. We also observed the highest shoot PNUE at maturity, which is in line with the idea that N is being translocated disproportionally to grain, which is known to occur in wheat [38]. Also known to occur in wheat in some environments, and with some varieties, is that vegetative biomass might increase without an associated increase in grain yields [39], demonstrating a concern that N fertilization does not provide an economic return in the form of more grain produced. Although we did not observe such an effect in this IWG study, the N use and allocation related to grain yields with future IWG varieties and with updated fertilization best practices will be important to consider.
The decreased root PNUE with fertilization in the 1-year-old stand and trend of increase in root PNUE with fertilization in the 2-year-old stand, suggests that N allocation and use changes with stand age. Decreased PNUE in the 1-year-old stand with any fertilization is in line with the discrepancy between our N concentration versus N yield results, namely that IWG is taking up more N with fertilization but not translating it into measurable biomass changes. Increased root PNUE in the 2-year-old stand with fertilization suggests that as stands age, they are investing less N per unit of root biomass as they invest in root growth. The importance of the turnover of N-rich plant materials as a long-term source of ecologically sustainable N for IWG has been the source of interest in intercropping IWG with legumes [15,24]. We demonstrate that root-derived N could become a less important soil N pool as plants invest less N per unit of root biomass over time, also potentially affecting N mineralization rates and the seasonal timing of soil available N. Therefore, investment in N-rich intercrops may be a valuable strategy where there is a goal to reduce mineral N inputs.
Although plant biomass yield responses to fertilization treatments were minimal, we found that IWG N allocation and uptake changed over the growing season. Root biomass remained consistent throughout the growing season in the 1-year-old stand and was similar at fall regrowth compared with spring regrowth in the 2-year-old stand. The persistence of root biomass post maturity coupled with evidence that defoliating IWG increases root biomass [12] suggests there is little-to-no tradeoff between the agronomic management of IWG and its ability to maintain large root systems in 1-year-old IWG. This is important because higher root biomass is associated with increased subsurface water protection and carbon sequestration [40,41]. However, the lower root biomass observed at fall regrowth in the 2-year-old IWG compared with root biomass at maturity suggests that root mortality and decomposition may be greater than productivity at certain times of the year. The implications of net root turnover on N cycling are discussed below.
A net decrease in root biomass in the 2-year-old stand in between maturity and regrowth suggests greater root mortality than productivity during that period. In the 1-year-old stand, comparable root biomass at maturity and fall regrowth suggests that either root growth stagnates post maturity or that root productivity is matched by root mortality. However, given that IWG root production can increase in response to defoliation [12], it is likely some degree of mortality occurred between maturity and fall regrowth. Root mortality is a known result of defoliation in other cool-season perennial grasses [42,43], so it is possible that although defoliation stimulates root growth overall in IWG, defoliation may cause a temporary loss of root biomass. Seeing that 0.9 to 1.1% of root biomass was N, this mortality may have contributed to the net mineralization observed in soil between maturity and fall regrowth. Additionally, the average total soil N approximately doubled between maturity and fall regrowth in the 1-year-old stand throughout the soil profile. The gains in total soil N in the subsoil suggest that residue left after harvest alone is not responsible for this increase. Given that mineral N did not also increase during those time periods, the increased soil N was likely organic, possibly due to an increase in organic matter from root turnover.
Our findings suggest that IWG may be enriching root tissue with N between spring regrowth and maturity, but not between maturity and fall regrowth. IWG root tissue had greater N concentration in the spring fertilization treatment than in the control treatment in the 1-year-old stand, suggesting IWG allocates abundant N to root tissue. Our results are similar to previous findings in a perennial system, where fertilization treatment increased root N content [44]. However, this is contrary to previous findings that perennial grasses translocate nutrients to belowground tissue prior to senescence [45]. Harvest timing at physiological maturity might also be related to the translocation of N to the roots of perennial cropping systems—more N may be translocated with later maturity dates [46]. Current IWG populations are harvested relatively soon after physiological maturity to avoid seed shatter; thus, that harvested biomass with N might be removed before re-translocation is significant.
Soil N pools and mineralization both changed in response to treatment and over the growing season. The greater soil mineral N concentration in the spring fertilization treatment at spring regrowth and in the split-fertilization treatment at spring regrowth and anthesis was related to having added mineral fertilizer to the soil before sampling. Relatively higher levels of mineral N in the unfertilized control plots can come from mineralization over the winter and in the early spring [47,48]. Due to the greater concentration of mineral N at spring regrowth, immobilization exceeded mineralization between spring regrowth and anthesis. Later in the season, the positive net mineralization between maturity and fall regrowth suggested an influx of organic N, possibly from post-grain harvest crop residue or root mortality. In other species, defoliation has been shown to reduce root dry-matter yield [49]. If root dieback occurred in the 1-year-old stand, regrowth also occurred, since the total root biomass was not different at maturity and fall regrowth. In that case, soil N could have been from rhizodeposition, which is known to occur alongside root development [50]. More work is needed to understand the implications of this N influx on N mineralization, in particular if mineralized N is taken up by IWG or if it is leached during the freeze/thaw cycles in winter.
Since the amount of soil mineral N at fall regrowth and maturity were similar, it can be inferred that any NH4+-N or NO3−-N produced by mineralization (and subsequent nitrification) was taken up by IWG. Alternatively, NO3−-N produced by mineralization and subsequent nitrification may have leached deeper into the soil profile. However, previous studies have found that NO3−-N leaching below IWG is orders of magnitude lower than that of corn, wheat, and soybean, with rates of leaching being higher in 1-year-old stands and stands fertilized at rates double those that were used in this study [6,7,15]. Mineralized soil NO3−-N was likely assimilated by IWG to meet the N demands of production between maturity and fall regrowth.
Net mineralized N was more negative in the split-fertilization treatment compared with the 1-year-old spring fertilization treatment from spring regrowth to anthesis. The greater immobilization (more negative rates and amounts) during this time period can be explained by the greater amount of urea applied at spring regrowth in the 1-year-old spring fertilization treatment. Because soil mineralization cores were capped after fertilization application, soil in split-fertilization treatment plots had a greater concentration of N at anthesis.
5. Conclusions
This is among the first studies to examine the intra-annual N dynamics in intermediate wheatgrass, a new perennial grain crop potentially capable of retaining N and improving water quality through its N uptake and possibly its use efficiency. IWG is also a crop in need of fertility optimization because of its dual-use potential and need to continue growth and biomass production after grain harvest. Fertilization only increased root and shoot N concentration in the 1-year-old stand, and it decreased PNUE but not NIE. Across fertilization treatments, shoot and root tissue N concentration declined between spring regrowth and maturity, while PNUE and biomass N yield remained similar or increased over the growing season, suggesting conservative N use on the lead up to physiological maturity. Fertilization did not affect soil mineral N levels or net N mineralization beyond the initial spike in mineral N shortly after fertilization. Soil mineral N was generally lowest at maturity and fall regrowth, and N-mineralization rates were highest and only positive between maturity and fall regrowth. Total soil N was greater at fall regrowth than at other timepoints in the 1-year-old stand. Taken together, this suggests that there is an influx of organic N in the soil between maturity and fall regrowth, likely due to release of N-rich root exudates or a decreased amount of plant or microbial N immobilization. Based on our findings, 1 and 2-year old IWG stands may receive enough N from existing soil N pools in fertile soils such as those tested here, and that the seasonal N allocation patterns of IWG should be taken into consideration with N recommendations and best practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13020468/s1. Table S1. 30 year normal and 2018 average temperature and monthly rainfall for growing season in Rosemount, MN; Table S2. ANOVA results for NUE in detail; Table S3. Results of analysis of variance to test for significant nitrogen addition treatment, sampling time, and interaction effects on soil NO₃⁻-N (0–15), soil NH₄⁺-N (0–15), soil mineralized NO₃⁻-N (0–15 cm), and soil mineralized NH₄⁺-N (0–15 cm) in 1 year old and 2 year old intermediate wheatgrass stands.
Author Contributions
Conceptualization and methodology, M.D., with support of J.M.J. and J.L.M.G.; formal analysis, M.D. and J.L.M.G.; investigation, M.D.; data curation, M.D. and J.L.M.G.; writing—original draft preparation, M.D.; writing—review and editing, M.D., J.M.J. and J.L.M.G.; visualization, M.D. and J.L.M.G.; supervision, J.M.J. and J.L.M.G.; project administration, J.M.J.; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by internal graduate support funding from the Peter Graham memorial fund from the University of Minnesota College of Food, Agriculture, and Natural Resource Sciences, but received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data supporting reported results and information can be provided upon request of the authors.
Acknowledgments
We would like to thank Katherine Bohn, Nicole Tautges, Lindsay Wilson, and Joshua Larson for managing the experimental plots and providing assistance with sample collection and data curation. We also thank Carol Loopstra, Emily Locke, Christina Vor, and Matthew Leung for support with sample analysis in the lab. We thank Craig Sheaffer for a friendly review of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keeler, B.L.; Gourevitch, J.D.; Polasky, S.; Isbell, F.; Tessum, C.W.; Hill, J.D.; Marshall, J.D. The Social Costs of Nitrogen. Sci. Adv. 2016, 2, e160021. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; DeHaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for multifunctionality in perennial grain crops. Bioscience 2018, 68, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nutrient use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crops Res. 2008, 107, 89–101. [Google Scholar] [CrossRef]
- Reilly, E.C.; Gutknecht, J.L.M.; Sheaffer, C.C.; Jungers, J.M. Reductions in soil water nitrate beneath a perennial grain crop compared to an annual crop rotation on sandy soil. Front. Sustain. Food Syst. 2022, 6, 996586. [Google Scholar] [CrossRef]
- Jungers, J.M.; DeHaan, L.H.; Mulla, D.J.; Sheaffer, C.C.; Wyse, D.L. Reduced nitrate leaching in a perennial grain crop compared to maize in the Upper Midwest, USA. Agric. Ecosyst. Environ. 2019, 272, 63–73. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Ollenburger, M.; Basso, B.; DeHaan, L.R. Soil and water quality rapidly responds to the perennial grain Kernza wheatgrass. Agron. J. 2013, 105, 735–744. [Google Scholar] [CrossRef]
- Fagnant, L.; Duchêne, O.; Celette, F.; David, C.; Bindelle, J.; Dumont, B. Learning about the growing habits and reproductive strategy of Thinopyrum intermedium through the establishment of its critical nitrogen dilution curve. Field Crops Res. 2023, 291, 108802. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Culman, S.; Robertson, G.; Snapp, S. How does nitrogen and perenniality influence belowground biomass and nitrogen use efficiency in small grain cereals? Crop Sci. 2018, 58, 2110–2120. [Google Scholar] [CrossRef]
- Jungers, J.M.; Sheaffer, C.C.; Lamb, J.A. The Effect of Nitrogen, Phosphorus, and Potassium Fertilizers on Prairie Biomass Yield, Ethanol Yield, and Nutrient Harvest. Bioenerg. Res. 2015, 8, 279–291. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W.; Mikha, M. Root and soil total carbon and nitrogen under bioenergy perennial grasses with various nitrogen rates. Biomass Bioenergy 2017, 107, 326–334. [Google Scholar] [CrossRef]
- Pugliese, J.Y.; Culman, S.W.; Sprunger, C.D. Correction to: Harvesting forage of the perennial grain crop kernza (Thinopyrum intermedium) increases root biomass and soil nitrogen cycling. Plant Soil 2019, 437, 255. [Google Scholar] [CrossRef]
- Bergquist, G.; Gutknecht, J.; Sheaffer, C.C.; Jungers, J.M. Plant Suppression and Termination Methods to Maintain Intermediate Wheatgrass (Thinopyrum intermedium) Grain Yield. Agriculture 2022, 12, 1638. [Google Scholar] [CrossRef]
- Jungers, J.M.; DeHaan, L.R.; Betts, K.J.; Sheaffer, C.C.; Wyse, D.L. Intermediate wheatgrass grain and forage yield responses to nitrogen fertilization. Agron. J. 2017, 109, 462–472. [Google Scholar] [CrossRef]
- Reilly, E.C.; Gutknecht, J.L.M.; Tautges, N.E.; Sheaffer, C.C.; Jungers, J.M. Nitrogen transfer and yield effects of legumes intercropped with the perennial grain crop intermediate wheatgrass. Field Crops Res. 2022, 286, 108627. [Google Scholar] [CrossRef]
- Tautges, N.E.; Jungers, J.M.; DeHaan, L.R.; Wyse, D.L.; Sheaffer, C.C. Maintaining grain yields of the perennial cereal intermediate wheatgrass in monoculture v. bi-culture with alfalfa in the Upper Midwestern USA. J. Agric. Sci. 2018, 156, 758–773. [Google Scholar] [CrossRef]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Guretzky, J.A. Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. Bioenergy Res. 2012, 5, 61–70. [Google Scholar] [CrossRef]
- Garten, C.T.; Smith, J.L.; Tyler, D.D.; Amonette, J.E.; Bailey, V.L.; Brice, D.J.; Castro, H.F.; Graham, R.L.; Gunderson, C.A.; Izaurralde, R.C.; et al. Intra-annual changes in biomass, carbon, and nitrogen dynamics at 4-year old switchgrass field trials in west Tennessee, USA. Agric. Ecosyst. Environ. 2010, 136, 177–184. [Google Scholar] [CrossRef]
- Redmann, R.E.; van Kessel, C. Nitrogen Budget and 15N Translocation in a Perennial Wheatgrass. Funct. Ecol. 1992, 6, 221–225. [Google Scholar]
- Syme, H.; Acuna, T.B.; Abrecht, D.; Wade, L.J. Nitrogen contributions in a windmill grass (Chloris truncata)–wheat (Triticum aestivum L.) system in south-western Australia. Soil Res. 2007, 45, 635–642. [Google Scholar] [CrossRef]
- Smaje, C. The strong perennial vision: A critical review. Agroecol. Sustain. Food Syst. 2015, 5, 471–499. [Google Scholar] [CrossRef]
- Puka-Beals, J.; Sheaffer, C.C.; Jungers, J.M. Forage yield and profitability of grain-type intermediate wheatgrass under different harvest schedules. Agroecosyst. Geosci. Environ. 2022, 5, e20274. [Google Scholar] [CrossRef]
- Hunter, M.C.; Sheaffer, C.C.; Culman, S.W.; Lazarus, W.F.; Jungers, J.M. Effects of defoliation and row spacing on intermediate wheatgrass II: Forage yield and economics. Agron. J. 2020, 112, 1862–1880. [Google Scholar] [CrossRef]
- Crews, T.E.; Kemp, L.; Bowden, J.H.; Murrell, E.G. How the Nitrogen Economy of a Perennial Cereal-Legume Intercrop Affects Productivity: Can Synchrony Be Achieved? Front. Sustain. Food Syst. 2023, 6, 755548. [Google Scholar] [CrossRef]
- Dodds, D.; Carter, J.; Meyer, D.; Haas, R. Grass Seed Production in North Dakota. Coop. Extensive Serv. North Dakota State Univ. Agric. Appl. Sci. 1987, R917, 1–30. [Google Scholar]
- Horton, H.; Asay, K.H.; Glover, T.F.; Young, S.A.; Haws, B.A.; Dewey, S.A.; Evans, J.O. Grass Seed Production Guide for Utah; Utah State University: Logan, UT, USA, 1990. [Google Scholar]
- Koeritz, E.J.; Watkins, E.; Ehlke, N.J. A split application approach to nitrogen and growth regulator management for perennial ryegrass seed production. Crop Sci. 2013, 53, 1762–1777. [Google Scholar] [CrossRef]
- Vleugels, T.; Rijckaert, G.; Gislum, R. Seed yield response to N fertilization and potential of proximal sensing in Italian ryegrass seed crops. Field Crop Res. 2017, 211, 37–47. [Google Scholar] [CrossRef]
- Nakamura, K.; Harter, T.; Hirono, Y.; Horino, H.; Mitsuno, T. Assessment of Root Zone Nitrogen Leaching as Affected by Irrigation and Nutrient Management Practices. Vadose Zone J. 2004, 3, 1353–1366. [Google Scholar] [CrossRef]
- Lasisi, A.A.; Akinremi, O.O.; Tenuta, M.; Cattani, D. Below-ground plant biomass and nitrogen uptake of perennial forage grasses and annual crops fertilized with pig manures. Agric. Ecosyst. Environ. 2018, 268, 1–7. [Google Scholar] [CrossRef]
- Moore, K.J.; Moser, L.E. Quantifying Developmental Morphology of Perennial Grasses. Crop Sci. 1995, 35, 37–43. [Google Scholar] [CrossRef]
- Smucker, A.J.M.; McBurney, S.L.; Srivastava, A.K. Quantitative separation of roots from compacted soil profiles by the hydropneumatic elutriation system. Agron. J. 1982, 74, 500–503. [Google Scholar] [CrossRef]
- Raison, R.J.; Connell, M.J.; Khanna, P.K. Methodology for studying fluxes of soil mineral-N in situ. Soil Biol. Biochem. 1987, 19, 521–530. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Reynolds, H.; Long, T.M. Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biol. Biochem. 2000, 32, 2095–2097. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Schetter, A.; Lin, C.H.; Zumpf, C.; Jang, C.; Hoffmann, L.; Rooney, W.; Lee, D.K. Genotype-Environment-Management Interactions in Biomass Yield and Feedstock Composition of Photoperiod-Sensitive Energy Sorghum. Bioenergy Res. 2022, 15, 1017–1032. [Google Scholar] [CrossRef]
- Vogel, K.P.; Brejda, J.J.; Walters, D.T.; Buxton, D.R. Switchgrass Biomass Production in the Midwest USA. Agron. J. 2002, 94, 413–420. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Serret, M.D.; Araus, J.L. The nitrogen contribution of different plant parts to wheat grains: Exploring genotype, water, and nitrogen effects. Front. Plant Sci. 2017, 7, 1986. [Google Scholar] [CrossRef]
- Maeoka, R.E.; Sadras, V.O.; Ciampitti, I.A.; Diaz, D.R.; Fritz, A.K.; Lollato, R.P. Changes in the Phenotype of Winter Wheat Varieties Released Between 1920 and 2016 in Response to In-Furrow Fertilizer: Biomass Allocation, Yield, and Grain Protein Concentration. Front. Plant Sci. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Sullivan, M.W.; Jiang, Z.; Hull, R.J. Root morphology and its relationship with nitrate uptake in Kentucky bluegrass. Crop Sci. 2000, 40, 765–772. [Google Scholar] [CrossRef]
- Lal, R.; Augustin, B. Carbon Sequestration in Urban Ecosystems; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Huang, B.; Gao, H. Growth and Carbohydrate Metabolism of Creeping Bentgrass Cultivars. Crop Sci. 2000, 40, 1115–1120. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Mowing effects on root production, growth, and mortality of creeping bentgrass. Crop Sci. 2002, 42, 1241–1250. [Google Scholar] [CrossRef]
- Lemus, R.; Parrish, D.J.; Abaye, O. Nitrogen-use dynamics in switchgrass grown for biomass. BioEnergy Res. 2008, 1, 153–162. [Google Scholar] [CrossRef]
- Smith, S.D.; Monson, R.K.; Anderson, J.E. Physiological Ecology of North American Desert Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Wayman, S.; Bowden, R.D.; Mitchell, R.B. Seasonal Changes in Shoot and Root Nitrogen Distribution in Switchgrass (Panicum virgatum). Bioenergy Res. 2014, 7, 243–252. [Google Scholar] [CrossRef][Green Version]
- Ryan, M.C.; Kachanoski, R.G.; Gillham, R.W. Overwinter soil nitrogen dynamics in seasonally frozen soils. Can. J. Soil Sci. 2000, 80, 541–550. [Google Scholar] [CrossRef]
- Malhi, S.S.; Nyborg, M. Increase in Mineral N in Soils During Winter and Loss of Mineral N During Early Spring in North-Central Alberta. Can. J. Soil Sci. 2010, 66, 397–409. [Google Scholar] [CrossRef]
- Biswell, H.H.; Weaver, J.E. Effect of Frequent Clipping on the Development of Roots and Tops of Grasses in Prairie Sod. Ecol. Soc. Am. 1933, 14, 368–390. [Google Scholar] [CrossRef]
- Shamoot, S.; McDonald, L.; Batholomew, W.V. Rhizo-deposition of organic debris in soil. Soil Sci. Soc. Am. J. 1968, 32, 817–820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).