Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Management
2.3. Recorded Data
2.4. Statistical Analysis
3. Results
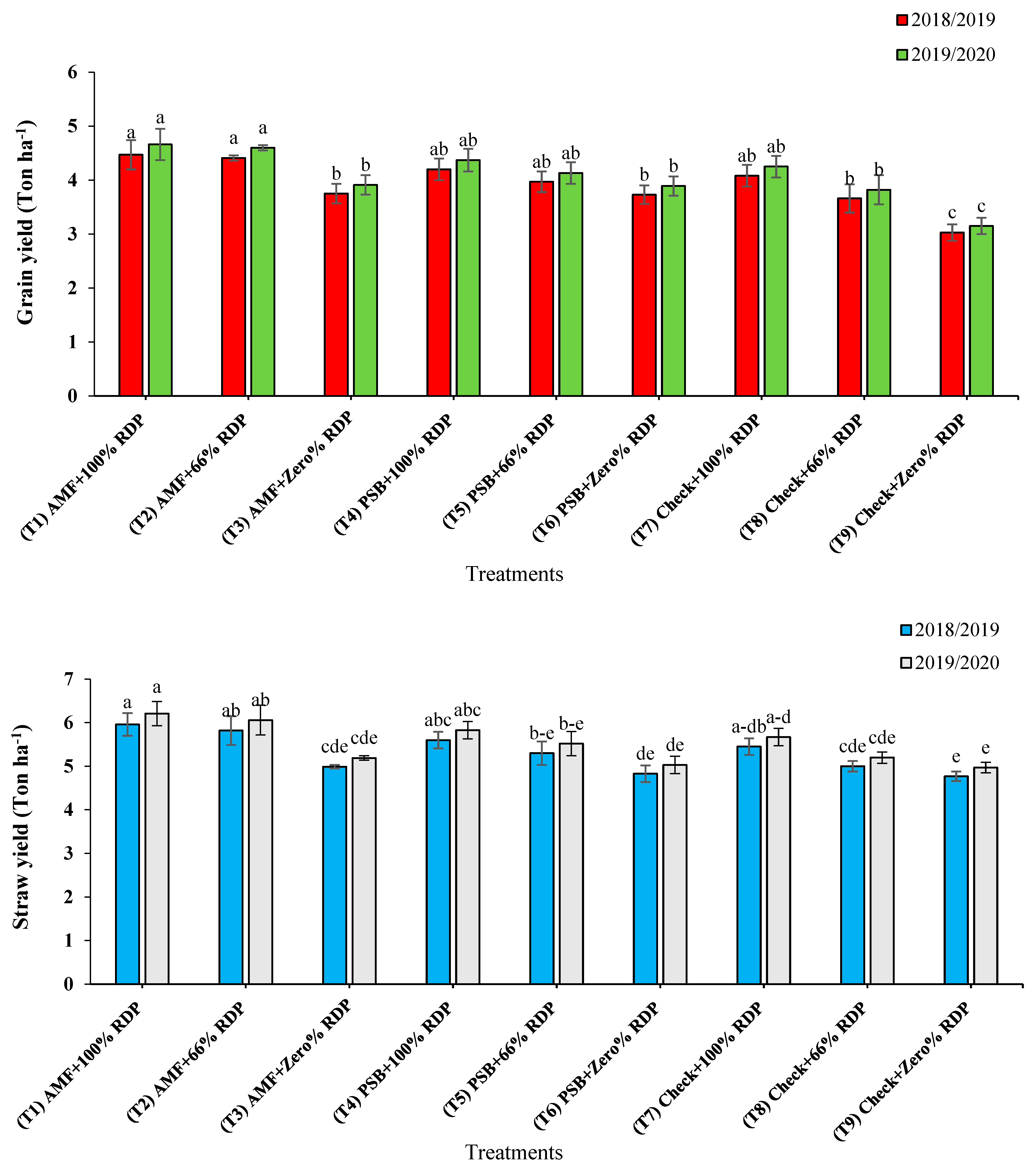
3.1. Agronomic and Yield Attributed Traits
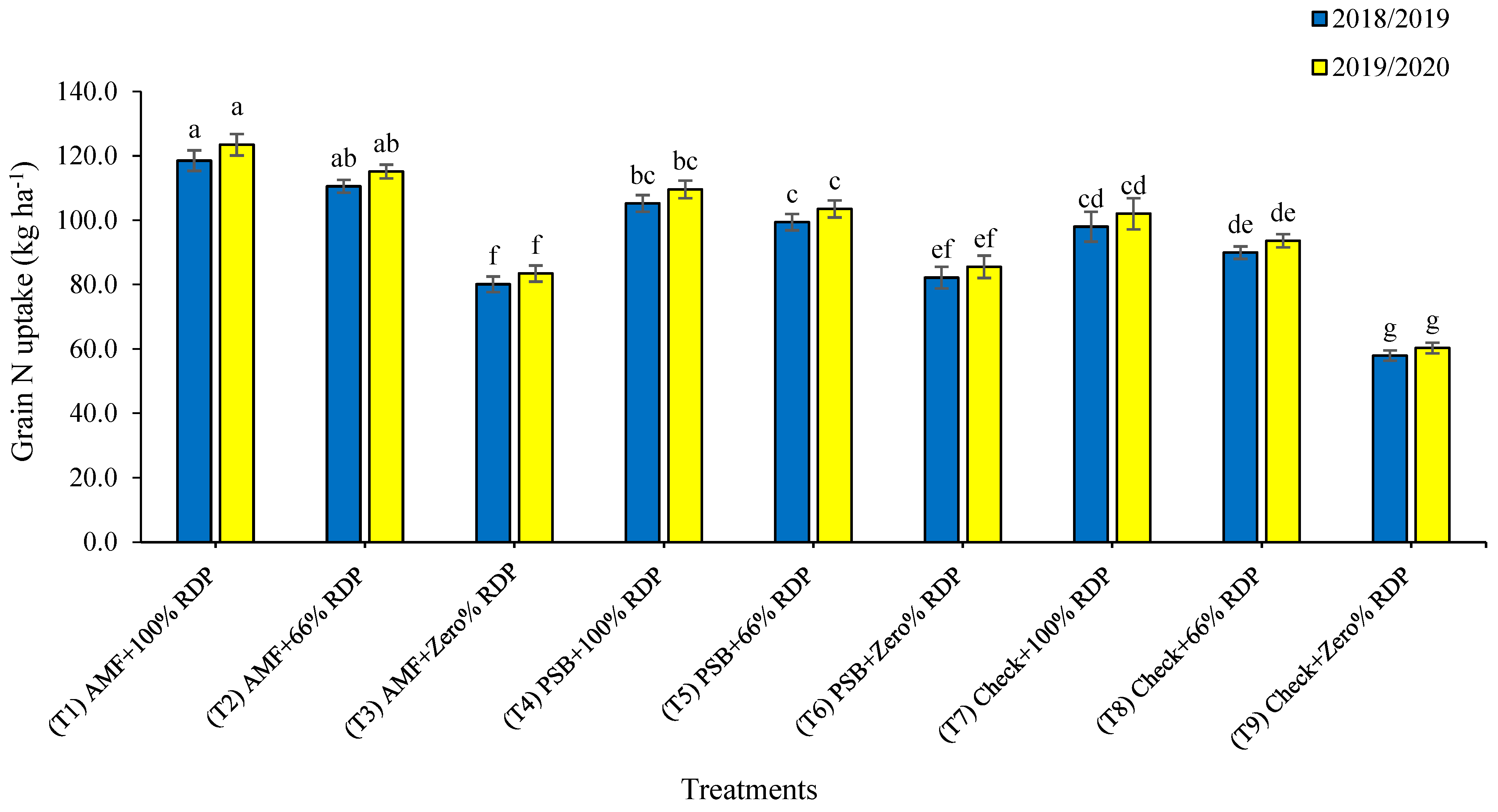
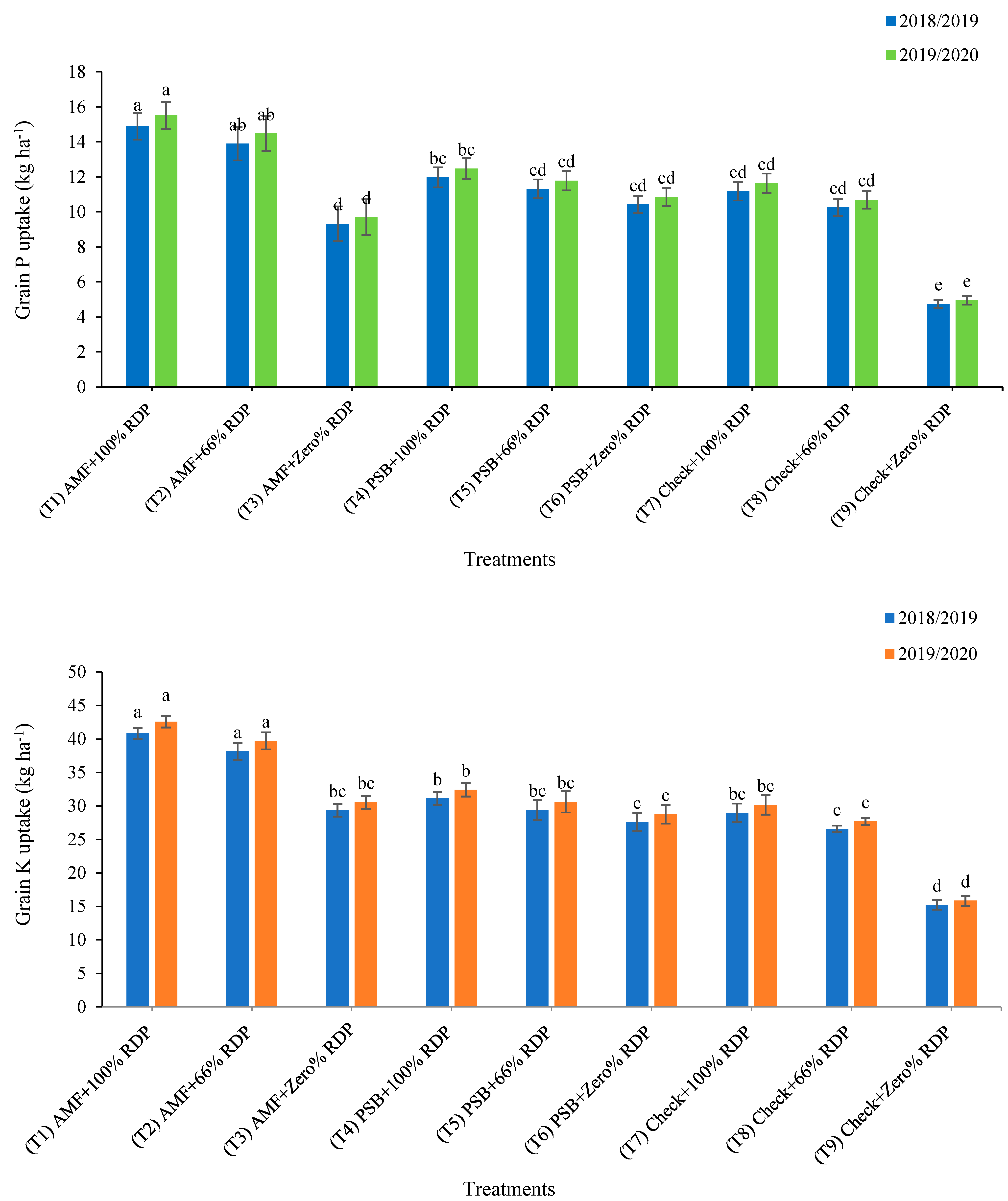
3.2. Nutrients Uptake in Grain and Straw Yields
3.3. Principal Component Analysis (PCA)
4. Discussion
4.1. Agronomic and Yield Attributed Traits
4.2. Nutrients Uptake in Grain and Straw Yields
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization. Faostat, FAO Statistics Division, January 2022. 2022. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 2 January 2023).
- Zhou, M.X. Barley Production and Consumption. In Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.; Ehsanzadeh, P. Photosynthetic Pigments, Ionic and Antioxidative Behaviour of Hulled Tetraploid Wheat in Response to NaCl. Photosynthetica 2016, 54, 340–350. [Google Scholar] [CrossRef]
- Masrahi, A.; Somenahally, A.; Gentry, T. Interactions of Arbuscular Mycorrhizal Fungi with Hyphosphere Microbial Communities in a Saline Soil: Impacts on Phosphorus Availability and Alkaline Phosphatase Gene Abundance. Soil Syst. 2020, 4, 63. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant Systems and O2.−/H2O2 Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 Mediates the Crosstalk of Brassinosteroid and Abscisic Acid in Tomato Responses to Heat and Oxidative Stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and Physiological Analysis of Drought Stress in Arabidopsis Reveals Early Responses Leading to Acclimation in Plant Growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal Transduction during Cold, Salt, and Drought Stresses in Plants. Mol. Biol. Rep. 2011, 39, 969–987. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible Solute Accumulation and Stress-Mitigating Effects in Barley Genotypes Contrasting in Their Salt Tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef]
- Theodorou, M.E.; Plaxton, W.C. Metabolic Adaptations of Plant Respiration to Nutritional Phosphate Deprivation. Plant Physiol. 1993, 101, 339–344. [Google Scholar] [CrossRef]
- Zen El-Dein, A.A.M.; Koriem, M.H.M.; Alsubeie, M.S.; Alsalmi, R.A.; Masrahi, A.S.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Awad-Allah, M.M.A.; Hefny, Y.A.A. Effect of Mycorrhiza Fungi, Preceding Crops, Mineral and Bio Fertilizers on Maize Intercropping with Cowpea. Agriculture 2022, 12, 1934. [Google Scholar] [CrossRef]
- Fernández Bidondo, L.; Bompadre, J.; Pergola, M.; Silvani, V.; Colombo, R.; Bracamonte, F.; Godeas, A. Differential Interaction between Two Glomus Intraradices Strains and a Phosphate Solubilizing Bacterium in Maize Rhizosphere. Pedobiologia 2012, 55, 227–232. [Google Scholar] [CrossRef]
- Gibson, T.S. Carbohydrate Metabolism and Phosphorus/Salinity Interactions in Wheat (Triticum aestivum L.). Plant Soil 1988, 111, 25–35. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Bargaz, A.; Nassar, R.M.A.; Rady, M.M.; Gaballah, M.S.; Thompson, S.M.; Brestic, M.; Schmidhalter, U.; Abdelhamid, M.T. Improved Salinity Tolerance by Phosphorus Fertilizer in Two Phaseolus Vulgaris Recombinant Inbred Lines Contrasting in Their P-Efficiency. J. Agron. Crop Sci. 2016, 202, 497–507. [Google Scholar] [CrossRef]
- Khosh Kholgh Sima, N.A.; Ahmad, S.T.; Alitabar, R.A.; Mottaghi, A.; Pessarakli, M. Interactive effects of salinity and phosphorus nutrition on physiological responses of two barley species. J. Plant Nutr. 2012, 35, 1411–1428. [Google Scholar] [CrossRef]
- Cakmakci, R.; Donmez, M.F.; Erdogan, U. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk. J. Agric. For. 2007, 31, 189–199. [Google Scholar]
- Relwani, L.; Krishna, P.; Sudhakara Reddy, M. Effect of Carbon and Nitrogen Sources on Phosphate Solubilization by a Wild-Type Strain and UV-Induced Mutants of Aspergillus Tubingensis. Curr. Microbiol. 2008, 57, 401–406. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of Salt-Tolerant Plant Growth-Promoting Rhizobacteria on Wheat Plants and Soil Health in a Saline Environment. Plant Biol. 2014, 17, 288–293. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Mustafa, E.M.A. Response of Guar to Bradyrhizobium inoculation and to nitrogen and phosphate fertilization. J. Agric. Sci. 2005, 13, 97–110. [Google Scholar]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular Mycorrhizal Symbiosis and Abiotic Stress in Plants: A Review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Gomaa, M.A.; Zen El-Dein, A.A.M.; El-Sorady, G.A.; Salama, N.G.A. Productivity of Maize in Relation to Preceding Crops. Egypt. Acad. J. Biol. Sci. H Bot. 2021, 12, 135–145. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops-a meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Mobasser, H.R.; Moradgholi, A. Mycorrhizal bio-fertilier applications on yield seed corn varieties in Iran. Ann. Biol. Res. 2012, 3, 1109–1116. [Google Scholar]
- Abdullahi, R.; Sheriff, H.H. Effect of arbuscular mycorrhizal fungi and chemical fertilizer on growth and shoot nutrients content of onion under field condition in Northern Sudan Savanna Nigeria. J. Agric. Vet. Sci. 2013, 3, 85–90. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi, P. Physiological interaction between symbionts in VA-mycorrhizal plants. Annu. Rev. Plant Physiol. 1988, 39, 221–224. [Google Scholar] [CrossRef]
- Sharifi, M.; Ghorbanli, M.; Ebrahimzadeh, H. Improved Growth of Salinity-Stressed Soybean after Inoculation with Salt Pre-Treated Mycorrhizal Fungi. J. Plant Physiol. 2007, 164, 1144–1151. [Google Scholar] [CrossRef]
- Baillie, I.C. Soil Survey Staff 1999, Soil Taxonomy: A basic system of soil classification for making and interpreting soil surveys, 2nd edition. Soil Use Manag. 2001, 17, 57–60. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez, Y. Manual for Identification of Vesicular Arbuscular Mycorrhizal Fungi (INVAM); University of Florida: Gainesville, FL, USA, 1990. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of A.O.A.C. International, 17th ed.; Horwitz, S.W., Ed.; AOAC: Rockville, MD, USA, 2000; Volume 2, pp. 66–68. [Google Scholar]
- Chapman, H.D.; Parker, F. Methods of analysis for soil, plant, and water. J. Plant Nutr. 1961, 22, 121–128. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis-Chemical and Microbiology Properties; American Society of Agronomy Inc.: Madison, WI, USA, 1982; 1159p. [Google Scholar]
- Casella, G. Statistical Design; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- CoStat Ver. 6.4, Cohort Software 798 Light House Ave. 2005. Available online: http://www.cohort.com/DownloadCoStatPart2.html (accessed on 19 March 2021).
- Duncan, S., Jr. Nonverbal Communication. Psychol. Bull. 1969, 72, 118–137. [Google Scholar] [CrossRef]
- Gabriel, K.R. The Biplot Graphic Display of Matrices with Application to Principal Component Analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- JMP®, Version 13.2.0; SAS Institute Inc.: Cary, NC, USA, 2017.
- Mehrvarz, S.; Chaichi, M.R. Effect of phosphate solubilizing microorganisms and phosphorus chemical fertilizer on forage and grain quality of barely (Hordeum vulgare L.). Am.-Eurasian J. Agric. Environ. Sci. 2008, 3, 855–860. [Google Scholar]
- Suri, V.K.; Choudhary, A.K. Comparative performance of mycorrhizal fungi and applied phosphorus in wheat under controlled environment in phosphorus deficient acid alfisol. J. Prog. Agric. 2010, 10, 23–28. [Google Scholar]
- Awasthi, R.; Tewari, R.; Nayyar, H. Synergy between plants and P-solubilizing microbes in soils: Effects on growth and physiology of crops. Inter. Res. J. Microbiol. 2011, 2, 484–503. [Google Scholar]
- Lone, A.A.; Mahdi, S.S.; Bhat, M.I.; Bhat, R.A.; Faisul, R.; Singh, O.P. Productivity and phosphorus use efficiency of wheat (Triticum aestivum L.) as influenced by biofertilizers and phosphorus under subtropical conditions of UP. Environ. Ecol. 2011, 29, 1321–1325. [Google Scholar]
- Şahin, F.; Çakmakçi, R.; Kantar, F. Sugar Beet and Barley Yields in Relation to Inoculation with N2-Fixing and Phosphate Solubilizing Bacteria. Plant Soil 2004, 265, 123–129. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Radzian, O.; Zaharah, A.R.; Sariah, M.; Razi, I.M. Role of phosphate solubilizing bacteria on rock phosphate solubility and growth of aerobic rice. J. Environ. Biol. 2011, 32, 607–612. [Google Scholar]
- Najafi, A.; Ardakani, M.R.; Rejali, F.; Sajedi, N. Response of winter barley to co-inoculation with Azotobacter and Mycorrhiza fungi influenced by plant growth promoting rhizobacteria. Ann Biol. Res. 2012, 3, 4002–4006. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and Their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, G.; Kismányoky, T.; Sárdi, K. Effect of Nitrogen Fertilization and Residue Management on the Productivity of Winter Barley (Hordeum vulgare L.). Acta Agron. Hung. 2013, 61, 101–111. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological Hormone Imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, G.; Garg, N. Alleviation of salt-induced ionic, osmotic and oxidative stresses in Cajanus cajan nodules by AM inoculation. Plant Biosyst. 2011, 145, 88–97. [Google Scholar] [CrossRef]
- Gundel, P.E.; Mart ínez-Ghersa, M.A.; Omacini, M.; Cuyeu, R.; Pagano, E.; Ríos, R.; Ghersa, C.M. Mutualism effectiveness and vertical transmission of symbiotic fungal endophytes in response to host genetic background. Evol. Appl. 2012, 5, 838–849. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought and stress tolerance. Photosynthetica 2000, 38, 187–206. [Google Scholar] [CrossRef]
- Kheirizadeh Arough, Y.; Seyed Sharifi, R.; Sedghi, M.; Barmaki, M. Effect of zinc and bio-fertilizers on antioxidant enzymes activity, chlorophyll content, soluble sugars and proline in Triticale under salinity condition. Not. Bot. Hort. Agrobo. 2016, 44, 116–124. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.S.; Li, X.L.; Tian, C.Y.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar] [CrossRef]
- Dadashzadeh, S.; Shariff, R.S.; Farzaneh, S. Physiological and Biochemical Responses of Barley to Application of Bio-Fertilizers and Nano Iron Oxide under Salinity Stress in Greenhouse. Bangladesh J. Bot. 2018, 47, 863–875. [Google Scholar] [CrossRef]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous Application of Mannitol and Thiourea Regulates Plant Growth and Oxidative Stress Responses in Salt-Stressed Maize (Zea mays L.). J. Plant Interact. 2013, 8, 234–241. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sayar, R.; Khemira, H.; Kamel, A.; Mosbahi, M. Physiological tests as predictive appreciation for drought tolerance in durum wheat (Triticum durum Desf.). Agron. Res. 2008, 6, 79–90. [Google Scholar]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt Tolerance in Zea mays (L.). Following Inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Zago, M.P.; Oteiza, P.I. The Antioxidant Properties of Zinc: Interactions with Iron and Antioxidants. Free. Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.-J. Trichoderma Reesei Improved the Nutrition Status of Wheat Crop under Salt Stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef]
- Bagheri, A.A.; Saadatmand, S.; Niknam, V.; Nejadsatari, T.; Babaeizad, V. Effect of endophytic fungus, Piriformospora indica, on growth and activity of antioxidant enzymes of rice (Oryza sativa L.) under salinity stress. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1337–1350. [Google Scholar]
- Pessarakli, M.; Huber, J.T. Biomass production and protein synthesis by alfalfa under salt stress. J. Plant Nutr. 1991, 14, 283–293. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Wang, G.; Liu, X.; Amombo, E. The fungus aspergillus aculeatus enhances salt-stress tolerance, metabolite accumulation, and improves forage quality in perennial ryegrass. Front. Microbiol. 2017, 8, 1664. [Google Scholar] [CrossRef] [PubMed]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.K.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Ripa, F.A.; Cao, W.; Tong, S.; Sun, J. Assessment of plant growth promoting and abiotic stress tolerance properties of wheat endophytic fungi. Biomed. Res. Int. 2019, 2019, 6105865. [Google Scholar] [CrossRef]
- Omar, M.N.A.; Osman, M.E.H.; Kasim, W.A.; Abd El-Daim, I.A. Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasilense. In Salinity and Water Stress; Springer: Berlin, Germany, 2009; pp. 133–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, B.; Li, H.; Zhou, W.; Takeuchi, Y.; Yoneyama, K. Effect of salinity on physiological characteristics, yield and quality of microtubers in vitro in potato. Acta Physiol. Plant 2005, 27, 481–489. [Google Scholar] [CrossRef]
- Zein, F.I.; Gaiza, E.A.; EL-Sanafawy, H.M.; Talha, N.I. Effect of specific ions, salinity and alkalinity on yield and quality of some Egyptian cotton genotypes. Egypt. J. Soil Sci. 2020, 60, 183–194. [Google Scholar] [CrossRef]
- Azcón, R.; Barea, J.-M. Mycorrhizosphere Interactions for Legume Improvement. Microbes Legume Improv. 2010, 59, 237–271. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Nehls, U.; Hampp, R.; Poralla, K.; Cardoso, E.J.B.N. Mycorrhiza and Soil Bacteria Influence Extractable Iron and Manganese in Soil and Uptake by Soybean. Plant Soil 2007, 298, 273–284. [Google Scholar] [CrossRef]
- Heydari, M.M.; Maleki, A. Effect of phosphorus sources and mycorrhizal inoculation on root colonization and phosphorus uptake of barley (Hordeum vulgare L.). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 235–248. [Google Scholar]
- Abou-Aly, H.E.; Mady, M.A. Complemented effect of humic acid and biofertilizers on wheat (Triticum aestivum L.) productivity. Ann. Agric. Sci. Moshtohor 2009, 47, 1–12. [Google Scholar]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms, and their role in crop production: A review paper. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Thalooth, T.; Bahr, A.; Tawfik, M.M. Productivity of some barley cultivars as affected by inoculation under water stress conditions. Elixir Appl. Bot. 2012, 51, 10743–10749. [Google Scholar]
- Wali Asal, M.; Shamseldin, A.; Radwan, F.I.; Abd El Lateef, E.M.; Zaki, N.M. Response of barley (Hordeum vulgare L) cultivars to humic acid, mineral and biofertilization under calcareous soil conditions. Middle East J. Agric. Res. 2018, 7, 71–82. [Google Scholar]
- Bekele, T.; HÖfner, W. Effects of Different Phosphate Fertilizers on Yield of Barley and Rape Seed on Reddish Brown Soils of the Ethiopian Highlands. Fertil. Res. 1993, 34, 243–250. [Google Scholar] [CrossRef]

| Season | Physical Property | Chemical Property | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand% | Silt% | Clay% | pH | EC (dS m−1) | SAR | ESP | Soluble Cation (meq 100 g−1 soil) | Soluble Anions (meq 100 g−1 soil) | ||||||
| Na+ | K+ | Ca++ | Mg++ | HCO3− | Cl− | SO4−− | ||||||||
| 2018/2019 | 28.34 | 23.45 | 48.21 | 8.21 | 10.53 | 18.64 | 42.23 | 43.40 | 1.14 | 9.86 | 29.63 | 58.30 | 40.90 | 14.30 |
| 2019/2020 | 25.32 | 26.44 | 48.24 | 8.22 | 10.65 | 18.76 | 42.21 | 43.70 | 1.15 | 9.88 | 29.65 | 58.60 | 40.30 | 14.60 |
| Month | Air Temperature (oc) | Relative Humidity (%) | Precipitation (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||||
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| December | 12.7 | 11.2 | 25.9 | 22.7 | 33.3 | 30.1 | 1.08 | 0.62 |
| January | 11.4 | 10.0 | 24.5 | 19.8 | 45.4 | 41.7 | 2.07 | 2.24 |
| February | 10.1 | 9.3 | 22.7 | 21.2 | 43.5 | 40.5 | 5.35 | 5.78 |
| March | 12.9 | 11.2 | 24.3 | 23.2 | 42.9 | 43.7 | 0.65 | 0.51 |
| April | 13.7 | 15.5 | 25.2 | 26.1 | 50.8 | 50.6 | 0.00 | 0.00 |
| Treatments | Plant Height (cm) | Spike Length (cm) | Spikes Weight (g) | |||
|---|---|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| (T1) AMF + 100% RDP | 111.50 ± 1.32 a | 116.14 ± 1.34 a | 10.47 ± 0.12 a | 10.91 ± 0.13 a | 3.46 ± 0.16 a | 3.61 ± 0.17 a |
| (T2) AMF + 66% RDP | 109.63 ± 1.27 a | 114.19 ± 1.33 a | 10.13 ± 0.62 a | 10.56 ± 0.64 a | 3.40 ± 0.16 ab | 3.54 ± 0.17 ab |
| (T3) AMF + Zero% RDP | 100.65 ± 1.24 cd | 104.82 ± 1.32 cd | 9.70 ± 0.27 abc | 10.10 ± 0.29 abc | 3.32 ± 0.16 ab | 3.45 ± 0.16 ab |
| (T4) PSB + 100% RDP | 105.17 ± 1.09 b | 109.51 ± 1.15 b | 10.04 ± 0.21 ab | 10.46 ± 0.22 ab | 3.34 ± 0.16 ab | 3.48 ± 0.17 ab |
| (T5) PSB + 66% RDP | 103.30 ± 1.21 bc | 107.56 ± 1.26 bc | 9.68 ± 0.49 abc | 10.08 ± 0.51 abc | 3.12 ± 0.15 ab | 3.26 ± 0.16 ab |
| (T6) PSB + Zero% RDP | 101.07 ± 1.07 cd | 105.22 ± 1.14 cd | 8.94 ± 0.39 bcd | 9.30 ± 0.41 bcd | 2.58 ± 0.12 cd | 2.69 ± 0.13 cd |
| (T7) Control + 100% RDP | 101.40 ± 1.59 bcd | 105.56 ± 1.66 bcd | 9.38 ± 0.42 abc | 9.77 ± 0.43 abc | 3.17 ± 0.15 ab | 3.30 ± 0.16 ab |
| (T8) Control + 66% RDP | 99.23 ± 1.79 d | 103.30 ± 1.87 d | 8.85 ± 0.42 cd | 9.21 ± 0.44 cd | 2.96 ± 0.14 bc | 3.08 ± 0.15 bc |
| (T9) Control + Zero% RDP | 93.10 ± 0.75 e | 96.90 ± 0.80 e | 7.86 ± 0.26 d | 8.41 ± 0.27 d | 2.47 ± 0.12 d | 2.57 ± 0.12 d |
| Treatments | Number of Spikes Plant−1 | 1000-Grain Weight (g) | ||
|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| (T1) AMF + 100% RDP | 59.03 ± 2.80 a | 61.49 ± 2.93 a | 53.55 ± 1.10 a | 55.78 ± 1.16 a |
| (T2) AMF + 66% RDP | 57.61 ± 2.74 ab | 60.00 ± 2.86 ab | 52.64 ± 0.97 abc | 54.82 ± 1.01 abc |
| (T3) AMF + Zero% RDP | 55.53 ± 2.64 ab | 57.84 ± 2.75 ab | 52.14 ± 1.66 abc | 54.30 ± 1.73 abc |
| (T4) PSB + 100% RDP | 58.29 ± 2.77 ab | 60.70 ± 2.89 ab | 53.18 ± 1.00 ab | 55.38 ± 1.05 ab |
| (T5) PSB + 66% RDP | 56.17 ± 2.67 ab | 58.49 ± 2.78 ab | 52.77 ± 0.54 abc | 54.94 ± 0.57 abc |
| (T6) PSB + Zero% RDP | 47.44 ± 2.25 cd | 49.39 ± 2.35 cd | 50.06 ± 0.63 c | 52.12 ± 0.65 c |
| (T7) Control + 100% RDP | 53.60 ± 2.55 abc | 55.80 ± 2.65 abc | 53.05 ± 1.24 ab | 55.22 ± 1.27 ab |
| (T8) Control + 66% RDP | 51.02 ± 2.42 bcd | 53.11 ± 2.53 bcd | 50.40 ± 0.71 abc | 52.46 ± 0.72 abc |
| (T9) Control + Zero% RDP | 44.13 ± 2.10 d | 43.94 ± 2.19 d | 46.64 ± 1.02 d | 48.54 ± 1.06 d |
| Treatments | Biological Yield (Ton ha−1) | Harvest Index (%) | ||
|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| (T1) AMF + 100% RDP | 11.22 ± 0.29 a | 11.69 ± 0.31 a | 39.79 ± 1.88 abc | 39.82 ± 1.89 abc |
| (T2) AMF + 66% RDP | 10.20 ± 0.25 abc | 10.63 ± 0.26 abc | 43.31 ± 1.57 a | 43.32 ± 1.52 a |
| (T3) AMF + Zero% RDP | 9.19 ± 0.43 cd | 9.57 ± 0.46 cd | 40.84 ± 0.01 ab | 40.88 ± 0.03 ab |
| (T4) PSB + 100% RDP | 10.58 ± 0.5 ab | 11.02 ± 0.51 ab | 39.66 ± 0.02 abc | 39.69 ± 0.08 abc |
| (T5) PSB + 66% RDP | 9.27 ± 0.44 cd | 9.65 ± 0.46 cd | 43.16 ± 3.93 a | 43.12 ± 3.94 a |
| (T6) PSB + Zero% RDP | 9.53 ± 0.45 bcd | 9.92 ± 0.47 bcd | 39.16 ± 0.02 abc | 39.20 ± 0.02 abc |
| (T7) Control + 100% RDP | 11.02 ± 0.53 a | 11.47 ± 0.55 a | 37.05 ± 0.01 bc | 37.03 ± 0.06 bc |
| (T8) Control + 66% RDP | 9.65 ± 0.46 bcd | 10.04 ± 0.48 bcd | 37.87 ± 0.88 bc | 37.94 ± 0.91 bc |
| (T9) Control + Zero% RDP | 8.58 ± 0.40 d | 8.92 ± 0.42 d | 35.37 ± 0.06 c | 35.32 ± 0.04 c |
| Treatments | Straw N Uptake (kg ha−1) | Straw P Uptake (kg ha−1) | Straw K Uptake (kg ha−1) | |||
|---|---|---|---|---|---|---|
| 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | 2018/2019 | 2019/2020 | |
| (T1) AMF + 100% RDP | 61.57 ± 2.95 ab | 64.13 ± 3.07 ab | 14.52 ± 0.77 a | 15.13 ± 0.8 a | 33.44 ± 1.38 a | 34.83 ± 1.44 a |
| (T2) AMF + 66% RDP | 60.21 ± 2.99 b | 62.71 ± 3.12 b | 14.21 ± 0.95 ab | 14.80 ± 0.99 ab | 32.71 ± 0.55 a | 34.06 ± 0.58 a |
| (T3) AMF + Zero% RDP | 33.79 ± 1.83 c | 35.18 ± 1.89 c | 10.48 ± 0.92 d | 10.92 ± 0.97 d | 24.77 ± 0.88 e | 25.79 ± 0.91 e |
| (T4) PSB + 100% RDP | 69.13 ± 3.28 a | 71.99 ± 3.42 a | 12.90 ± 0.98 abc | 13.43 ± 1.02 abc | 31.00 ± 0.97 ab | 32.28 ± 1.01 ab |
| (T5) PSB + 66% RDP | 65.44 ± 3.11 ab | 68.13 ± 3.24 ab | 12.21 ± 0.58 bcd | 12.71 ± 0.6 bcd | 29.34 ± 0.94 bc | 30.55 ± 0.97 bc |
| (T6) PSB + Zero% RDP | 59.61 ± 1.00 b | 62.06 ± 1.06 b | 10.48 ± 0.5 d | 10.91 ± 0.52 d | 22.93 ± 1.09 e | 23.88 ± 1.13 e |
| (T7) Control + 100% RDP | 63.60 ± 3.02 ab | 66.21 ± 3.15 ab | 11.79 ± 0.56 cd | 12.27 ± 0.58 cd | 28.02 ± 1.33 cd | 29.17 ± 1.39 cd |
| (T8) Control + 66% RDP | 58.35 ± 2.77 b | 60.74 ± 2.90 b | 10.83 ± 0.35 cd | 11.28 ± 0.36 cd | 25.71 ± 1.22 de | 26.76 ± 1.27 de |
| (T9) Control + Zero% RDP | 27.45 ± 1.30 c | 28.57 ± 1.36 c | 5.12 ± 0.24 e | 5.33 ± 0.25 e | 12.82 ± 0.61 f | 13.34 ± 0.63 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masrahi, A.S.; Alasmari, A.; Shahin, M.G.; Qumsani, A.T.; Oraby, H.F.; Awad-Allah, M.M.A. Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture 2023, 13, 537. https://doi.org/10.3390/agriculture13030537
Masrahi AS, Alasmari A, Shahin MG, Qumsani AT, Oraby HF, Awad-Allah MMA. Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture. 2023; 13(3):537. https://doi.org/10.3390/agriculture13030537
Chicago/Turabian StyleMasrahi, Abdurrahman S., Abdulrahman Alasmari, Mostafa G. Shahin, Alaa T. Qumsani, Hesham F. Oraby, and Mamdouh M. A. Awad-Allah. 2023. "Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil" Agriculture 13, no. 3: 537. https://doi.org/10.3390/agriculture13030537
APA StyleMasrahi, A. S., Alasmari, A., Shahin, M. G., Qumsani, A. T., Oraby, H. F., & Awad-Allah, M. M. A. (2023). Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture, 13(3), 537. https://doi.org/10.3390/agriculture13030537







