Nutritional Value and Sensory Quality of New Potatoes in Response to Silicon Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Design
2.3. Data Analysis
3. Results
3.1. Tuber Yield
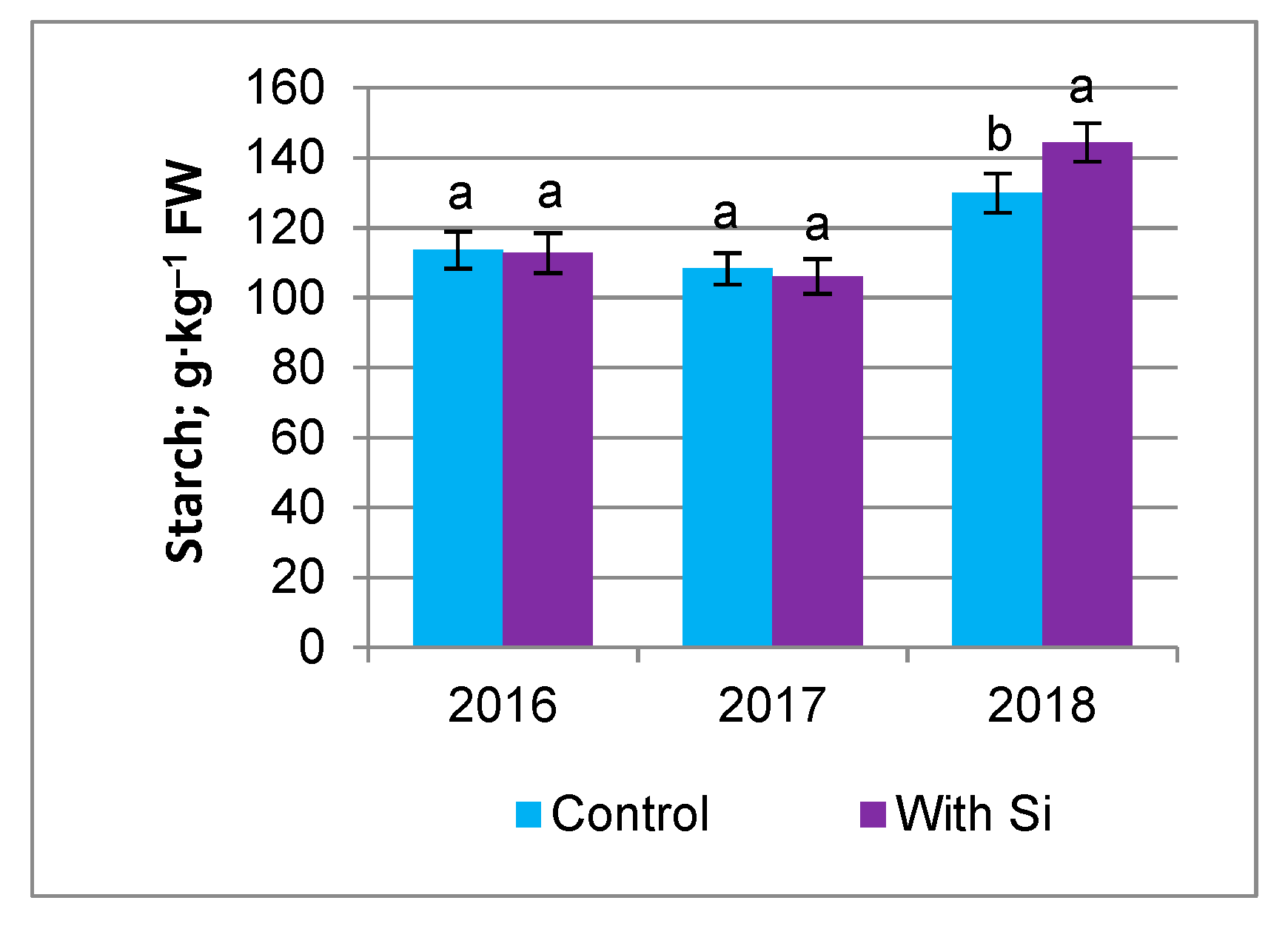
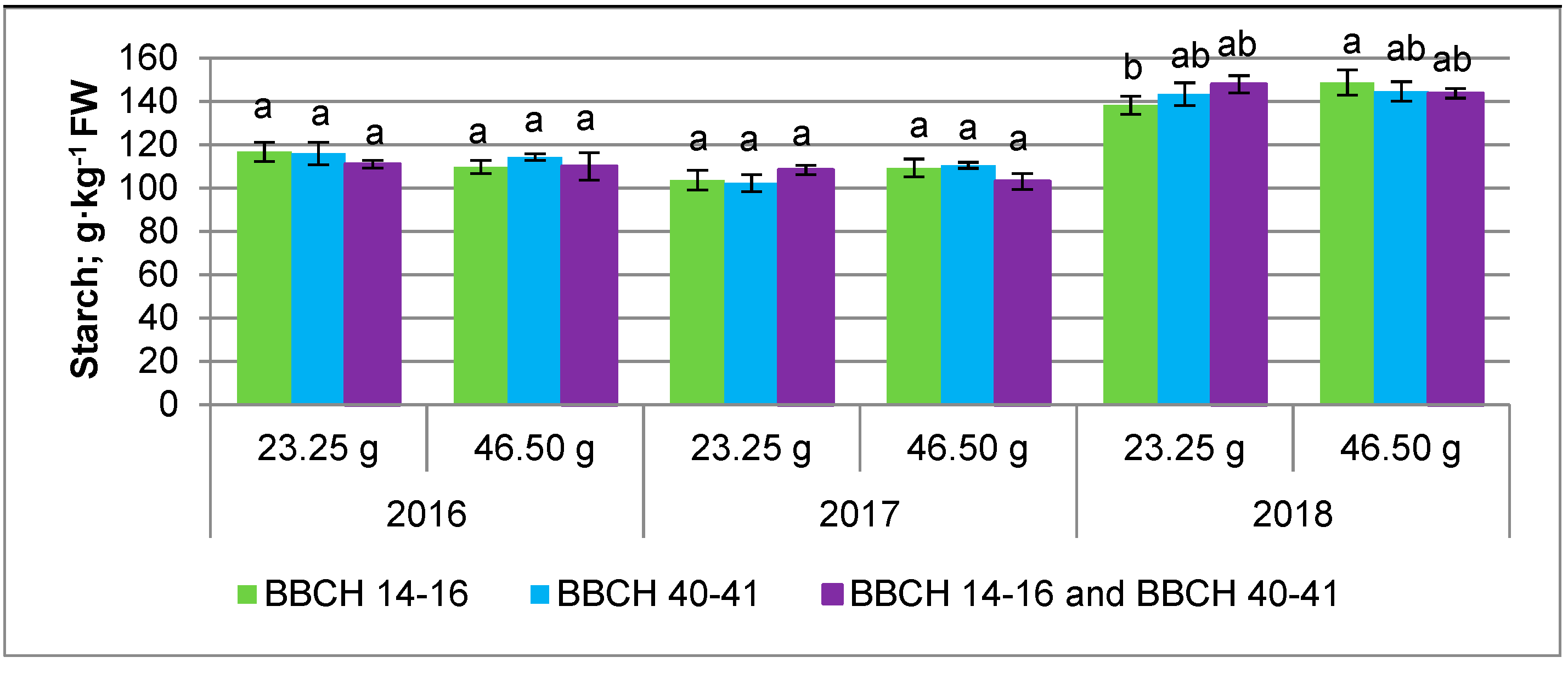
3.2. Nutrient Content
3.3. Darkening of Cooked Potatoes
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beals, K.A. Potatoes, nutrition and health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Navarre, D.A.; Brown, C.R.; Sathuvalli, V.R. Potato vitamins, minerals and phytonutrient from a plant biology perspective. Am. J. Potato Res. 2019, 96, 111–126. [Google Scholar] [CrossRef]
- Wijesinha-Bettoni, R.; Mouillé, B. The contribution of potatoes to global food security, nutrition and healthy diets. Am. J. Potato Res. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Ierna, A.; Melilli, M.G. Ascorbic acid and total phenolic content in early potatoes as affected by growing season, genotype and harvest time. Acta Hortic. (ISHS) 2014, 1040, 133–141. [Google Scholar] [CrossRef]
- Trawczyński, C. The influence of cultivars and weather conditions of vegetation period on the content of some nutritional and anti-nutritional components in potato tubers. Acta Agrophys. 2016, 23, 119–128. [Google Scholar]
- Leonel, M.; do Carmo, E.L.; Fernandes, A.M.; Soratto, R.P.; Ebúrneo, J.A.M.; Garcia, E.L.; dos Santos, T.P.R. Chemical composition of potato tubers: The effect of cultivars and growth conditions. J. Food Sci. Technol. 2017, 54, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Wegener, C.B.; Jürgens, H.U.; Jansen, G. Drought stress affects nutritional and bioactive compounds in potatoes (Solanum tuberosum L.) relevant to human health. Func. Food Health Dis. 2017, 7, 17–35. [Google Scholar] [CrossRef]
- Jayanty, S.S.; Diganta, K.; Raven, B. Effects of cooking methods on nutritional content in potato tubers. Am. J. Potato Res. 2019, 96, 183–194. [Google Scholar] [CrossRef]
- Wang-Pruski, G.; Nowak, J. Potato after-cooking darkening. Am. J. Potato Res. 2004, 81, 7–16. [Google Scholar] [CrossRef]
- Hussain, R.; Sanabil; Huali, X.; Kumar, A.; Perveen, R.; Fatima, I.; Tawfeuk, H.A. Discoloration of raw and cooked potatoes: Fundamentals of nature, mechanisms, causes, and controls. Am. J. Potato Res. 2022, 99, 287–306. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of drought stress on potato production: A review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Sawicka, B.; Michałek, W.; Pszczółkowski, P. The relationship of potato tubers chemical composition with selected physiological indicators. Zemdirb.-Agric. 2015, 102, 41–50. [Google Scholar] [CrossRef]
- Starck, Z. Effect of stress conditions on coordination of photosynthetic production and resources allocation. Post. Nauk Rol. 2010, 1, 9–26. (In Polish) [Google Scholar]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought stress-induced changes in starch yield and physiological traits in potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Caradonia, F.; Ronga, D.; Tava, A.; Francia, E. Plant biostimulants in sustainable potato production: An overviev. Potato Res. 2022, 65, 83–104. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Ahire, M.L.; Mundada, P.S.; Nikam, T.D.; Bapat, V.A.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef]
- Kovács, S.; Kutasy, E.; Csajbók, J. The multiple role of silicon in alleviating environmental stresses in sustainable crop production. Plants 2022, 11, 1223. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional role of silicon to activate resilient plant growth and mitigate abiotic stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Laane, H.M. The effect of the application of foliar sprays with stabilized silicic acid: An overview of the results from 2003–2014. Silicon 2017, 9, 803–807. [Google Scholar] [CrossRef]
- Ługowska, M. Effect of bio-stimulants on the yield of cucumber fruits and on nutrient content. Afr. J. Agric. Res. 2017, 14, 2112–2118. [Google Scholar] [CrossRef]
- Shehata, S.A.; Saad, M.E.-M.; Saleh, M.A.; Atala, S.A. Effect of foliar spray with potassium silicate on growth, yield, quality and storability of cucumber fruits. Ann. Agric. Sci. Moshtohot 2018, 56, 385–396. [Google Scholar] [CrossRef]
- Al-Najjar, S.A.L.M.A.-M.; Khairy, A.-S.A.F.M. Effect of silicon on yield and fruit quality of tomato grown under sandy soil conditions. J. Biol. Agric. Healthc. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Chakma, R.; Ullah, H.; Sonprom, J.; Biswas, A.; Himanshu, S.K.; Datta, A. Effect of silicon and organic manure on growth, fruit yield, and quality of grape tomato under water-deficit stress. Silicon 2023, 15, 763–774. [Google Scholar] [CrossRef]
- Nada, M.M. Effect of foliar spray with potassium silicate and glycine betaine on growth and early yield quality of strawberry plants. J. Plant Prod. 2020, 11, 1295–1302. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kucińska, K. The effect of silicon foliar fertilization in sugar beet—Beta vulgaris (L.) ssp. vulgaris conv. crassa (Alef.) prov. altissima (Döll). Turk. J. Field Crops 2015, 20, 115–119. [Google Scholar] [CrossRef]
- Vulavala, V.K.R.; Elbaum, R.; Yermiyabu, U.; Fogelman, E.; Kuma, A.; Ginzberg, I. Silicon fertilization of potato: Expression of putative transporters and tuber skin quality. Planta 2016, 243, 217–229. [Google Scholar] [CrossRef]
- Pilon, C.; Soratto, R.P.; Moreno, L.A. Effect of soil and folia application of soluble silicon on mineral nutrition, gas exchange, and growth of potato plants. Crop Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Dorneles, A.O.S.; Pereira, A.S.; Possebom, G.; Sasso, V.M.; Rossato, I.V.; Tabaldi, L.A. Growth of potato genotypes under different silicon concentrations. Adv. Hortic. Sci. 2018, 32, 289–295. [Google Scholar]
- Wadas, W. Potato (Solanum tuberosum L.) growth in response to foliar silicon application. Agronomy 2021, 11, 2423. [Google Scholar] [CrossRef]
- Soratto, R.P.; Fernandes, A.M.; Crusciol, C.A.C.; Souza-Schlick, G.D. Yield, tuber quality, and disease incidence on potato crops as affected by silicon leaf application. Pesq. Agropec. Bras. 2012, 47, 1000–1006. (In Portuguese) [Google Scholar] [CrossRef]
- Kafi, M.; Nabati, J.; Saadatain, B.; Oskoueian, A.; Shabahang, J. Potato response to silicone compounds (micro- and nanoparticles) and potassium as affected by salinity stress. Ital. J. Agron. 2019, 14, 162–169. [Google Scholar] [CrossRef]
- Trawczyński, C. Assess of tuber yield and quality after foliar application of silicon and microelements. Agron. Sci. 2021, 76, 9–20. [Google Scholar] [CrossRef]
- Wadas, W. Possibility of increasing early potato yield with foliar application of silicon. Agron. Sci. 2022, 77, 61–75. [Google Scholar] [CrossRef]
- Wróbel, S. Effects of fertilization of potato cultivar Jelly with foliar fertilizers Yara Vita Ziemniak and Actisil. Biul. IHAR 2012, 266, 295–306. (In Polish) [Google Scholar]
- Bansal, K.; Hooda, V.; Verma, N.; Kharewal, T.; Tehri, N.; Dhull, V.; Gahlaut, A. Stress alleviation and crop improvement using silicon nanoparticles in agriculture: A review. Silicon 2022, 14, 10173–10186. [Google Scholar] [CrossRef]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y. Silicon as a smart fertilizer for sustainability and crop improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef]
- Al-juthery, H.W.A.; Al-tace, R.A.H.G.; Al-Obaidi, Z.H.H.; Ali, E.A.H.; Nal-Shami, Q.M. Influence of foliar application of some nano-fertilizers in growth and yield of potato under drip nirrigation. J. Phys. Conf. Ser. 2019, 1294, 092024. [Google Scholar] [CrossRef]
- Lebedeva, G.; Solodovnik, V.; Telysheva, G.; Vigovskis, J.; Švarta, A. Use of lignosilicon to improve the harvest and quality parameters of potato. In Environment Technology Resources, Proceedings of the 8th International Scientific and Practical Conference; Ansone, V., Ed.; Rezekne Higher Education Institute—Rezeknes Augstskola: Rezekne, Latvia, 2015; Volume 2, pp. 282–286. [Google Scholar] [CrossRef]
- Nowacki, W. Characteristic of Native Potato Cultivars Register, 23rd ed.; Plant Breeding Acclimatization Institute-National Research Institute: Jadwisin, Poland, 2020; p. 44. [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1997–2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- PN-EN 12145:2001P; Fruit and Vegetable Juices-Determination of Total Dry Matter—Gravimetric Method with Loss of Mass on Drying. Polish Committee for Standardization: Warsaw, Poland, 2001. (In Polish)
- PN-EN ISO 10520:2002; Native Starch-Determination of Starch Content-Ewers Polarimetric Method. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- PN-A-79011:1998; Dry Food Mixes-Test Methods-Determination of Sugar Content. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- ISO 1871:2009; Food and Feed Products-General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- PN-A-04019:1998; Food Products–Determination of Vitamin C Content. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- ISO 6635:1984; Fruits, Vegetables and Derived Products-Determination of Nitrite and Nitrate Content-Molecular Absorption Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984.
- Pokluda, R. An assesment of the nutritional value of vegetables using an ascorbate-nitrate index. Veg. Crops Res. Bull. 2006, 64, 29–37. [Google Scholar]
- Roztropowicz, S. Methodology of Observation, Measurements and Sampling in Agronomic Experiments with Potatoes; Plant Breeding and Acclimatization Institute: Jadwisin, Poland, 1999; p. 50. (In Polish) [Google Scholar]
- Wadas, W.; Dębski, H. Effect of silicon foliar application on the assimilation area and photosynthetic pigment contents of potato (Solanum tuberosum L.). Appl. Ecol. Environ. Res. 2022, 20, 1369–1384. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Lin, B.; Guo, D.-J.; Singh, M.; Rajput, V.D.; Snigh, R.K.; Singh, P.; Sharma, A.; Malviya, M.K.; et al. Silicon induced drought tolerance in crop plants: Physiological adaptation strategies. Silicon 2022, 14, 2473–2487. [Google Scholar] [CrossRef]
- Fernández, V.; Elbert, G. Foliar iron fertilization: A critical review. J. Plant. Nutr. 2005, 28, 2113–2124. [Google Scholar] [CrossRef]
- Morales-Fernández, S.D.; Mora-Aguilar, R.; Salinas-Moreno, Y.; Rodriguez-Pérez, J.E.; Colinas-León, M.T.; Lozoya-Saldaña, H.L. Growth, yield and sugar content of potato tubers at different physiological ages. Rev. Chapingo Ser. Hortic. 2015, 21, 129–146. [Google Scholar] [CrossRef]
- Trawczyński, C.; Wierzbicka, A. Relationship between vitamin C and nitrates contents in tubers of potato cultivars belonging to different maturity groups. Biul. IHAR 2012, 266, 143–150. (In Polish) [Google Scholar]
- Ierna, A. Influence of harvest date on nitrate contents of free potato varieties for offseason production. J. Food Compost. Anal. 2009, 22, 551–555. [Google Scholar] [CrossRef]
- Geigenberger, P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. Bot. 2003, 54, 457–465. [Google Scholar] [CrossRef]
- Umar, A.S.; Iqbal, M. Nitrate accumulation in plants, factor affecting the process, and human health implication. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Mazurczyk, W.; Lis, B. Variation of chemical composition of tubers of potato table cultivars grown under deficit and excess of water. Pol. J. Food Nutr. Sci. 2001, 51, 27–30. [Google Scholar]
| Year | Temperature; °C | Rainfall; mm | Hydrothermal Conditions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| April | May | June | April | May | June | April | May | June | |
| 2016 | 9.1 | 15.1 | 18.4 | 28.7 | 54.8 | 36.9 | rather dry | rather dry | very dry |
| 2017 | 6.9 | 13.9 | 17.8 | 59.6 | 49.5 | 57.9 | very humid | rather dry | rather dry |
| 2018 | 13.1 | 17.0 | 18.3 | 34.5 | 27.3 | 31.5 | dry | very dry | very dry |
| Treatment | Dry Matter % FW | Starch g·kg−1 FW | Total Sugars g·kg−1 FW | Monosaccharides g·kg−1 FW |
|---|---|---|---|---|
| Control | 18.65 ± 2.92 a | 117.3 ± 10.32 a | 6.42 ± 0.45 a | 2.00 ± 0.24 a |
| With Si | 18.82 ± 2.70 a | 121.1 ± 17.69 b | 6.52 ± 0.44 a | 2.11 ± 0.26 a |
| Dosage and Time of Silicon Application | Dry Matter % FW | Starch g·kg−1 FW | Total Sugars g·kg−1 FW | Monosaccharides g·kg−1 FW |
|---|---|---|---|---|
| Silicon dosage; g Si·ha−1 | ||||
| 23.25 g | 18.93 ± 2.82 a | 120.9 ± 17.29 a | 6.56 ± 0.42 a | 2.13 ± 0.24 a |
| 46.50 g | 18.71 ± 2.63 a | 121.3 ± 18.41 a | 6.50 ± 0.48 a | 2.10 ± 0.27 a |
| Time of silicon application | ||||
| BBCH 14–16 | 18.89 ± 2.84 a | 120.7 ± 18.01 a | 6.50 ± 0.52 a | 2.07 ± 0.25 a |
| BBCH 40–41 | 18.83 ± 2.14 a | 121.8 ± 15.39 a | 6.68 ± 0.34 a | 2.13 ± 0.24 a |
| BBCH 14–16 and BBCH 40–41 | 18.73 ± 2.72 a | 120.8 ± 18.18 a | 6.42 ± 0.44 a | 2.15 ± 0.27 a |
| Treatment | Protein g·kg−1 FW | L-Ascorbic Acid mg·kg−1 FW | Nitrates mg·kg−1 FW | Ascorbate–Nitrate Index (IAN) |
|---|---|---|---|---|
| Control | 15.25 ± 1.51 a | 116.6 ± 6.20 a | 92.22 ± 6.38 a | 1.27 ± 0.12 a |
| With Si | 15.64 ± 1.69 a | 116.4 ± 8.05 a | 89.24 ± 7.10 a | 1.31 ± 0.10 a |
| Dosage and Time of Silicon Application | Protein g·kg−1 FW | L-Ascorbic Acid mg·kg−1 FW | Nitrates mg·kg−1 FW | Ascorbate–Nitrate Index (IAN) |
|---|---|---|---|---|
| Silicon dosage; g Si·ha−1 | ||||
| 23.25 g | 15.74 ± 1.69 a | 117.4 ± 8.9 a | 90.11 ± 6.69 a | 1.30 ± 0.09 a |
| 46.50 g | 15.53 ± 1.71 a | 115.4 ± 7.1 a | 88.37 ± 7.52 a | 1.31 ± 0.12 a |
| Time of silicon application | ||||
| BBCH 14–16 | 15.72 ± 1.54 a | 113.7 ± 7.8 b | 90.61 ± 7.61 a | 1.26 ± 0.10 a |
| BBCH 40–41 | 15.66 ± 1.66 a | 116.5 ± 8.5 ab | 89.00 ± 7.12 a | 1.31 ± 0.10 a |
| BBCH 14–16 and BBCH 40–41 | 15.53 ± 1.94 a | 119.0 ± 7.4 a | 88.11 ± 6.72 a | 1.36 ± 0.09 a |
| Years | Dry Matter % FW | Starch g·kg−1 FW | Total Sugars g·kg−1 FW | Monosaccharides g·kg−1 FW |
|---|---|---|---|---|
| 2016 | 17.61 ± 0.82 b | 112.9 ± 5.37 b | 6.74 ± 0.39 a | 1.95 ± 0.22 a |
| 2017 | 16.46 ± 1.05 c | 106.4 ± 4.68 c | 6.20 ± 0.47 b | 2.15 ± 0.28 a |
| 2018 | 22.32 ± 0.83 a | 142.4 ± 7.47 a | 6.61 ± 0.27 a | 2.20 ± 0.20 a |
| Years | Protein g·kg−1 FW | L-Ascorbic Acid mg·kg−1 FW | Nitrates mg·kg−1 FW | Ascorbate-Nitrate Index (IAN) |
|---|---|---|---|---|
| 2016 | 15.43 ± 0.81 b | 117.3 ± 5.09 a | 87.8 ± 6.96 b | 1.34 ± 0.13 a |
| 2017 | 14.02 ± 0.91 c | 110.5 ± 4.91 b | 85.5 ± 4.98 b | 1.30 ± 0.10 a |
| 2018 | 17.27 ± 1.21 a | 121.5 ± 2.44 a | 95.8 ± 4.35 a | 1.27 ± 0.07 a |
| Treatment | After-Cooking Darkening; 9-Point Danish Scale | |
|---|---|---|
| 10 min after Cooking | 2 h after Cooking | |
| Control | 8.97 ± 0.05 a | 8.87 ± 0.04 a |
| With Si | 8.94 ± 0.07 a | 8.85 ± 0.06 a |
| Dosage and Time of Silicon Application | After-Cooking Darkening; 9-Point Danish Scale | |
|---|---|---|
| 10 min after Cooking | 2 h after Cooking | |
| Silicon dosage; g Si·ha−1 | ||
| 23.25 g | 8.96 ± 0.06 a | 8.87 ± 0.07 a |
| 46.50 g | 8.92 ± 0.08 a | 8.83 ± 0.12 a |
| Time of silicon application | ||
| BBCH 14–16 | 8.94 ± 0.06 a | 8.85 ± 0.08 a |
| BBCH 40–41 | 8.94 ± 0.07 a | 8.86 ± 0.09 a |
| BBCH 14–16 and BBCH 40–41 | 8.93 ± 0.08 a | 8.83 ± 0.12 a |
| Years | After-Cooking Darkening; 9-Point Danish Scale | |
|---|---|---|
| 10 min after Cooking | 2 h after Cooking | |
| 2016 | 8.92 ±0.07 a | 8.82 ± 0.09 b |
| 2017 | 8.96 ± 0.06 a | 8.89 ± 0.05 a |
| 2018 | 8.94 ± 0.08 a | 8.85 ± 0.08 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadas, W. Nutritional Value and Sensory Quality of New Potatoes in Response to Silicon Application. Agriculture 2023, 13, 542. https://doi.org/10.3390/agriculture13030542
Wadas W. Nutritional Value and Sensory Quality of New Potatoes in Response to Silicon Application. Agriculture. 2023; 13(3):542. https://doi.org/10.3390/agriculture13030542
Chicago/Turabian StyleWadas, Wanda. 2023. "Nutritional Value and Sensory Quality of New Potatoes in Response to Silicon Application" Agriculture 13, no. 3: 542. https://doi.org/10.3390/agriculture13030542
APA StyleWadas, W. (2023). Nutritional Value and Sensory Quality of New Potatoes in Response to Silicon Application. Agriculture, 13(3), 542. https://doi.org/10.3390/agriculture13030542







