Duguetia lanceolata A. St.-Hil. (Annonaceae) Essential Oil: Toxicity against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and Selectivity for the Parasitoid Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and EOs
2.2. Chemical Characterization of the EOs
2.3. Bioassays with the FAW
2.3.1. FAW Maintenance Creation
2.3.2. Acute Toxicity of EOs against the FAW in a Topical Application Test
2.3.3. Dose-Response and Time-Response Curves
2.3.4. Chronic Toxicity of EOs against the FAW in an Ingestion Test
2.3.5. Food Preference Test of the FAW by EOs
2.3.6. Toxicity of Major Compounds from Duguetia lanceolata (Leaves) EO against the FAW
2.4. Selectivity of EOs for Trichogramma pretiosum
2.4.1. General Procedures
2.4.2. Selectivity of EOs on the Immature Phases of Trichogramma pretiosum
2.4.3. Selectivity of EOs for Trichogramma pretiosum Adults
2.4.4. Classification of EOs According to the International Organization for Biological Control
2.5. Statistical Analysis
3. Results
3.1. Chemical Characterization of EOs
3.2. Bioassays with the FAW
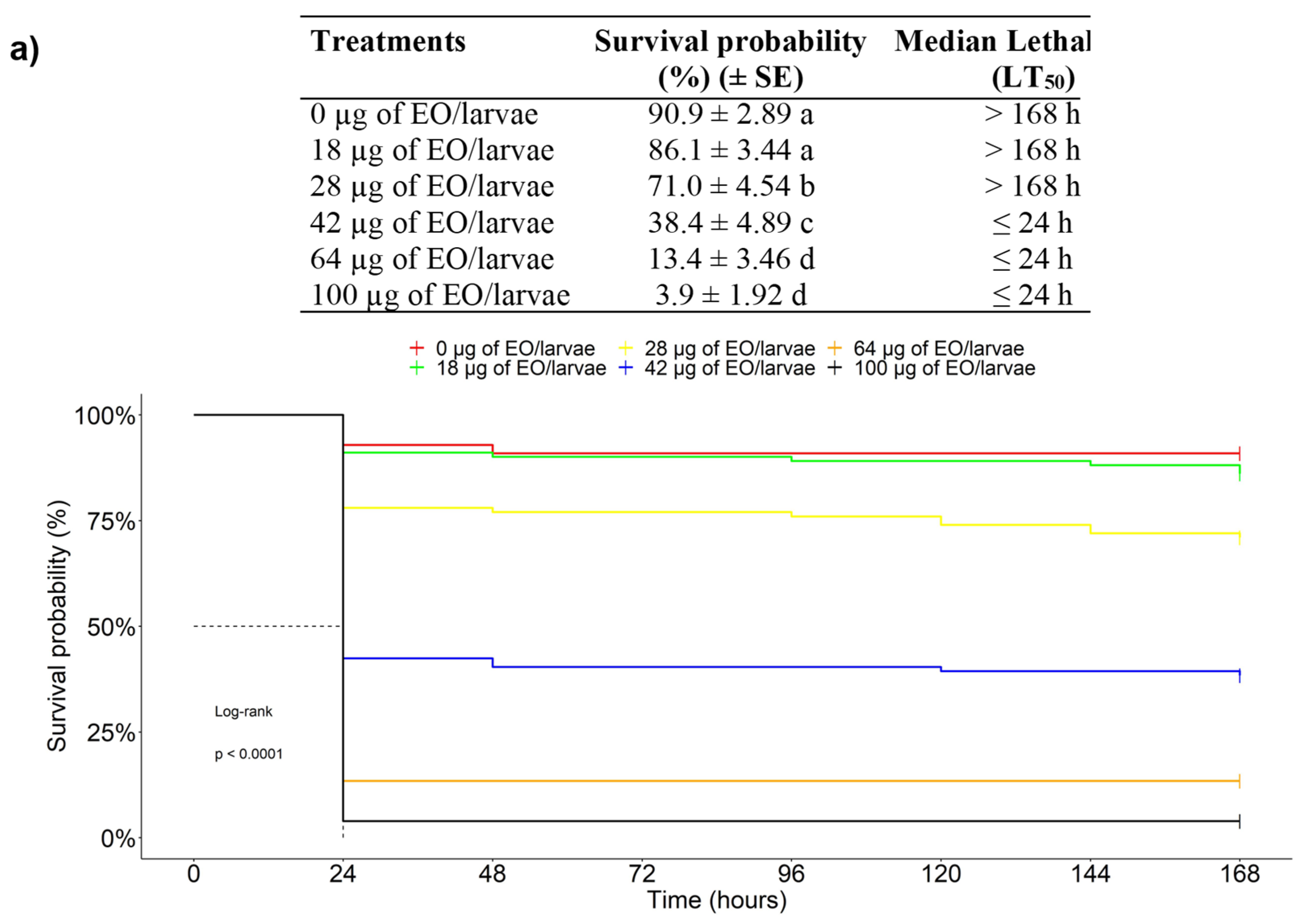
3.2.1. Acute Toxicity of EOs against the FAW in a Topical Application Test
3.2.2. Dose-Response and Time-Response Curves
3.2.3. Chronic Toxicity of EOs against the FAW in an Ingestion Test
3.2.4. Food Preference Test of the FAW by EOs
3.2.5. Toxicity of Major Compounds from Duguetia lanceolata (Leaves) EO against the FAW
3.3. Selectivity of EOs for Trichogramma pretiosum
3.3.1. Selectivity of EOs on the Immature Phases of Trichogramma pretiosum
3.3.2. Selectivity of EOs for Trichogramma pretiosum Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shylesha, A.N.; Jalali, S.K.; Ankita, G.; Richa, V.; Venkatesan, T.; Pradeeksha, S.; Rakshit, O.; Ganiger, P.C.; Omprakash, N.; Subaharan, K.; et al. Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control 2018, 32, 145–151. [Google Scholar] [CrossRef] [Green Version]
- EPPO. Spodoptera frugiperda (LAPHFR)—Distribution, EPPO Global Database. Available online: https://gd.eppo.int/taxon/LAPHFR/distribution (accessed on 12 December 2022).
- Kasoma, C.; Shimelis, H.; Laing, M.D. Fall armyworm invasion in Africa: Implications for maize production and breeding. J. Crop Improv. 2021, 35, 111–146. [Google Scholar] [CrossRef]
- Valicente, F.H. Manejo Integrado de Pragas na Cultura do Milho. Available online: http://www.infoteca.cnptia.embrapa.br/handle/doc/1017489 (accessed on 20 October 2022).
- Kumar, R.M.; Gadratagi, B.G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Yang, F.; Williams, J.; Huang, F.; Kerns, D.L. Genetic basis and cross-resistance of Vip3Aa resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) derived from Texas, USA. Crop Prot. 2021, 147, 105702. [Google Scholar] [CrossRef]
- Banerjee, R.; De Bortoli, C.P.; Huang, F.; Lamour, K.; Meagher, R.; Buntin, D.; Ni, X.; Reay-Jones, F.P.F.; Stewart, S.; Jurat-Fuentes, J.L. Large genomic deletion linked to field-evolved resistance to Cry1F corn in fall armyworm (Spodoptera frugiperda) from Florida. Sci. Rep. 2022, 12, 13580. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Z.; Yang, S.; Lin, Y.; Zhong, H.; Jin, T. Insecticide resistance and its underlying synergism in field populations of Spodoptera frugiperda (J. E. Smith) from Hainan Island, China. Phytoparasitica 2022, 50, 933–945. [Google Scholar] [CrossRef]
- Okuma, D.M.; Cuenca, A.; Nauen, R.; Omoto, C. Large-scale monitoring of the frequency of ryanodine receptor target-site mutations conferring diamide resistance in Brazilian field populations of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 626. [Google Scholar] [CrossRef]
- Section 25(b) Chemicals | CropWatch. 2022. Available online: https://cropwatch.unl.edu/potato/section25b_chemicals (accessed on 15 November 2022).
- Demortain, D. The science behind the ban: The outstanding impact of ecotoxicological research on the regulation of neonicotinoids. Curr. Opin. Insect Sci. 2021, 46, 78–82. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Isman, M.B.; Seffrin, R. Natural insecticides from the Annonaceae: A unique example for developing biopesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: New Delhi, India, 2014; pp. 21–33. [Google Scholar]
- Blessing, L.T.; Colom, O.Á.; Popich, S.; Neske, A.; Bardón, A. Antifeedant and toxic effects of acetogenins from Annona montana on Spodoptera frugiperda. J. Pest Sci. 2004, 83, 307–310. [Google Scholar] [CrossRef]
- Freitas, A.F.; Pereira, F.F.; Formagio, A.S.N.; Lucchetta, J.T.; Vieira, M.C.; Mussury, R.M. Effects of methanolic extracts of Annona species on the development and reproduction of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ansante, T.F.; Ribeiro, L.P.; Bicalho, K.U.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Vendramim, J.D. Secondary metabolites from Neotropical Annonaceae: Screening, bioguided fractionation, and toxicity to Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Ind. Crops Prod. 2015, 74, 969–976. [Google Scholar] [CrossRef]
- Ruiz Hidalgo, J.; Gilabert, M.; Cabedo, N.; Cortes, D.; Neske, A. Montanacin-L and montanacin-K two previously non-described acetogenins from Annona montana twigs and leaves. Phytochem. Lett. 2020, 38, 78–83. [Google Scholar] [CrossRef]
- Alves, D.S.; Machado, A.R.T.; Campos, V.A.C.; Oliveira, D.F.; Carvalho, G.A. Selection of Annonaceae species for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) and metabolic profiling of Duguetia lanceolata using nuclear magnetic resonance spectroscopy. J. Econ. Entomol. 2016, 109, 649–659. [Google Scholar] [CrossRef]
- Alves, D.S.; Costa, V.A.; Machado, A.R.T.; Oliveira, D.F.; Carvalho, G.A. Duguetia lanceolata A. St.-Hil. Stem bark produces phenylpropanoids lethal to Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Crop Prot. 2020, 127, 104965. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Baldassarri, C.; Ferrati, M.; Maggi, F.; Pavela, R.; Tsabang, N.; Petrelli, R.; Ricciardi, R.; Desneux, N.; Benelli, G. Essential oils from Cameroonian aromatic plants as effective insecticides against mosquitoes, houseflies, and moths. Plants 2022, 11, 2353. [Google Scholar] [CrossRef]
- Cherif, A.; Mansour, R.; Grissa-Lebdi, K. The egg parasitoids Trichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 2021, 31, 661–693. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, Present Status, and Perspectives. Ann. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Parra, J.R.P.; Coelho, A. Applied biological control in Brazil: From laboratory assays to field application. J. Insect Sci. 2019, 19, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Filho, A.T. NeoTropTree: Flora Arbórea dos Neotrópicos: Um Banco de Dados Envolvendo Biogeografia, Diversidade e Conservação. Available online: http://prof.icb.ufmg.br/treeatlan (accessed on 8 September 2022).
- The Plant List. Available online: http://www.theplantlist.org (accessed on 15 September 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Parra, J.R.P. Técnicas de Criação de Insetos Para Programas de Controle Biológico, 3rd ed.; FEALQ: Piracicaba, Brasil, 2001. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Cônsoli, F.L.; Botelho, P.S.M.; Parra, J.R.P. Selectivity of insecticides to the egg parasitoid Trichogramma galloi Zucchi, 1988, (Hym., Trichogrammatidae). J. Appl. Entomol. 2001, 125, 37–43. [Google Scholar] [CrossRef]
- Rampelotti-Ferreira, F.T.; Coelho, A.; Parra, J.R.P.; Vendramim, J.D. Selectivity of plant extracts for Trichogramma pretiosum Riley (Hym.: Trichogrammatidae). Ecotoxicol. Environ. Saf. 2017, 138, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Parreira, D.S.; la Cruz, R.A.; Dimaté, F.A.R.; Batista, L.D.; Ribeiro, R.C.; Ferreira, G.A.R.; Zanuncio, J.C. Bioactivity of ten essential oils on the biological parameters of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) adults. Ind. Crops Prod. 2019, 127, 11–15. [Google Scholar] [CrossRef]
- Hassan, S.A. The initiative of the IOBC/WPRS working group on pesticides and beneficial organisms. In Pesticides and Beneficial Organisms; Haskell, P.T., McEwen, P., Eds.; Springer: Boston, MA, USA, 1998; pp. 22–27. [Google Scholar]
- Sterk, G.; Hassan, S.A.; Baillod, M.; Bakker, F.; Bigler, F.; Blümel, S.; Bogenschütz, H.; Boller, E.; Bromand, B.; Brun, J.; et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group “Pesticides and Beneficial Organisms”. BioControl 1999, 44, 99–117. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Felix, S.F.; Rodrigues, A.M.; Rodrigues, A.L.M.; Freitas, J.C.C.; Alves, D.R.; Silva, A.A.; Santos, D.L.; Oliveira, K.R.L.; Montes, R.A.; Silva, M.V.F.; et al. Chemical composition, larvicidal activity, and enzyme inhibition of the essential oil of Lippia grata Schauer from the caatinga biome against dengue vectors. Pharmaceuticals 2021, 14, 250. [Google Scholar] [CrossRef]
- Piri, A.; Sahebzadeh, N.; Zibaee, A.; Sendi, J.J.; Shamakhi, L.; Shahriari, M. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Chemosphere 2020, 256, 127103. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Insecticidal and fungicidal efficacy of essential oils and nanoencapsulation approaches for the development of next generation ecofriendly green preservatives for management of stored food commodities: An overview. Int. J. Pest Manag. 2021. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Belton, P.; Nicholson, R.A.; Lowenberger, C.A. Effects of the essential oil constituent thymol and other neuroactive chemicals on flight motor activity and wing beat frequency in the blowfly Phaenicia sericata. Pest Manag. Sci. 2010, 66, 277–289. [Google Scholar] [CrossRef]
- Haj Darwich, C.M.; Chrzanowski, M.M.; Bernatowicz, P.P.; Polanska, M.A.; Joachimiak, E.; Bebas, P. Molecular oscillator affects susceptibility of caterpillars to insecticides: Studies on the egyptian cotton leaf worm—Spodoptera littoralis (Lepidoptera: Noctuidae). Insects 2022, 13, 488. [Google Scholar] [CrossRef]
- Ling, R.; Yang, R.; Li, P.; Zhang, X.; Shen, T.; Li, X.; Yang, Q.; Sun, L.; Yan, J. Asatone and isoasatone A against Spodoptera litura Fab. by acting on Cytochrome P450 monoxygenases and glutathione transferases. Molecules 2019, 24, 3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wilson, A.E.; Liu, N. A new method to address the importance of detoxified enzyme in insecticide resistance—Meta-analysis. Front. Physiol. 2022, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, X.; Li, T.; Cheng, P.; Gong, M. New insights into cypermethrin insecticide resistance mechanisms of Culex pipiens pallens by proteome analysis. Pest Manag. Sci. 2022, 78, 4579–4588. [Google Scholar] [CrossRef] [PubMed]
- Voirol, L.R.P.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial symbionts in lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.G.; Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef] [Green Version]
- Lv, N.; Ma, K.; Li, R.; Liang, P.; Gao, X. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2021, 212, 111969. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century—Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R.; Maggi, F.; Mazzara, E.; Torresi, J.; Cianfaglione, K.; Benelli, G.; Canale, A. Prolonged sublethal effects of essential oils from non-wood parts of nine conifers on key insect pests and vectors. Ind. Crops Prod. 2021, 168, 113590. [Google Scholar] [CrossRef]
- Alves, D.S.; Morejón, R.C.; Machado, A.R.T.; Carvalho, G.A.; Pina, O.; Oliveira, D.F. Acaricidal activity of Annonaceae fractions against Tetranychus tumidus and Tetranychus urticae (Acari: Tetranychidae) and the metabolite profile of Duguetia lanceolata (Annonaceae) using GC-MS. Semin. Ciências Agrárias 2015, 36, 4119. [Google Scholar] [CrossRef] [Green Version]
- Pares, R.B.; Alves, D.S.; Alves, L.F.A.; Godinho, C.C.; Gobbo Neto, L.; Ferreira, T.T.; Nascimento, M.M.; Ascari, J.; Oliveira, D.F. Acaricidal activity of annonaceae plants for Dermanyssus gallinae (Acari: Dermanyssidae) and metabolomic profile by HPLC-MS/MS. Neotrop. Entomol. 2021, 50, 662–672. [Google Scholar] [CrossRef]
- Maia, D.S.; Lopes, C.F.; Saldanha, A.A.; Silva, N.L.; Sartori, Â.L.B.; Carollo, C.A.; Sobral, M.G.; Alves, S.N.; Silva, D.B.; Siqueira, J.M. Larvicidal effect from different Annonaceae species on Culex quinquefasciatus. Environ. Sci. Pollut. Res. 2020, 27, 36983–36993. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Domingues, V.C.; Gonçalves, G.L.P.; Fernandes, J.B.; Glória, E.M.; Vendramim, J.D. Essential oil from Duguetia lanceolata St.-Hil. (Annonaceae): Suppression of spoilers of stored-grain. Food Biosci. 2020, 36, 100653. [Google Scholar] [CrossRef]
- Domingues, V.D.C.; Ribeiro, L.D.P.; Gonçalves, G.L.P.; Forim, M.R.; Silva, M.F.G.F.; Fernandes, J.B.; Vendramim, J.D. Grain-protectant compounds from Duguetia lanceolata (Annonaceae) derivatives: Bioassay-guided searching and toxicity against the maize weevil. J. Stored Prod. Res. 2020, 85, 101549. [Google Scholar] [CrossRef]
- Gonçalves, G.L.P.; Domingues, V.C.; Ribeiro, L.; Fernandes, J.B.; Silva, M.; Forim, M.R.; Vendramim, J.D. Compounds from Duguetia lanceolata St.- Hil. (Annonaceae) bioactive against Zabrotes subfasciatus (Boheman) (Coleoptera: Chrysomelidae: Bruchinae). Ind. Crops Prod. 2017, 97, 360–367. [Google Scholar] [CrossRef]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Alves, M.S.; Araújo, A.A.L.; Pinto, M.A.O.; Amaral, M.P.H.; Rodarte, M.P.; Kaplan, M.A.C. Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules 2012, 17, 11056–11066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, J.; Zhang, N.; Yu, W.; Jiang, J.; Dai, G. Insecticidal activities of Salvia hispanica L. essential oil and combinations of their main compounds against the beet armyworm Spodoptera exigua. Ind. Crops Prod. 2021, 162, 113271. [Google Scholar] [CrossRef]
- Wu, L.; Huo, X.; Zhou, X.; Zhao, D.; He, W.; Liu, S.; Liu, H.; Feng, T.; Wang, C. Acaricidal activity and synergistic effect of thyme oil constituents against carmine spider mite (Tetranychus cinnabarinus (Boisduval)). Molecules 2017, 22, 1873. [Google Scholar] [CrossRef] [Green Version]
- Kumrungsee, N.; Dunkhunthod, B.; Manoruang, W.; Koul, O.; Pluempanupat, W.; Kainoh, Y.; Yooboon, T.; Piyasaengthong, N.; Bullangpoti, V.; Nobsathian, S. Synergistic interaction of thymol with Piper ribesioides (Piperales: Piperaceae) extracts and isolated active compounds for enhanced insecticidal activity against Spodoptera exigua (Lepidoptera: Noctuidae). Chem. Biol. Technol. Agric. 2022, 9, 38. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Na, Y.; Hou, Z.-B.; Wang, X.-D.; Zhou, F.; Zhang, J.; Wang, J.-L. Acute toxicity of Zanthoxylum bungeanum against two stored product insects and synergistic interactions between two major compounds limonene and linalool. J. Environ. Sci. Health B 2022, 57, 739–744. [Google Scholar] [CrossRef]
- Dutra, K.A.; Teixeira, V.W.; Cruz, G.S.; Silva, C.T.S.; Assunção, C.G.D.; Ferreira, C.G.M.; Monteiro, A.L.B.; Agra Neto, A.C.; Lapa Neto, C.J.C.; Teixeira, A.A.C.; et al. Morphological and immunohistochemical study of the midgut and fat body of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) treated with essential oils of the genus Piper. Biotech. Histochem. 2019, 94, 498–513. [Google Scholar] [CrossRef]
- Cárdenas-Ortega, N.C.; González-Chávez, M.M.; Figueroa-Brito, R.; Flores-Macías, A.; Romo-Asunción, D.; Martínez-González, D.E.; Pérez-Moreno, V.; Ramos-López, M.A. Composition of the essential oil of Salvia ballotiflora (Lamiaceae) and its insecticidal activity. Molecules 2015, 20, 8048–8059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alva, M.; Popich, S.; Borkosky, S.; Cartagena, E.; Bardón, A. Bioactivity of the essential oil of an Argentine collection of Acanthospermum hispidum (Asteraceae). Nat. Prod. Comm. 2012, 7, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.E.C.; Shivaji, R.; Williams, W.P.; Luthe, D.S.; Sandoya, G.V.; Smith, C.L.; Sparks, D.L.; Brown, A.E. A maize line resistant to herbivory constitutively releases (E)-β-Caryophyllene. J. Econ. Entomol. 2012, 105, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Yuan, K.; Xu, H.; Zhang, Y.; Tian, J.; Li, Q.; Zhu, W.; Ye, H. Proteomic and metabolomic revealed differences in the distribution and synthesis mechanism of aroma precursors in yunyan 87 tobacco leaf, stem, and root at the seedling stage. ACS Omega 2022, 7, 33295–33306. [Google Scholar] [CrossRef]
- Vukić, M.D.; Vuković, N.L.; Mladenović, M.; Tomašević, N.; Matić, S.; Stanić, S.; Sapienza, F.; Ragno, R.; Božović, M.; Kačániová, M. Chemical composition of various Nepeta cataria plant organs’ methanol extracts associated with in vivo hepatoprotective and antigenotoxic features as well as molecular modeling investigations. Plants 2022, 11, 2114. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Vendramim, J.D.; Gonçalves, G.L.P.; Ansante, T.F.; Gloria, E.M.; Lopes, J.C.; Mello-Silva, R.; Fernandes, J.B. Searching for promising sources of grain protectors in extracts from Neotropical Annonaceae. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 215–232. [Google Scholar]
- Lago, J.H.G.; Moreira, I.C.; Tanizaki, T.M.; Moreno, P.R.H.; Roque, N.F.; Limberger, R.P.; Apel, M.A.; Henriques, A.T. Mono and Sesquiterpenes from the leaf essential oil of Xylopia brasiliensis Spreng. (Annonaceae). J. Essent. Oil Res. 2003, 15, 406–407. [Google Scholar] [CrossRef]
- Pimenta, L.P.S.; Nascimento, F.C.; Boaventura, M.A.D. Acetogenins from the leaves of Rollinia laurifolia. Helv. Chim. Acta 2005, 88, 3225–3231. [Google Scholar] [CrossRef]
- Nascimento, F.D.C.; Boaventura, M.A.D.; Assunção, A.C.S.; Pimenta, L.P.S. Acetogeninas de anonáceas isoladas de folhas de Rollinia laurifolia. Quim. Nova 2003, 26, 319–322. [Google Scholar] [CrossRef]
- Pimenta, L.P.S.; Nascimento, F.C.; Assunção, A.C.S.; Oliveira, A.B.; Boaventura, M.A.D. Laurifolin, a novel acetogenin from Rollinia laurifolia leaves. Tetrahedron Lett. 2001, 42, 8433–8434. [Google Scholar] [CrossRef]
- Campbell, B.E.; Pereira, R.M.; Koehler, P.G. Complications with controlling insect eggs. In Insecticides Resistance; Trdan, S., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Blossman-Myer, B.L.; Burggren, W.W. Metabolic allometry during development and metamorphosis of the silkworm Bombyx mori: Analyses, patterns, and mechanisms. Physiol. Biochem. Zool. 2010, 83, 215–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, R.F. The Insects Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]








| Species/Synonyms | Mass of Fresh Material (kg) | Collected Part | Oil Mass (g)/yield (%) | Number of Exsiccate | Geographic Coordinates |
|---|---|---|---|---|---|
| Annona neolaurifolia H. Rainer Sin. Rollinia laurifolia Schlt [26] | 2.2 | Leaves | 3.69 (0.19%) | 27,638 | S 21°13.648′; W 044°57.405′ |
| 1.1 | Stem barks | 0 | |||
| 1.2 | Branches | 0 | |||
| Duguetia lanceolata A. St.-Hil. Sin. Aberemoa lanceolata var. parvifolia R.E.Fr. [27] | 1.9 | Leaves | 3.69 (0.19%) | 27,629 | S 21°13.567′; W 044°57.575′ |
| 4.3 | Stem barks | 2.54 (0.058%) | |||
| 1.1 | Branches | 0 | |||
| Xylopia brasiliensis R.E.Fr Sin. Xylopia gracilis (R.E.Fr.) R.E.Fr [26] | 2.9 | Stem barks | 0.13 (0.009%) | 27,636 | S 21°13.732′; W 044°58.064′ |
| 1.4 | Branches | 0.82 (0.028%) |
| Compound | Peak Area a (%) | Probability b (%) | Molecular Formula | AI (Calculated) ** | AI (Literature) *** |
|---|---|---|---|---|---|
| Annona neolaurifolia (leaves) | |||||
| α-pinene | 6.80 | 97 | C10H16 | 931 | 932 |
| Sabinene | 1.47 | 94 | C10H16 | 970 | 969 |
| β-pinene | 6.35 | 97 | C10H16 | 974 | 974 |
| Linalool | 5.45 | 93 | C10H18O | 1099 | 1095 |
| α-terpineol | 1.55 | 94 | C10H18O | 1188 | 1186 |
| α-copaene | 1.57 | 94 | C15H24 | 1371 | 1374 |
| β-cubebene | 1.07 | 94 | C15H24 | 1386 | 1387 |
| (E)-caryophyllene | 13.70 | 94 | C15H24 | 1414 | 1417 |
| α-humulene | 2.93 | 95 | C15H24 | 1448 | 1452 |
| γ-muurolene | 1.44 | 92 | C15H24 | 1473 | 1478 |
| NI * | 5.94 | - | - | 1476 | - |
| NI * | 1.19 | - | - | 1490 | |
| δ-cadinene | 1.26 | 89 | C15H24 | 1520 | - |
| Spathulenol | 2.65 | 92 | C15H24O | 1572 | 1577 |
| Caryophyllene oxide | 7.98 | 95 | C15H24O | 1577 | 1582 |
| NI * | 1.00 | - | - | 1602 | - |
| Duguetia lanceolata (leaves) | |||||
| α-pinene | 1.80 | 97 | C10H16 | 931 | 932 |
| β-pinene | 3.38 | 98 | C10H16 | 975 | 974 |
| β-caryophyllene | 7.43 | 94 | C15H24 | 1414 | 1417 |
| α-caryophyllene | 1.11 | 95 | C15H24 | 1448 | 1452 |
| NI * | 1.28 | - | - | 1467 | - |
| NI * | 1.47 | - | - | 1476 | - |
| NI * | 1.02 | - | - | 1483 | - |
| NI * | 2.48 | - | - | 1521 | - |
| NI * | 4.07 | - | - | 1550 | - |
| Spathulenol | 4.59 | 92 | C15H24O | 1572 | 1577 |
| Caryophyllene oxide | 9.42 | 94 | C15H24O | 1577 | 1582 |
| Elemol | 1.16 | 91 | C15H26O | 1587 | 1548 |
| Guaiol | 2.27 | 92 | C15H26O | 1594 | 1600 |
| NI * | 3.92 | - | - | 1608 | - |
| NI * | 1.53 | - | - | 1624 | - |
| NI * | 1.40 | - | - | 1634 | - |
| NI * | 1.78 | - | - | 1636 | - |
| NI * | 4.44 | - | - | 1644 | - |
| NI * | 1.63 | - | - | 1652 | - |
| NI * | 1.08 | - | - | 1664 | - |
| NI * | 1.81 | - | - | 1682 | - |
| NI * | 1.03 | - | - | 1757 | - |
| NI * | 1.75 | - | - | 1773 | - |
| NI * | 1.19 | - | - | 1783 | - |
| NI * | 1.32 | - | - | 1897 | - |
| Duguetia lanceolata (stem bark) | |||||
| α-pinene | 9.70 | 97 | C10H16 | 931 | 932 |
| β-pinene | 13.55 | 97 | C10H16 | 975 | 974 |
| α-Cubebene | 1.15 | 95 | C15H24 | 1346 | 1348 |
| NI * | 2.66 | - | - | - | |
| γ-muurolene | 1.25 | 92 | C15H24 | 1472 | 1479 |
| δ-cadinene | 1.55 | 90 | C15H24 | 1520 | 1522 |
| Spathulenol | 3.18 | 92 | C15H24O | 1572 | 1577 |
| Caryophyllene oxide | 1.80 | 94 | C15H24O | 1576 | 1582 |
| Guaiol | 1.01 | 91 | C15H26O | 1593 | 1600 |
| NI * | 2.46 | - | - | - | |
| NI * | 4.15 | - | - | - | |
| NI * | 1.94 | - | - | 1637 | 1645 |
| NI * | 6.36 | - | - | - | |
| NI * | 12.47 | - | - | - | |
| NI * | 1.81 | - | - | - | |
| NI * | 1.18 | - | - | - | |
| NI * | 1.82 | - | - | - | |
| NI * | 1.16 | - | - | - | |
| NI * | 1.24 | - | - | - | |
| Xylopia brasiliensis (branches) | |||||
| α-pinene | 1.10 | 97 | C10H16 | 931 | 932 |
| Camphene | 1.60 | 97 | C10H16 | 945 | 946 |
| β-pinene | 1.00 | 97 | C15H24 | 974 | 974 |
| Eucalyptol | 2.43 | 95 | C10H18O | 1028 | 1026 |
| Nopinone | 1.47 | 90 | C9H14O | 1135 | 1135 |
| Myrtenal | 1.73 | 95 | C10H14O | 1194 | 1195 |
| Verbenone | 1.44 | 94 | C10H14O | 1206 | 1204 |
| β-elemene | 1.57 | 96 | C15H24 | 1388 | 1389 |
| β-caryophyllene | 1.58 | 94 | C15H24 | 1414 | 1417 |
| Spathulenol | 43.14 | 94 | C15H24O | 1576 | 1577 |
| NI * | 7.92 | - | - | 1579 | 1582 |
| NI * | 5.26 | - | - | 1626 | - |
| NI * | 2.45 | - | - | 1635 | - |
| NI * | 1.18 | - | - | 1639 | - |
| NI * | 1.61 | - | - | 1650 | - |
| NI * | 1.03 | - | - | 1674 | - |
| Xylopia brasiliensis (stem bark) | |||||
| α-pinene | 5.70 | 97 | C10H16 | 931 | 932 |
| Camphene | 6.10 | 97 | C10H16 | 946 | 946 |
| β-pinene | 7.00 | 98 | C10H16 | 975 | 974 |
| Trans-pinocarveol/Pinocarveol | 1.03 | 95 | C10H16O | 1135 | 1135 |
| Myrtenal | 1.03 | 95 | C10H14O | 1194 | 1195 |
| NI * | 3.19 | - | - | 1372 | - |
| NI * | 2.59 | - | - | 1440 | - |
| NI * | 1.21 | - | - | 1473 | - |
| α-curcumene | 1.60 | 93 | C15H22 | 1480 | 1479 |
| NI * | 1.34 | - | - | 1509 | - |
| NI * | 1.98 | - | - | 1520 | - |
| NI * | 1.75 | - | - | 1550 | - |
| NI * | 1.35 | - | - | 1561 | - |
| Spathulenol | 7.94 | 94 | C15H24O | 1573 | 1577 |
| Caryophyllene oxide | 5.24 | 89 | C15H24O | 1577 | 1582 |
| NI * | 1.40 | - | - | 1587 | - |
| NI * | 1.28 | - | - | - | - |
| NI * | 2.21 | - | - | 1607 | - |
| NI * | 2.27 | - | - | 1623 | - |
| NI * | 1.54 | - | - | 1627 | - |
| Cubenol | 2.82 | 85 | C15H26O | 1638 | 1645 |
| NI * | 1.83 | - | - | 1602 | - |
| NI * | 2.44 | - | - | 1607 | 1652 |
| β-bisabolol | 4.51 | 83 | C15H26O | 1623 | 1674 |
| NI * | 4.67 | - | - | 1627 | - |
| NI * | 2.26 | - | - | 1638 | - |
| NI * | 1.06 | - | - | 1643 | - |
| NI * | 1.22 | - | - | 1648 | 1579 |
| Treatment | df | χ2 | p | b * | e * | LD50 (µg of EO/Insect) | LD90 (µg of EO/Insect) |
|---|---|---|---|---|---|---|---|
| Duguetia lanceolata (stem bark) | 500 | 543.79 | 0.0858 | −1.34 | 24.75 | 24.75 ± 2.0589 | 127.14 ± 27.4170 |
| Duguetia lanceolata (leaves) | 498 | 477.05 | 0.7428 | −3.58 | 38.33 | 38.33 ± 1.3423 | 70.76 ± 4.3037 |
| Cypermethrin | 498 | 571.11 | 0.0514 | −0.83 | 0.01 | 0.01 ± 0.0019 | 0.14 ± 0.0421 |
| Treatment | Emergence (%) | ER (%) | TC * | SR | Emergence (%) | ER (%) | TC * | SR | Emergence (%) | ER (%) | TC * | SR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | 80.7 ± 5.02 a | - | - | 0.54 ns | 80.2 ± 3.89 a | - | - | 0.63 ± 0.07 ns | 78.70 ± 4.39 a | - | - | 0.53 ± 0.03 |
|
Essential oil from D. lanceolata (stem bark) | 5.0 ± 3.7 b | 93.8 | III | ** | 0.0 ± 0.00 c | 100 | IV | 4.00 ± 4.00 b | 94.9 | III | ** | |
|
Essential oil from D. lanceolata (leaves) | 68.3 ± 6.4 a | 14.9 | I | 0.40 ns | 24.2 ± 3.46 b | 69.8 | II | 0.45 ± 0.10 ns | 3.71 ± 2.03 b | 95.3 | III | ** |
| Treatment | Number of Parasitized Eggs | ER (%) | TC * |
|---|---|---|---|
| Acetone | 27.40 ± 1.94 a | - | - |
| Essential oil from D. lanceolata (stem bark) | 0.00 ± 0.00 b | 100.0 | IV |
| Essential oil from D. lanceolata (leaves) | 0.03 ± 0.03 b | 99.9 | IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Paula Rosetti, M.K.; Alves, D.S.; Luft, I.C.; Pompermayer, K.; Scolari, A.S.; de Souza e Silva, G.T.; de Oliveira, M.S.; Vanegas, J.A.G.; Pacule, H.B.; Silva, G.H.; et al. Duguetia lanceolata A. St.-Hil. (Annonaceae) Essential Oil: Toxicity against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and Selectivity for the Parasitoid Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Agriculture 2023, 13, 488. https://doi.org/10.3390/agriculture13020488
de Paula Rosetti MK, Alves DS, Luft IC, Pompermayer K, Scolari AS, de Souza e Silva GT, de Oliveira MS, Vanegas JAG, Pacule HB, Silva GH, et al. Duguetia lanceolata A. St.-Hil. (Annonaceae) Essential Oil: Toxicity against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and Selectivity for the Parasitoid Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Agriculture. 2023; 13(2):488. https://doi.org/10.3390/agriculture13020488
Chicago/Turabian Stylede Paula Rosetti, Mayara Ketllyn, Dejane Santos Alves, Isabela Caroline Luft, Katiane Pompermayer, Andressa Soares Scolari, Gabriela Trindade de Souza e Silva, Murilo Silva de Oliveira, Javier Andrés García Vanegas, Horácio Bambo Pacule, Geraldo Humberto Silva, and et al. 2023. "Duguetia lanceolata A. St.-Hil. (Annonaceae) Essential Oil: Toxicity against Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and Selectivity for the Parasitoid Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae)" Agriculture 13, no. 2: 488. https://doi.org/10.3390/agriculture13020488








