Abstract
Since climate changes have caused water restrictions, safflower stands out as an alternative crop due to its adaptability to restrictive soil and climate conditions. Thus, this research aimed to evaluate the physiological and yield performance of four safflower lines (IMA 02, IMA 04, IMA 14, and IMA 21) under two water regimes [without water deficiency—around 22% soil moisture content—100% of field capacity (FC); and with water deficiency—50% of FC]. The water regimes were imposed for 30 days during the flowering phase, followed by rehydration for 20 days. Water deficiency decreased relative water content, water potential, photosynthetic pigment contents, photosynthetic performance, maximum variable and potential quantum yield of PSII, electron transport rate, and photochemical quenching. In contrast, it increased electrolyte leakage, water use efficiency, and non-photochemical quenching. The decreases in photochemical efficiency and photosynthetic performance as a function of water deficiency caused reductions in the number of capitula, 100-grain mass, and harvest index, with more significant reductions in IMA 02, which was considered susceptible to soil water changes. IMA 04, IMA 14, and IMA 21 were considered tolerant because their physiological variables and yield components were less affected by water restriction, and they also showed recovery after rehydration compared to IMA 02. Thus, these lines can be recommended for commercial use, and safflower breeding programs aiming to select superior genotypes under drought conditions.
1. Introduction
Water scarcity due to climate variations limits agricultural production. Safflower (Carthamus tinctorius L.) emerges as a planting alternative due to its adaptability to several edaphoclimatics conditions and ensured productivity in adverse environments [1]. Its cultivation has increased due to its potential for development and growth under semi-arid conditions during the Brazilian off-season and use in biofuel production [2].
Due to recent safflower breeding in Brazil, research has been conducted to characterize the accessions for morphological and nutritional characteristics, yield potential, and selection of promising materials [3,4,5]. Identifying cultivars with high yields is essential for establishing this crop compared to other oilseeds [6].
When exposed to adverse agricultural environments, safflower plants show changes in their morphology and physiology [7]. Despite its tolerance to drought, studies have shown decreased safflower grain yield under water restriction [8,9,10,11,12], which may be related to physiological changes [13], by inducing less photoassimilate allocation in grains, which leads to reduced crop yields [14,15].
Plant responses to water deficit and recovery from this stress differ among crops [3,16,17] and vary with plant age, species, and stress intensity, attributed to morphological, physiological, and biochemical aspects [18,19,20].
Abiotic stresses significantly and negatively impact plants’ photosynthetic rate by altering the organelles’ structure and the concentration of pigments and metabolites, which prevents carbon assimilation and damages the photosynthetic apparatus [21].
Stomatal closure is one of the first steps in the plant’s adaptation to water deficit, allowing the maintenance of water status [22]. Thus, by adjusting stomatal openings, plants can control water loss by reducing transpiration flux and limiting CO2 diffusion. The decline in intercellular CO2 after stomata closure and lower light use efficiency under water stress decrease the functioning of the photosynthetic apparatus to match the available carbon substrate [23].
Gas exchange assessments help determine the effects of stress on plant–water relations. Analysis of chlorophyll contents and chlorophyll a fluorescence also help evaluate the stress level induced by damage to photosynthetic structures [24]. Water deficiency causes changes in safflower plant functioning, such as decreased leaf water potential [3], relative water content [3,25,26,27,28], stomatal closure [28], photosynthetic rate [28,29,30], and chloroplast pigments [3,27,28,30,31] due to shoot decrease [28,29,32,33], as well as changes in chlorophyll a fluorescence, and anatomical modifications leading to reduced productivity [34,35,36]. Therefore, it is ideal to consider more than one physiological parameter in water-deficient plants, being essential to determine the different responses of plants and their genotypes [37,38]. Thus, understanding these responses, mechanisms, and traits related to the tolerance of safflower lines to water deficiency is essential. However, studies in the flowering phase, which is the most critical for loss of production, still need to be made available.
In this context, this research was based on the hypothesis that there is genetic variability in the safflower crop regarding tolerance to stress caused by water deficiency and its respective recovery, which is expressed through the physiology of the plant, resulting in differences in productivity. Thus, this research aimed to evaluate the physiological and yield variables of four safflower lines in response to water deficit and rehydration capacity in the flowering phase to assist in selecting lines that best adapt to arid and semi-arid regions.
2. Material and Methods
2.1. Description of the Experimental Area
The experiment was conducted for five months in a protected environment in the Department of Crop Production of the School of Agricultural Sciences—FCA/UNESP, located in Botucatu (22°51′01″ S, 48°25′55″ W, 786 m asl), Sao Paulo, Brazil. The protected environment was a steel structure with galvanized steel arches. It had a high ceiling of 3 m and a total area of 63 m2, with anti-aphid fabric (2 mm diameter) on the sides and a transparent plastic roof (150 μm diameter).
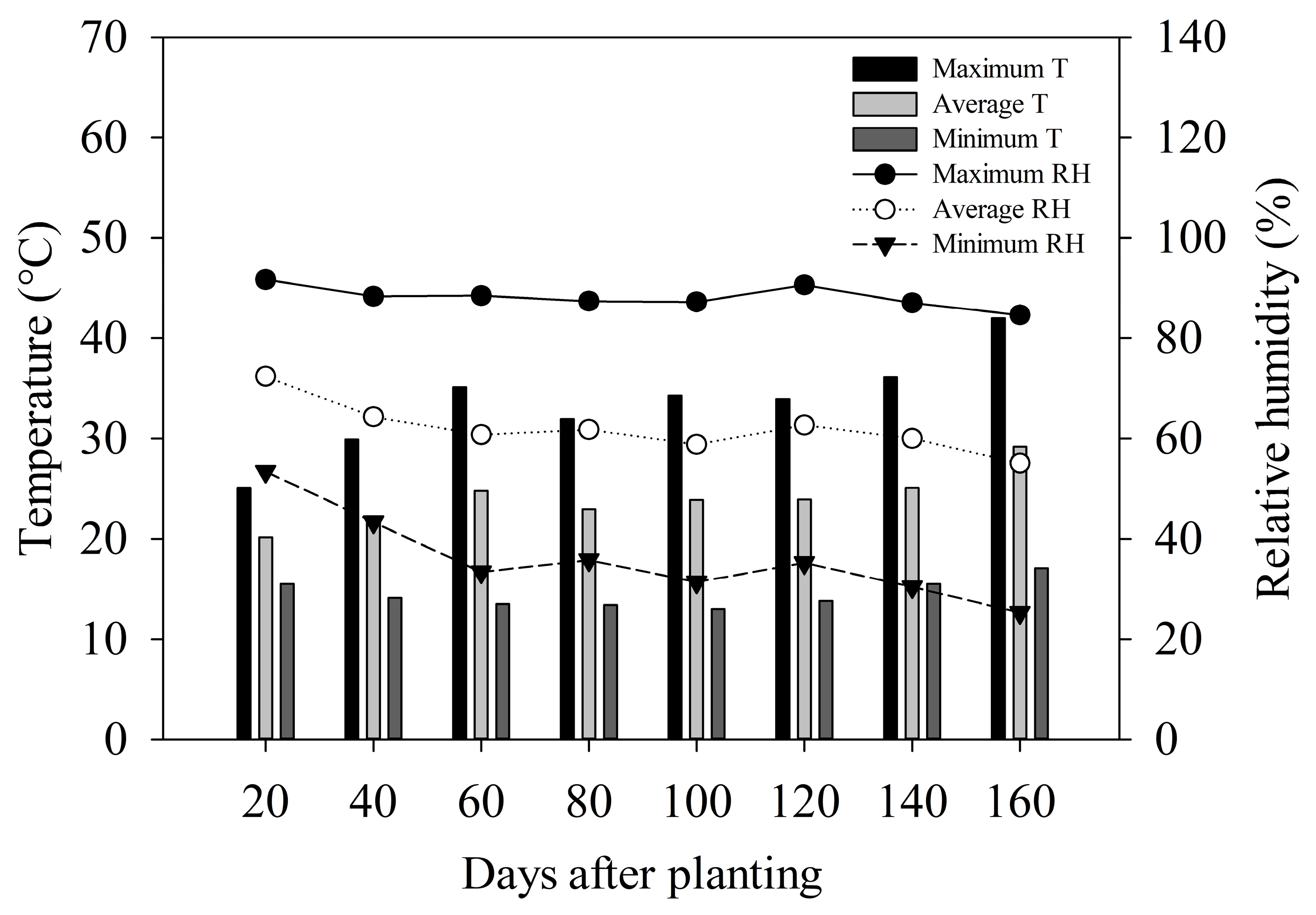
The temperature data and air humidity inside the protected environment during the experiment conduction were obtained with a datalogger (Instrutherm, HT-500, Sao Paulo, SP, Brazil) (Figure 1).
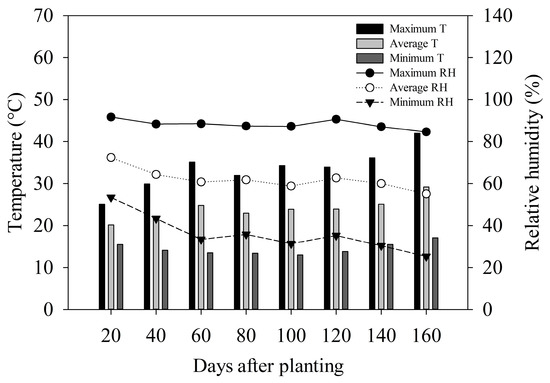
Figure 1.
Minimum, maximum, and average temperature (T) and relative humidity (RH) in the protected environment during the experiment conduction.
The used soil is classified as medium-textured Red Latosol [39]. The physical and chemical characteristics are described in Table 1. Liming and fertilizing were performed based on the sunflower crop [40] since there has yet to be a fertilizer recommendation for Brazil’s safflower crop. Eight seeds of the safflower genotypes were sown in 40-L plastic pots with 64.0 kg of soil, and five plants were maintained during the experiment.

Table 1.
Physical-chemical attributes of the soil.
2.2. Soil Water Retention Curve, Irrigation Management, and Soil Water Content Monitoring
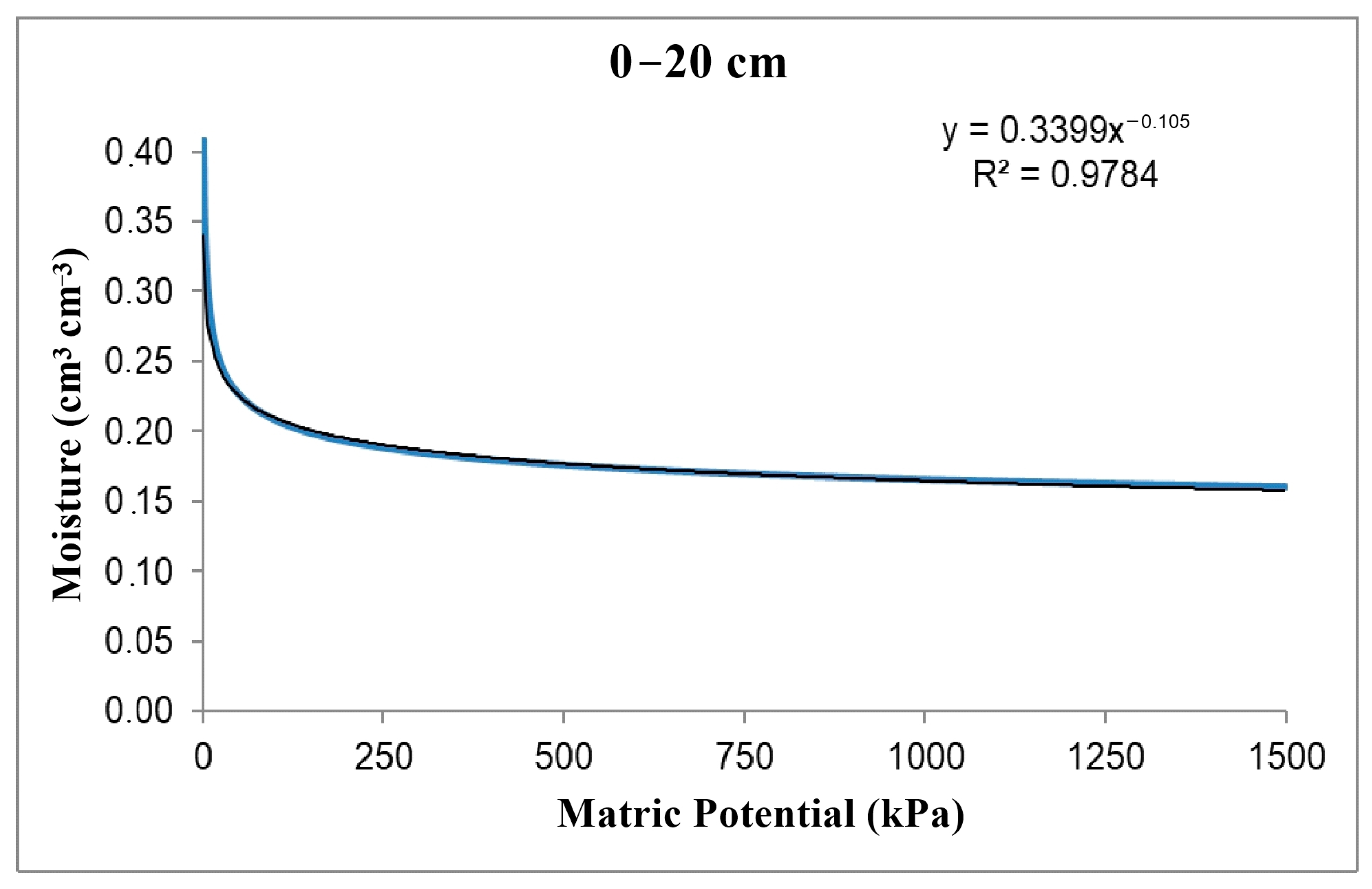
Through soil chemical and physical characterization, the soil–water retention curve was obtained (Figure 2) using the model described by Van Genutchen [41].
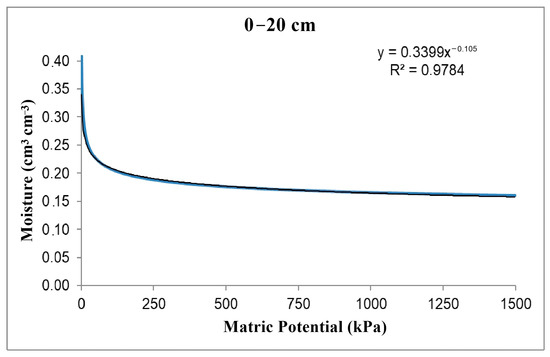
Figure 2.
Soil–water retention curve.
Irrigation was performed by an automatic system, using drippers (Netafim, PCJ-CNL 2 l/h, Ribeirão Preto, SP, Brazil), controlling the flow with registers.
The soil moisture control was performed through puncture tensiometers installed close to the plant in all the pots at 20 cm of soil depth, and the tension was measured by a digital tensimeter (SondaTerra, Piracicaba, SP, Brazil). The plants were inspected and irrigated daily between 8 and 9 am, and soil moisture was maintained at field capacity until the beginning of the water regimes.
The estimation of the irrigation layer was carried out based on the available water capacity (AWC), aiming to maintain the water tension in the soil according to each stage through Equation (1):
where AWC is the available water capacity in the soil (mm), FC is the volumetric water content in the field capacity (cm3 cm−3), PWP is the volumetric water content at the permanent wilting point (cm3 cm−3), and R is the effective depth of the root system (mm) [42].
AWC = (FC − PWP) × R
2.3. Experimental Design, Treatments, and Plant Material
The experimental design used was a completely randomized 4 × 2 factorial scheme consisting of four safflower lines (IMA 02, IMA 04, IMA 14, and IMA 21) and two water regimes (without and with water deficiency), with six replicates. The plants were maintained under field capacity (FC) until 60 days after sowing (DAS). The water regimes were imposed when the lines reached 90% of the floral bud burst. In the regime “without deficiency (−D)”, plants were hydrated with 100% of FC (around 22% soil moisture content) during the entire cycle. In contrast, in the regime “moderate deficiency (+D)”, plants were maintained for 30 days under 50% of FC. Soon after, plants were rehydrated for 20 days.
The lines used are in the breeding process and came from the Western Regional Plant Introduction Station (WRPIS) germplasm bank in the United States, obtained through the Germplasm Resources Information Network (GRIN), from which the Instituto Matogrossense do Algodão (imported them IMA-MT), and subsequently transferred to the Safflower Breeding Program of the School of Agricultural Sciences of Botucatu.
2.4. Physiological Traits
Leaf water potential (Ψleaf) was evaluated between 11:30 am and 1:00 pm using a Scholander pressure chamber (SoilMoisture Equipment, Santa Barbara, CA, USA). The reading was taken on the second leaf expanded from the plant’s apex.
The relative water content (RWC) was determined in six leaf discs of 1.1671 cm2, taken from the middle third leaf of plants. The mass of fresh matter (FM), the mass of turgid matter (TM) after hydration in deionized water for 24 h, and the mass of dry matter (DM) after drying for 48 h at 80 °C in a forced air circulation oven were determined on a precision analytical balance (Shimadzu, BL-3200H, Piracicaba, SP, Brazil). The RWC was calculated according to Equation (2) [43]:
RWC = (FM − DM)/(TM − DM) × 100
The electrolyte leakage (EL) was evaluated by indirect determination. Ten leaf discs of 0.5 cm2 in diameter were collected from the middle third leaf of each plant, placed in test tubes containing 10 mL of deionized water, and immersed for 24 h. The initial conductivity (Ci) was obtained after 24 h of incubation, and the final conductivity (Cf) was obtained after the discs remained in a water bath at 60 °C for three hours. The calculation was performed using Equation (3) proposed by Campos e Thi [44]:
where EL is represented in % and Ci and Cf in μS cm−1.
EL = (Ci/Cf) × 100
The photosynthetic pigment contents (chlorophylls a and b—Chl a and b, and carotenoids) were determined in two leaf discs of 1.1671 cm2 from the plants’ middle third leaf. The discs were immersed for 48 h in dimethylformamide solution (DMF), protected from light. Then, 1 mL of the chlorophyll extract was diluted in 1 mL of deionized water, and the absorbance of the samples was determined in a spectrophotometer (Shimadzu, UV-2700, Kyoto, Japan) at wavelengths of 480, 647, and 664 nm. The chlorophyll a, b, and carotenoid concentrations were calculated based on the methodology described by Wellburn [45], and the results were expressed in μg cm−2.
From the evaluation of gas exchange, we obtained the net CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E), and intercellular CO2 concentration (Ci). The measurements were obtained on plants’ fully expanded middle third leaves with an infrared gas analyzer (IRGA) (LI-COR Biosciences Inc., LI-6400XT, Lincoln, Nebraska, EUA). Measurements were performed between 09:00 and 11:30 am, using constant photosynthetic photon flux density of 1500 μmol m−2 s−1 in the leaf chamber, atmospheric CO2 concentration (400.12 ppm ± 20 ppm), ambient temperature (27.47 °C ± 3 °C), and humidity (61.94% ± 10%). The instantaneous water use efficiency (WUE) was calculated by the ratio A/E, and the instantaneous carboxylation efficiency (CE) by the ratio A/Ci.
The parameters of chlorophyll a fluorescence, the maximum variable quantum yield of photosystem II (PSII) (Fv/Fm), PSII effective quantum yield (φPSII), apparent electron transport rate (ETR), photochemical quenching (qP), quantum yield of regulated energy dissipation (Y(NPQ)), and unregulated energy dissipation ((Y(NO)) were obtained on the same leaf used for the gas exchange measurements, using a 6400-40 leaf chamber fluorometer coupled to the IRGA. The measurements were performed in the morning period, after acclimatization of the leaves in the dark with specific clips for one hour. The photochemical quenching (qP) [46] was calculated from fluorescence in a light-adapted sample before the saturation pulse (F) and maximum fluorescence in a light-adapted sample (Fm’). φPSII, Y(NPQ), and Y(NO) were calculated according to Genty et al. [47], and φPSII was also used to estimate ETR [48].
2.5. Yield Components
For the analysis of the yield components, three plants were collected from each pot. The number of capitula per plant (NC) was obtained by direct counting. After threshing, the grains were weighed, and the 100-grain mass (100 GM) was obtained in precision analytical balance (Shimadzu, BL-3200H, Piracicaba, SP, Brazil). The yield was obtained after correcting the moisture content of the grains to 10%, and the results were expressed in g plant−1. The harvest index (HI) was obtained by dividing the mass of grains by the shoot fresh matter mass (stem + branches + leaves + grains).
2.6. Statistical Analysis
The data were submitted to the Shapiro–Wilk normality test. Once this assumption was met, we proceeded with the two-factor analysis of variance (ANOVA). The means were compared by Tukey test (p ≤ 0.05) using the AgroEstat statistical software (AgroEstat, version 2015, Jaboticabal, SP, Brazil).
3. Results
3.1. Physiological Traits
Ψleaf and EL were affected by the factors water regime (Wr) and lines (L) under adequate water regime; on the other hand, Wr, L, and Wr × L affected Ψleaf and EL under water deficiency and at rehydration (Table 2). RWC was only affected by the L factor under adequate water regime, the two factors, and the interaction between them under water deficiency, and by L and Wr × L at rehydration. Chl a, Chl b, Chl a + b, and carotenoids were affected by the L factor under adequate water regime and by Wr, L, and Wr × L under water deficiency and at rehydration. Additionally, Chl a/Chl b was only affected by the L factor under adequate water regime and at rehydration, and by the two factors and the interaction between them under water deficiency (Table 2).

Table 2.
Analysis of variance of leaf water potential, relative water content, electrolyte leakage (EL), chlorophyll a content (Chl a), chlorophyll b content (Chl b), total chlorophyll content (Chl a + b), chlorophyll a/b ratio (Chl a/Chl b), and carotenoids of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration.
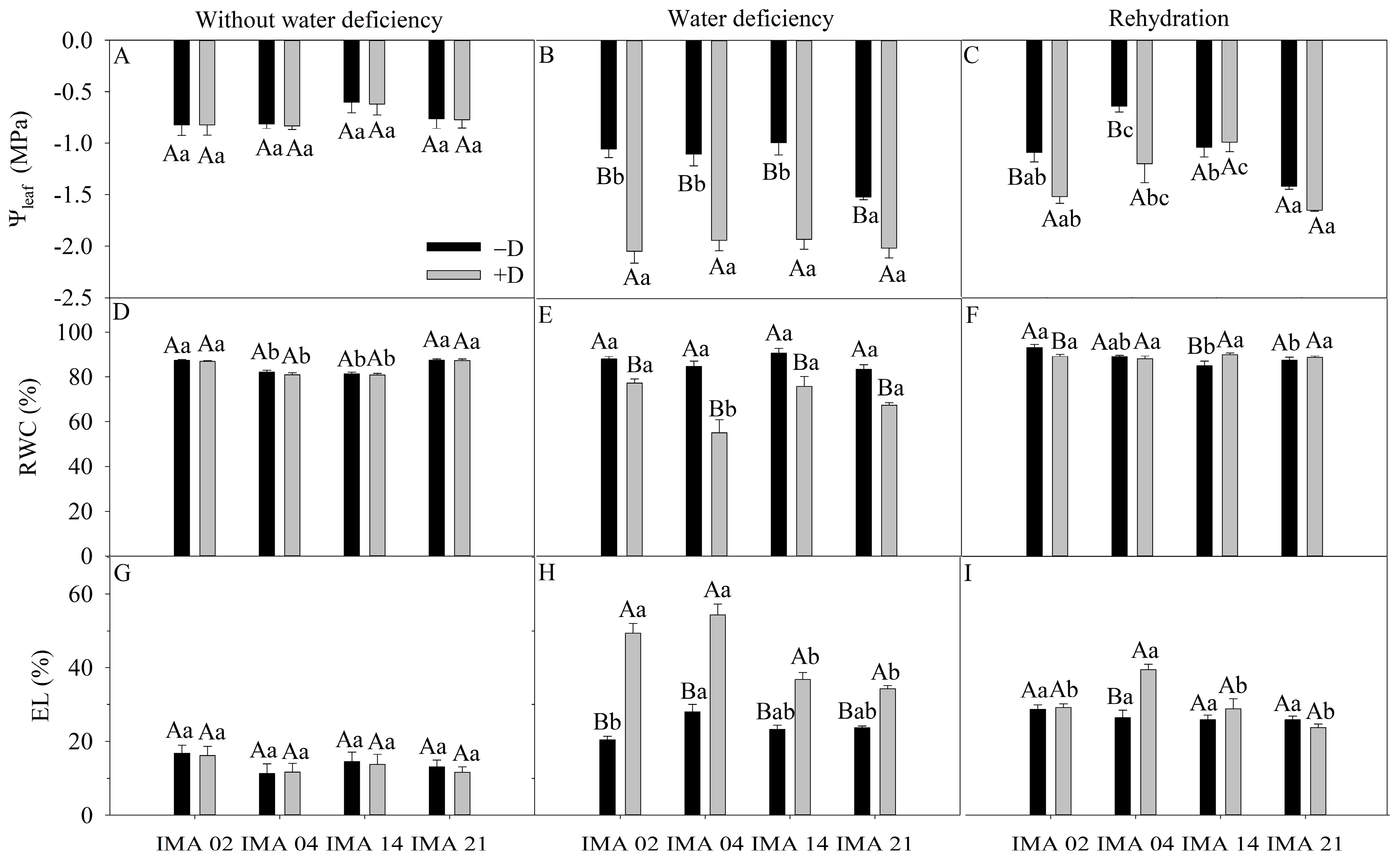
The initial evaluation recorded an average Ψleaf value of −0.75 MPa (Figure 3A). The water deficiency decreased the Ψleaf of lines IMA 02, IMA 04, IMA 14, and IMA 21 by 48.5%, 43.1%, 48.4%, and 24.6%, respectively (Figure 3B). After rehydration, lines IMA 02 and IMA 04 still showed reductions in Ψleaf of 28.3% and 46.7%, respectively. The strains IMA 14 and IMA 21 showed less pronounced declines in Ψleaf and matched the adequate water regime (−D) (Figure 3C).
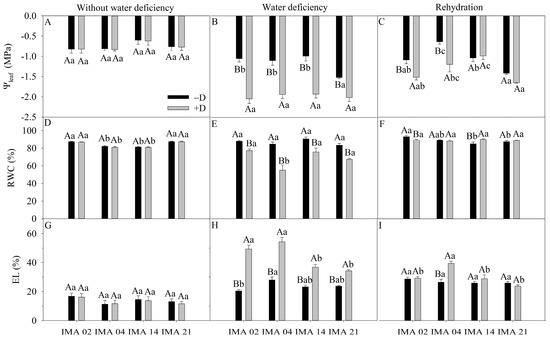
Figure 3.
Leaf water potential (Ψleaf) (A–C), relative water content (RWC) (D–F), and electrolyte leakage (EL) (G–I) of four safflower lines without water deficiency 30 days after the imposition of water regimes, and 20 days after rehydration. Means followed by the same letter in each evaluation period, lower case between lines and within water regime, and upper case within each line and between water regimes, do not differ by the Tukey test (p ≤ 0.05).
Under an adequate water regime, average RWC values between 78.7% and 88.7% were recorded (Figure 3D). After the water deficiency period, the RWC of the lines was reduced by approximately 68.9%. The IMA 04 line showed the most significant reduction (34.8%), followed by lines IMA 21 (19.0%) and IMA 14 (16.4%), while the IMA 02 showed the lowest reduction in RWC (7.6%) (Figure 3E). After rehydration, the RWC of IMA 04, IMA 14, and IMA 21 lines equaled −D, contrary to what was observed for IMA 02 (Figure 3F).
The EL showed an average value of 13.5% among treatments at initial evaluation (Figure 3G). Imposition of water deficiency increased EL by 58.8%, 48.6%, and 39.6% in IMA 04, IMA 02, and IMA 14 lines, respectively. The lowest extravasation of cell membranes was observed in IMA 21 (31.1%) (Figure 3H). After rehydration, only line IMA 04 did not show recovery, as, under +D, it still provided 36.1% of released electrolytes (Figure 3I).
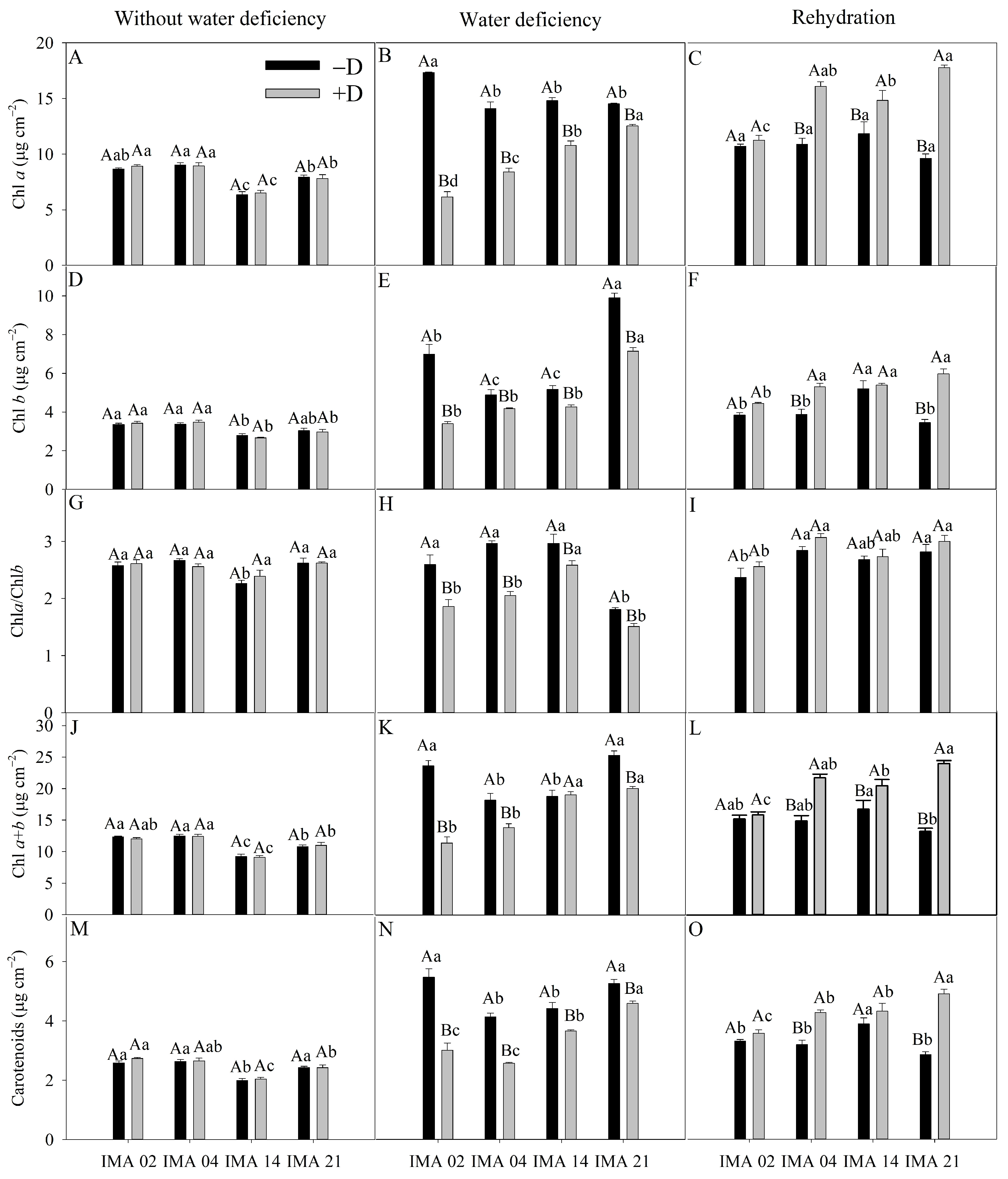
Under −D, differences were observed among lines for Chl a, Chl b, and total chlorophyll (Chl a + b) in which IMA 14 showed the lowest values (6.34, 2.79, and 9.13 μg cm−2, respectively), while the highest values were observed in lines IMA 02 (8.66, 3.36, and 12.02 μg cm−2, respectively) and IMA 04 (9.02, 3.37, and 12.36 μg cm−2, respectively) (Figure 4A,D,J). Additionally, no differences existed between the lines for the Chl a/Chl b ratio (Figure 4G).
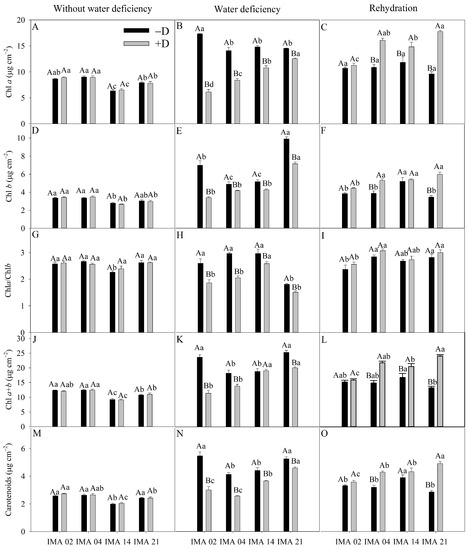
Figure 4.
Chlorophyll a content (Chl a) (A–C), chlorophyll b content (Chl b) (D–F), chlorophyll a/b ratio (Chl a/Chl b) (G–I), total chlorophyll content (Chl a + b) (J–L), and carotenoids content (M–O) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration. Means followed by the same letter in each evaluation period, lower case between lines and within water regime, and upper case within each line and between water regimes, do not differ by the Tukey test (p ≤ 0.05).
However, the imposition of water deficiency provided reductions in Chl a, Chl b, and Chl a + b in all the lines, with higher decreases verified in IMA 02 (64.4%, 51.4%, and 60.7%, respectively). In contrast, the lowest declines were verified in lines IMA 14 and IMA 21 (Figure 4B,E,K). Additionally, after rehydration, treatments under +D showed increases in Chl a, Chl b, and Chl a + b, with the highest increases verified in IMA 21 and IMA 04 (Figure 4C,F,L). Water deficiency decreases the Chl a/Chl b ratio in all the lines, but after rehydration, all lines recovered, establishing an average of 2.76 (Figure 4H,I).
Under an adequate water regime, line IMA 14 showed the lowest average value (2.00 μg cm−2), while the average value of the other lines was 2.55 μg cm−2 (Figure 4M). Water deficiency negatively affected the carotenoid contents of the lines, with reductions of 45.0%, 38.0%, 17.0%, and 12.8% in IMA 02, IMA 04, IMA 14, and IMA 21, respectively (Figure 4N). After rehydration, carotenoid content was recovered in all lines, with an average increase of 21.1% under +D compared to −D. There were highlights for lines IMA 21 and IMA 04 under +D, which, after rehydration, showed increases of 41.8% and 25.3% in carotenoid contents, respectively, compared to the same lines under −D (Figure 4O).
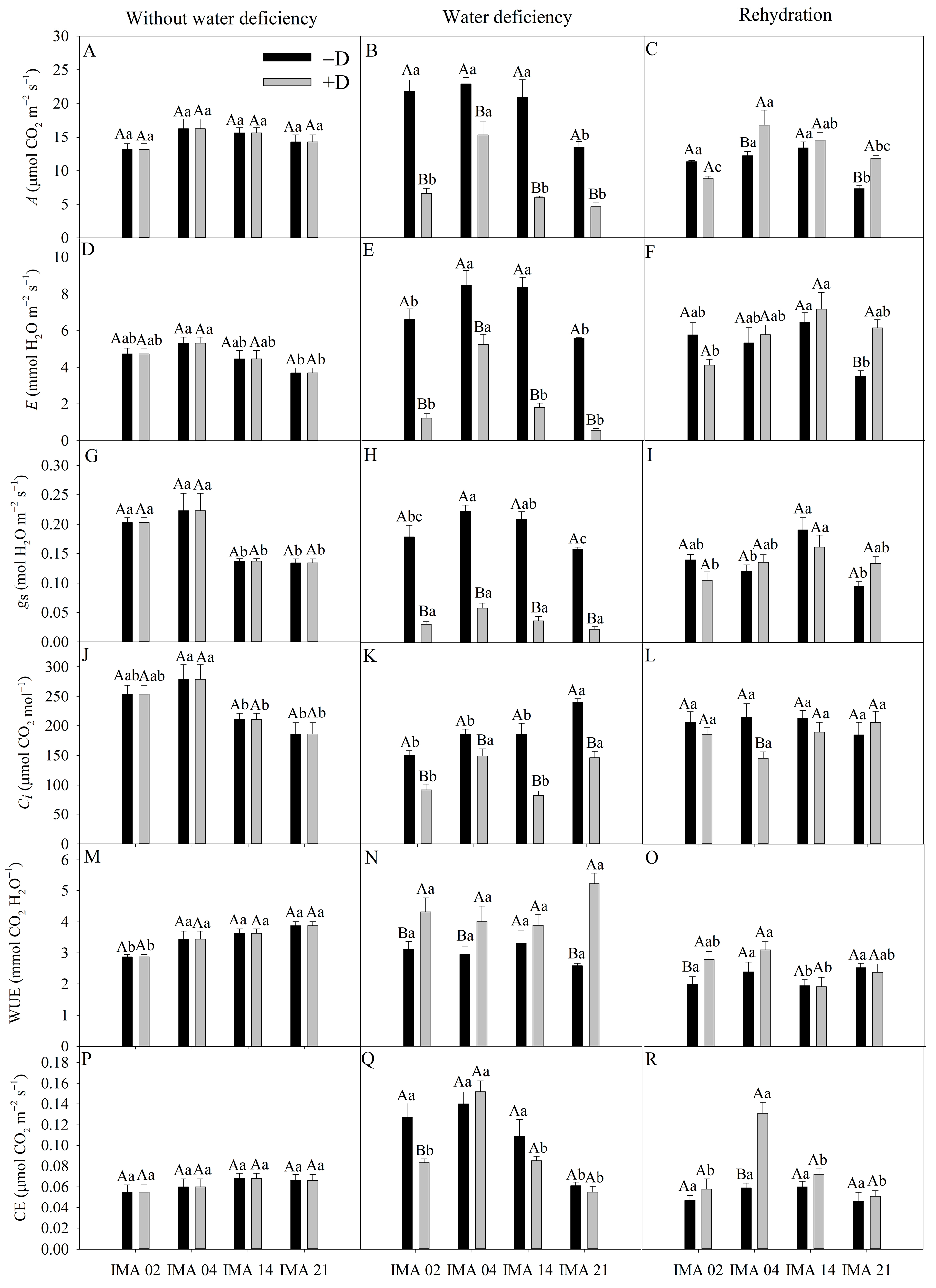
At the first evaluation, A, E, gs, Ci, and WUE were affected only by the L factor, while CE was unaffected by any of the factors (Table 3). Under water deficiency, the factors Wr, L, and Wr × L affected A, E, gs, Ci, and CE, while WUE was affected by Wr and Wr × L. Additionally, at rehydration, A, E, gs, and CE were affected by Wr, L, and Wr × L, while Ci and WUE were affected only by Wr and L factor, respectively (Table 3).

Table 3.
Analysis of variance of CO2 assimilation rate (A), transpiration rate (E), stomatal conductance (gs), intercellular CO2 concentration (Ci), water use efficiency (WUE), and carboxylation efficiency (CE) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration.
Under adequate water regime, an average A value of 14.82 µmol CO2 m−2 s−1 was recorded (Figure 5A). After the imposition of water deficiency, A decreased 69.6%, 71.3%, and 65.5% for the lines IMA 02, IMA 14, and IMA 21, respectively. Despite a reduction of 33.0% verified in IMA 04, this line maintained the highest A under +D (15.35 µmol CO2 m−2 s−1) (Figure 5B). The rehydration of plants enabled the recovery of A in all lines. However, the plants of IMA 04 and IMA 21 under +D showed A 37.8% and 61.5% higher than under −D, respectively (Figure 5C).
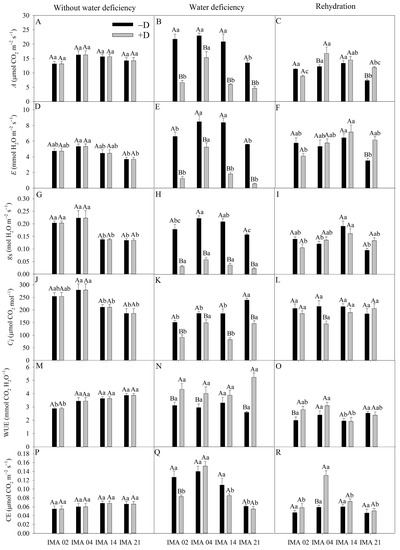
Figure 5.
CO2 assimilation rate (A) (A–C), transpiration rate (E) (D–F), stomatal conductance (gs) (G–I), intercellular CO2 concentration (Ci) (J–L), water use efficiency (WUE) (M–O), carboxylation efficiency (CE) (P–R) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration. Means followed by the same letter in each evaluation period, lower case between lines and within water regime, and upper case within each line and between water regimes, do not differ by the Tukey test (p ≤ 0.05).
The highest E value, under an adequate water regime, was verified in IMA 04 (5.33 mmol H2O m−2 s−1) and the lowest in IMA 21 (3.70 mmol H2O m−2 s−1) (Figure 5D). The imposition of water deficiency provided reductions in E of 90.1%, 81.3%, 78.5%, and 38.3% in the lines IMA 21, IMA 02, IMA 14, and IMA 04, respectively (Figure 5E). With rehydration, there was the recovery of the safflower plants as far as E was concerned, with a highlight to the IMA 21 under +D that outperformed the −D condition by 74.9%, followed by the IMA 14 and IMA 04 lines under +D that showed increases of 11.2% and 8.3% in E compared to the −D condition (Figure 5F).
Under adequate water regime, lines IMA 02 and IMA 04 showed gs, on average, 57.1% higher than that verified in lines IMA 14 and IMA 21 (Figure 5G). Water deficiency decreased gs in 85.3%, 82.6%, 82.2%, and 73.7% in lines IMA 21, IMA 02, IMA 14, and IMA 04, respectively (Figure 5H). After rehydration, all lines recovered gs (Figure 5I).
Under adequate water regime, the highest values of Ci were verified in lines IMA 02 and IMA 04 (Figure 5J). However, after the water deficiency period, a significant difference was observed among water regimes within each line, with a decrease of 39% in IMA 21, 21.2% in IMA 02, 55.4% in IMA 14, and 39.1% in IMA 04, compared to −D condition (Figure 5K). After rehydration, only line IMA 04 showed no Ci recovery, resulting in a difference of 32.3% between −D and +D (Figure 5L).
IMA 02 showed the lowest WUE under adequate water regime, averaging 2.89, while IMA 14 and IMA 21 provided the highest average value (3.76 mmol CO2 H2O−1) (Figure 5M). Under water deficiency, line IMA 14 was not influenced, resulting in an average of 3.59 mmol CO2 H2O−1; on the other hand, the imposition of water deficiency raised the WUE in other lines, causing increases of 50.6% in IMA 21, 28.0% in IMA 02, and 26.4% in IMA 04 (Figure 5N). After rehydration, the WUE values of the lines under −D e +D were similar, except for line IMA 02 under +D, which still maintained higher WUE than the −D condition (Figure 5O).
CE showed an average of 0.06 µmol CO2 m−2 s−1 among treatments in the initial evaluation (Figure 5P). The imposition of water deficiency provided reductions of, on average, 33% and 12.5% in CE of the lines IMA 02 and IMA 21, respectively, compared to the −D condition (Figure 5Q). After the rehydration period, the CE of IMA 02 and IMA 21 recovered, while there was an increase of 54.6% in the CE of IMA 04 conducted under +D, compared to the −D condition (Figure 5R).
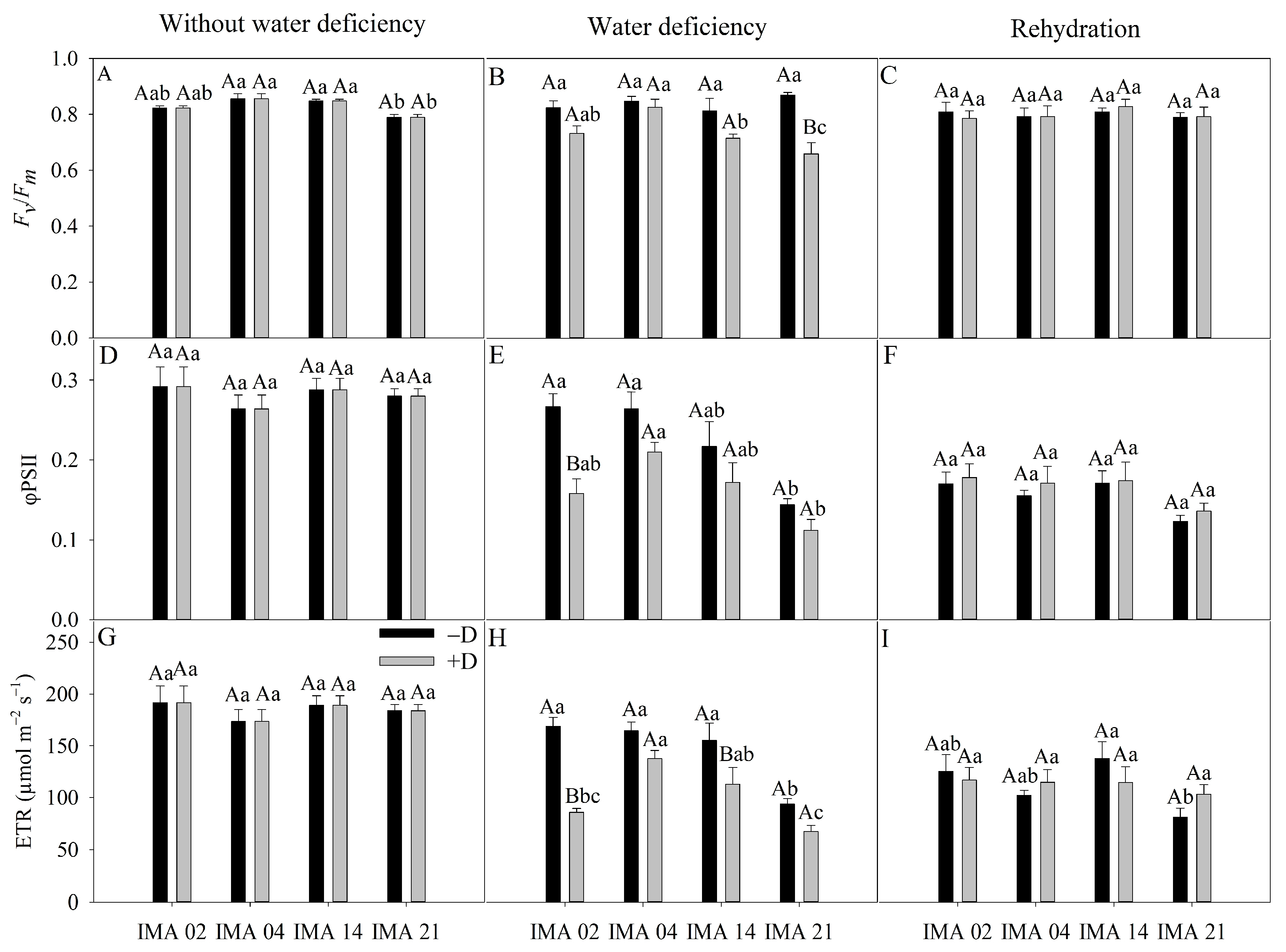
Under adequate water regime, Fv/Fm, NQP, Y(NQP), and Y(NO) were affected only by the L factor, while φPSII, ETR, and qP were unaffected by any of the factors (Table 4). Under water deficiency, Fv/Fm, φPSII, ETR, and Y(NQP) were affected by Wr, L, and Wr × L; on the other hand, qP was affected only by Wr × L, and NQP and Y(NO) were affected by Wr and Wr × L. Additionally, at rehydration, ETR, NQP, and Y(NO) were affected only by the L factor, while the other variables were affected by none of the factors (Table 4).

Table 4.
Analysis of variance of maximum variable quantum yield of photosystem II (PSII) (Fv/Fm), the effective quantum yield of the PSII (φPSII), electron transport rate (ETR), photochemical quenching (qP), non-photochemical quenching (NQP), the quantum yield of regulated dissipation (Y(NQP)), and quantum yield of unregulated dissipation (Y(NO)) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration.
Under adequate water regime, the lowest average value of Fv/Fm was verified in IMA 21 (0.79). At the same time, the other lines showed an average value of 0.84 (Figure 6A). The imposition of water deficiency promoted a significant difference only in IMA 21 line, causing a reduction of 24.2% compared to the −D condition (Figure 6B). After rehydration, the Fv/Fm values were similar among lines and water regimes (Figure 6C).
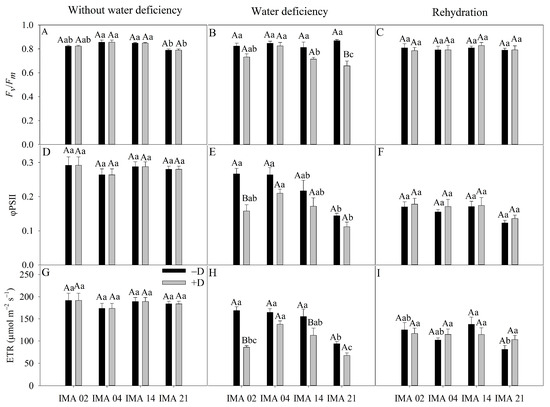
Figure 6.
Maximum variable quantum yield of photosystem II (PSII) (Fv/Fm) (A–C), effective quantum yield of the PSII (φPSII) (D–F), and electron transport rate (ETR) (G–I) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration. Means followed by the same letter in each evaluation period, lower case between lines and within water regime, and upper case within each line and between water regimes, do not differ by the Tukey test (p ≤ 0.05).
φPSII showed an average of 0.28 among treatments in the initial evaluation (Figure 6D). The imposition of water deficiency promoted significant reduction only in the IMA 02 line (40.7%), reaching a value of 0.15, while hydrated plants showed an average value of φPSII of 0.27 (Figure 6E). After rehydration, there was no difference between treatments for φPSII (Figure 6F).
Under adequate water regime, an average ETR value of 184 µmol m−2 s−1 was recorded (Figure 6G). On the other hand, the imposition of water deficiency promoted reductions of 49.2% and 27.2% in ETR of the IMA 02 and IMA 14 lines, respectively, while no significant decrease was observed in other lines (Figure 6H). After the rehydration period, the ETR was similar between safflower plants under −D and +D (Figure 6I).
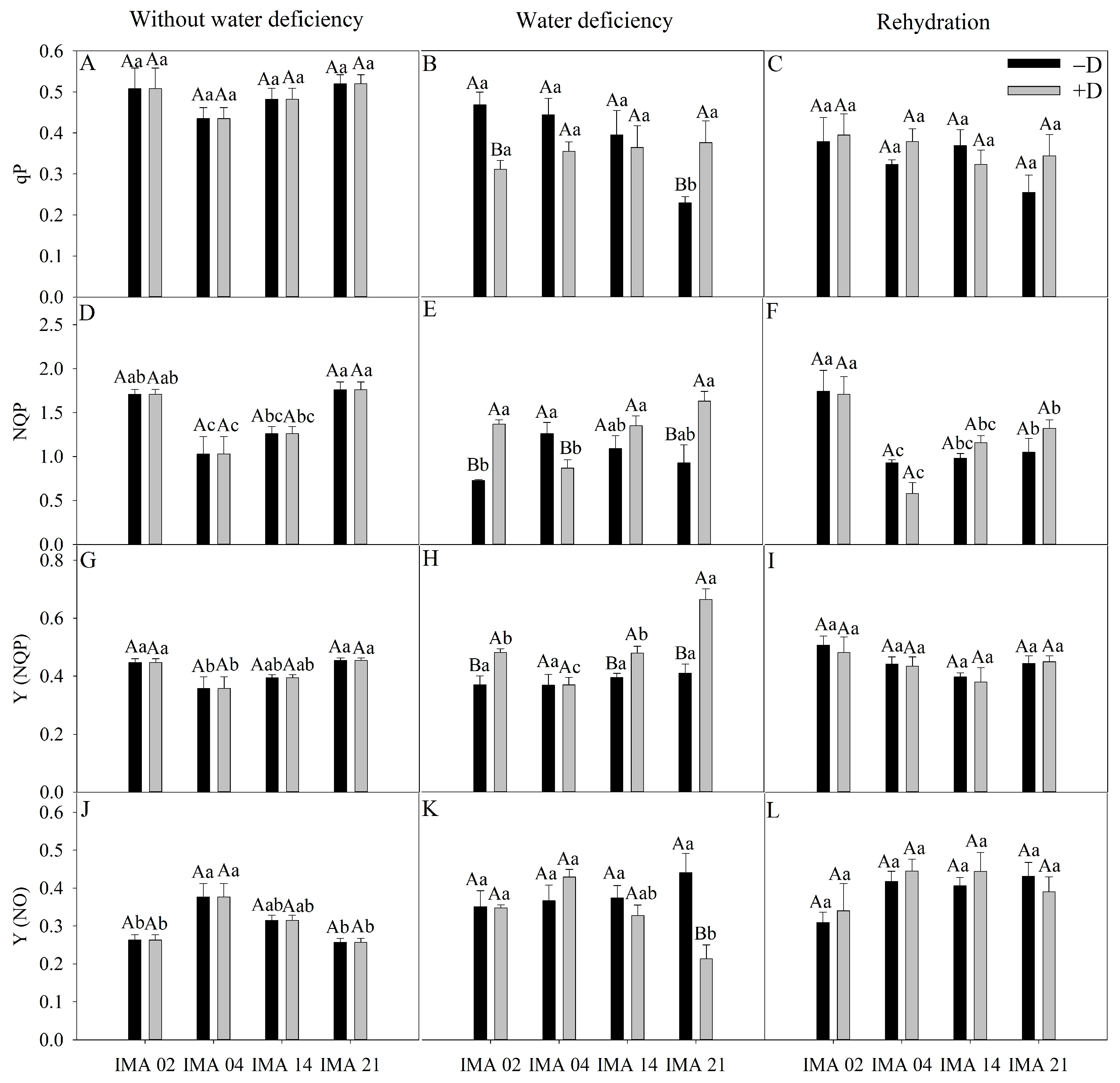
In the initial evaluation, qP showed an average of 0.49 among treatments (Figure 7A). After the imposition of water deficiency, there was a reduction of 33.4% in qP of the IMA 02 line compared to the −D condition. In comparison, there was an increase of 64.1% in qP of IMA 21, and no effect was verified in IMA 04 and IMA 14 lines (Figure 7B). After rehydration, there was the recovery of IMA 02 as to qP, and the values were similar among the lines, averaging 0.35 (Figure 7C).
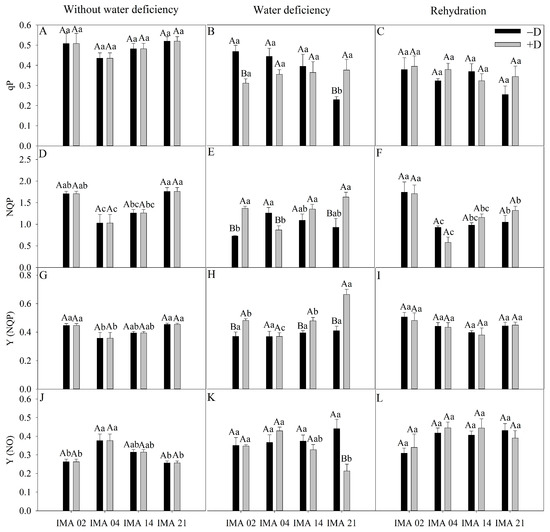
Figure 7.
Photochemical quenching (qP) (A–C), non-photochemical quenching (NQP) (D–F), the quantum yield of regulated dissipation (Y(NQP)) (G–I), and quantum yield of unregulated dissipation (Y(NO)) (J–L) of four safflower lines without water deficiency, 30 days after the imposition of water regimes, and 20 days after rehydration. Means followed by the same letter in each evaluation period, lower case between lines and within water regime, and upper case within each line and between water regimes, do not differ by the Tukey test (p ≤ 0.05).
Under adequate water regime, the highest values of NPQ were verified in IMA 02 and IMA 21 (1.74), and the lowest average value was verified in IMA 04 (1.03) (Figure 7D). Under water deficiency, there was a reduction of 30.7% in NPQ of IMA 04 and increases of 87.1% and 75.6% in NPQ of IMA 02 and IMA 21 lines, respectively, compared to −D (Figure 7E). After rehydration, the NPQ values were similar within lines between plants under +D and −D (Figure 7F).
The lowest average value of Y(NQP) was verified in IMA 04 (0.36), while the lines IMA 02 and IMA 21 showed an average value of 0.45 under adequate water regime (Figure 7G). After 30 days of water deficiency, there were increases of 21.2%, 29.9%, and 38.2% in Y(NQP) of the lines IMA 14, IMA 02, and IMA 21 under +D, respectively, compared to the −D condition (Figure 7H), which did not occur in IMA 04. Additionally, there was no difference among treatments for Y(NQP) after rehydration, with an average value of 0.44 (Figure 7I).
In the initial evaluation, there was a highlight to IMA 04 that showed the highest value of Y(NO) (0.38) (Figure 7J). Water deficiency negatively impacted only IMA 21, causing a decrease of 51.7% in Y(NO) (Figure 7K). After rehydration, there was the recovery of IMA 21, which equaled the other lines (Figure 7L).
3.2. Yield Components
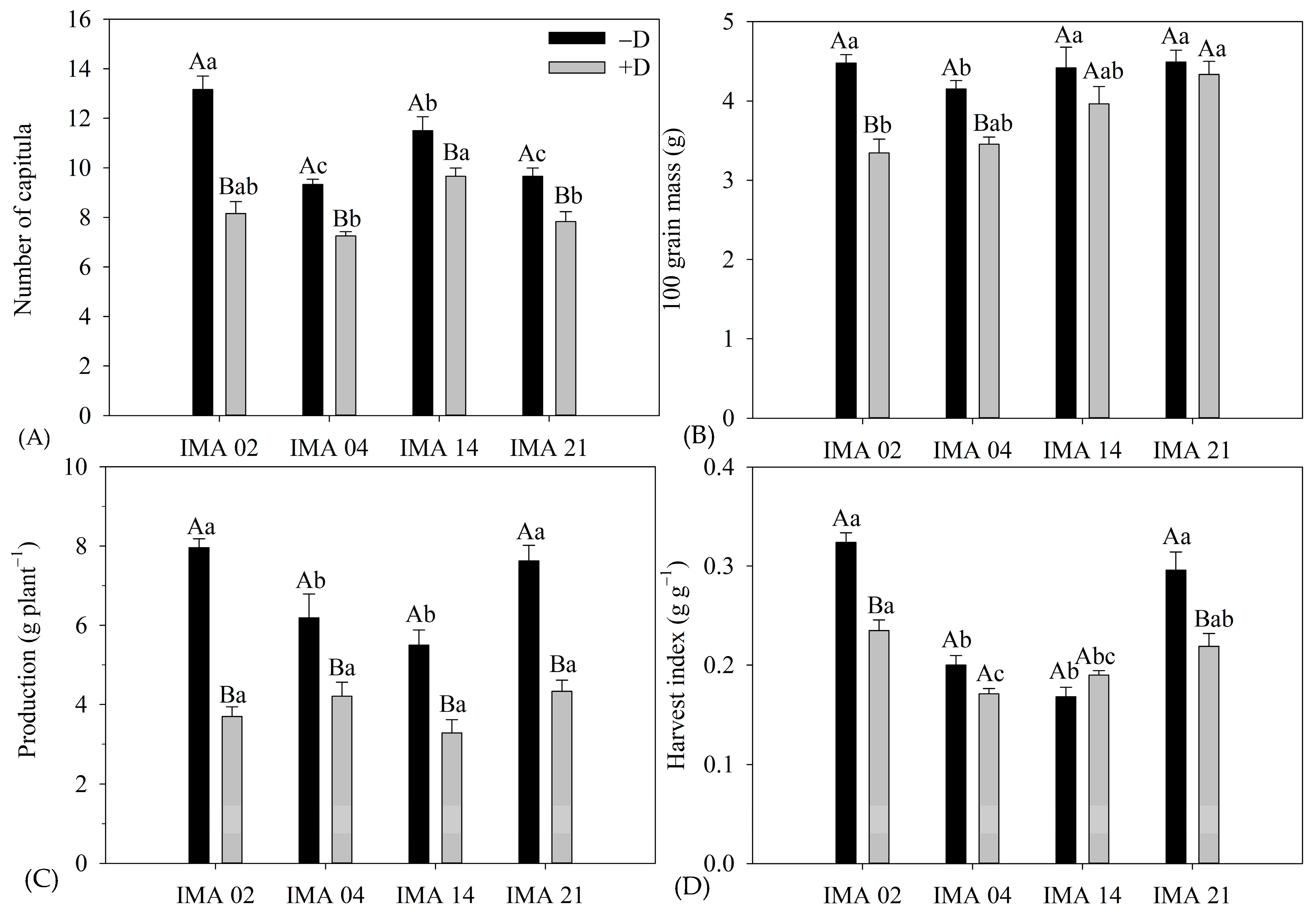
NC, yield, and HI were affected by Wr, L, and Wr × L, while 100-grain mass was affected only by Wr × L (Table 5).

Table 5.
Analysis of variance of the number of capitula (NC), 100-grain mass (100 GM), yield and harvest index (HI) of four safflower lines under two water regimes (with and without water deficiency).
The imposition of water deficiency promoted reductions of 38.0%, 21.5%, 19.6%, and 16.0% in NC of the lines IMA 02, IMA 04, IMA 21, and IMA 14, respectively, resulting in 8.2 capitula plant−1, 7.3 capitula plant−1, 7.8 capitula plant−1, and 9.7 capitula plant−1, respectively (Figure 8A).
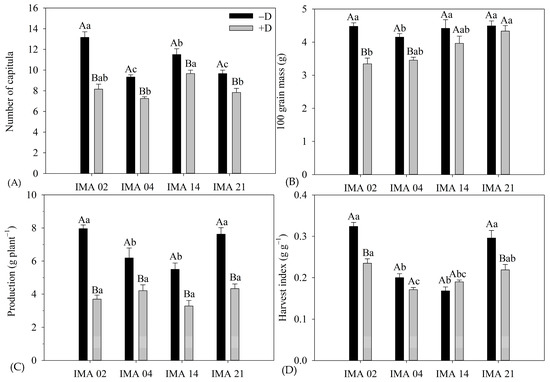
Figure 8.
Number of capitula (NC) (A), 100-grain mass (100 GM) (B), yield (C), and harvest index (HI) (D) of four safflower lines as a function of two water regimes (with water deficiency—+D; without water deficit—−D). Means followed by the same letter in each evaluation period, lower case between lines and within the water regime, and upper case within each line and between water regimes do not differ by Tukey’s test.
Whereas, 100 GM was negatively affected by water deficiency in IMA 02 and IMA 04 lines, with reductions of 25.2% and 16.6%, resulting in 100 GM of 3.35 g and 4.15 g, respectively (Figure 8B). The 100 GM of the IMA 14 and IMA 21 lines were unaffected by water regimes, showing averages of 4.19 g and 4.41 g, respectively.
Water deficiency negatively affected the yield of safflower lines (Figure 8C). The IMA 02 line showed the highest yield among the lines under −D (7.96 g plant−1); however, the highest reduction was verified under +D, 53.6%, resulting in 3.70 g plant−1. The second highest yield under −D was verified in IMA 21 (7.62 g plant−1), which showed a decrease of 41.9% in its yield under +D, resulting in 4.33 g plant−1. The lowest yield under −D was verified in IMA 14 (5.49 g plant−1), which had its yield reduced by 41.5% under +D. Additionally, the IMA 04 produced 6.19 g plant−1 under −D and showed the lowest yield reduction under +D (30.1%), resulting in 4.21 g plant−1. However, despite the observed differences in yield magnitudes between the water regimes, the yield was not significantly different among the lines under +D.
The HI differed among the water regimes, except for line IMA 14, which averaged 0.19 g g−1. The imposition of water deficiency negatively affected HI in lines IMA 02, IMA 21, and IMA 04, with reductions of 27.5%, 26.0%, and 14.5%, resulting in HI of 0.23 g g−1, 0.24 g g−1, and 0.17 g g−1, respectively (Figure 8D).
4. Discussion
Water deficiency decreased the Ψleaf of safflower lines, with decreases in RWC and increases in EL. Reduction of the plant water content results in cell contraction, decreased turgescent pressure against cell walls, and increased solute concentration [49]. Several studies have shown that Ψleaf and relative water content are reduced by water deficit in safflower [3,7,9,25,26,50], which are variables widely used as indicators of plant water status. Thus, as it is an effective parameter in reflecting the effects of water deficit in safflower, the relative water content can be used to select drought-tolerant genotypes [38,51].
Although the Ψleaf and RWC were reduced in all lines due to water deficiency, IMA 14 and IMA 21 showed the highest recovery rates of these parameters after rehydration, as they matched the plants conducted in the adequate water regime, indicating higher resilience capacity. After rehydration, plant cultivars with high recovery capacity can be considered water deficiency tolerant [52].
One of the major impacts of water stress is cell membrane modification, which reduces its function or causes total dysfunction. Cell membrane dysfunction due to stress is expressed by increased permeability and ion leakage, readily measured by electrolyte leakage [53]. Thus, the increased EL suggests damage by oxidative stress on the cell membrane. The stress caused by water deficit increases EL due to the increased fluidity of the cell membrane, which consequently leads to its disruption from the formation of reactive oxygen species, which reduce enzyme activity and promote lipid peroxidation [54,55]. Maintaining membrane integrity after a water deficit is an essential indicator of stress tolerance [53,56]. In the present study, the IMA 14 and IMA 21 lines showed the lowest EL values under stress, evidencing an excellent ability to maintain plasma membrane stability and indicating drought tolerance [57].
Photosynthetic efficiency is conditioned by the photosynthetic pigment concentration and the water status of the plant [58]. Photosynthetic pigments are essential in absorbing, concentrating, and storing light energy and producing reduced power [59]. Thus, pigments are critical chloroplast components to photosynthesis; consequently, the relative chlorophyll content positively relates to the photosynthetic rate.
The imposition of water deficiency usually promotes chlorophyll degradation, a typical oxidative symptom that can result from photoinhibition [60,61]. This process reduces the efficiency of light energy absorption, electron transfer, and ATP and NADPH production in the photochemical phase of photosynthesis [62].
Despite being considered a water deficiency tolerant species, studies have already reported reductions in the photosynthetic pigment contents of safflower plants under moderate and severe stress conditions [3,27,30,63,64]. In our study, the water restriction reduced the contents of Chl a, Chl b, Chl a + b, Chl a/Chl b, and carotenoids. However, after the rehydration period, the lines were recovered, evidenced by the increase in photosynthetic pigment levels.
Carotenoids are accessory pigments that play a crucial role in plant protection against photosynthetic photoinhibition by preventing oxidative damage caused by stress [65,66,67]. The content of photosynthetic pigments in leaves is used as an index to show the plants’ photosynthetic capacity and physiological status [68,69]. Thus, pigment content may be a valuable marker for selecting water-stress-tolerant varieties [7,70]. After rehydration, the IMA 21 line increased photosynthetic pigment indices, so it can be considered drought tolerant due to this.
Between 60 DAS and 140 DAS, there was an increase in air temperature, reaching higher levels, which was associated with plant aging. This provided an increase in photosynthetic metabolism, increasing the content of photosynthetic pigments and gas exchange activity to ensure the formation and filling of grains.
Water deficiency decreased the CO2 assimilation rate in safflower plants, which may have resulted from reduced chlorophyll synthesis or damage to its molecules [21,71,72]. Stomatal closure is one of the mechanisms to alleviate drought, being one of the first steps in plant adaptation to water deficit, allowing the maintenance of water status [22]. The adjustment of the stomatal aperture, evidenced by the considerable reduction in gs in plants under +D, allows controlling water loss by reducing transpiration flux and limiting CO2-diffusion [73,74], which explains the decreases in A, gs, and E associated with significant increases in Ci.
Lower light use efficiency under water stress reduces the functioning of the photosynthetic machinery to match the available carbon substrate [23]. Research has already demonstrated reduced photosynthetic capacity of safflower plants under water stress, with the reduction in photosynthetic rate being genotype dependent, as observed in our results [29,30,75], in which all lines were affected by the imposition of water deficiency, with a highlight on IMA 04 that showed the best performance under water deficiency. However, after rehydration, there was the recovery of lines IMA 04, IMA 14, and IMA 21, which did not occur with IMA 02.
High values of WUE indicate an efficient relation between A and E in plants that can maintain a balanced relationship between water saving and metabolism maintenance [76], represented by the ratio of dry matter mass produced per gram of transpired water [77]. As observed in our study, water deficit usually promotes high values for this variable by stomatal closure, which reduces critical metabolic processes for plant tissue functioning, such as CO2 assimilation and transpiration.
This study demonstrated a reduction of CE in plants subjected to water stress due to decreases in CO2 assimilation rates and CO2 levels in the leaf mesophyll after stomatal closure, causing biochemical damage to the photosynthetic apparatus due to the low regeneration of the RUBISCO enzyme, which hinders the carboxylation process [77,78,79].
The reductions in CE observed in IMA 02, IMA 14, and IMA 21 under +D indicate non-stomatal limitation mechanisms of photosynthesis, which can directly interfere with productive yield. The rehydration period favored the recovery and maintenance of the stability of IMA 04 as a function of CE.
Water stress inhibits photosynthetic activity in tissues due to an imbalance between light uptake and its use. In general, water deficiency directly affects the photochemical efficiency of PSII in safflower lines, the phase responsible for light energy absorption and electron transfer by the photosystems I and II to produce ATP and NADPH [80,81,82]. The reductions verified in the parameters φPSII, ETR, and Fv/Fm indicate impairment of the photosynthetic apparatus by reducing its efficiency.
The PSII is highly light-sensitive, and the negative regulation of photosynthesis under water stress causes an energy imbalance in the PSII reaction center, leading to photoinhibition [83]. Mechanisms have evolved in the plant to protect it from photoinhibition, such as non-photochemical quenching, electron (e−) transport to molecules other than CO2, such as to oxygen, which leads to photorespiration and Mehler reaction [84,85], non-radiative energy dissipation mechanisms [80,86], and chlorophyll concentration changes [81]. However, these processes ultimately lead to a lower quantum yield of PSII [85].
The reduction in φPSII and increase in NQP implies a decrease in the rate of capture and conversion of excitation energy by the PSII reaction center. Thus, reductions in ETR and PSII photochemical efficiency indicate the disorganization of PSII reaction centers under water stress conditions [87,88].
The qP can indicate physiological differences between crop genotypes [89]. Additionally, the NQP is a mechanism that plants use to protect themselves against the harmful impacts of high light intensity [90]. Thus, the excess energy absorbed reduces the impairment of the functioning of the photosynthetic apparatus [91]. Our results show an increase in NQP in the lines under +D, except IMA 04, which did not have its NPQ affected by water deficiency, maintaining the lowest values under this condition, which indicates higher efficiency of this line in using the energy absorbed by the antenna complex of PSII [92].
Under +D, the leaves of safflower lines increased the (Y(NQP)), indicating the efficiency of this mechanism in the photosynthetic apparatus photoprotection of these plants. These results evidence the activation of the dissipation of excess energy, being part of the energy used in the photosynthetic process, lost in the form of heat, a process associated with thermal dissipation through the xanthophyll cycle [93].
The quantum yield of unregulated non-photochemical energy dissipation in PSII, Y(NO), represents constitutive energy loss [88]. IMA 02 and IMA 04 were unchanged in Y(NO) under +D, suggesting that these lines have an effective system for dissipating energy in the unregulated form, i.e., energy lost constitutively in the antennae of PSII and by fluorescence, which is dissipated to non-photochemical processes [94]. On the other hand, decreases in Y(NO) were observed in IMA 21, indicating susceptibility to withstanding excess light energy.
As expected, water restriction caused decreases in the number of capitula and mass of 100 grains, which resulted in lower yield and harvest index. However, the minor reductions in yield components were verified in the IMA 04, IMA 14, and IMA 21 lines. Thus, these can be considered tolerant.
According to Shahrokhnia and Sepaskhah [11], the decrease in seed yield in safflower occurs mainly through the reduction in the number of capitula per plant and the number of seeds per capitula. Moreover, other authors have found a positive correlation between the number of capitula, number of seeds per capitula, 100-grain mass, and productivity [12,95].
Our results corroborate those of Silva et al. [64], who also found a reduction in capitula number, 100-grains mass, and productivity of safflower under moderate water deficit (−50 kPa), and Loghmani et al. [96] and Joshan et al. [97], who also reported that water stress negatively affected the 100-grain mass and grain yield in different safflower cultivars.
Reduced safflower seed yield under water deficiency conditions has also been reported by Santos et al. [10], Mohammadi et al. [12], Yeloojeh et al. [13], Eslam [51], and Movahhedy-Dehnavy et al. [98]. In addition, Amini et al. [30] evaluated safflower genotypes under water deficit and observed that cultivars with low yields were characterized by presenting low chlorophyll values, results like those found in the present work.
The harvest index demonstrates the efficiency of photoassimilate transport to the grains through the source–drain relationship. Except for IMA 14, the other lines had the HI reduced as a function of water deficiency. Silva et al. [64] and Heydarian et al. [99] also reported reduced HI in safflower plants after the imposition of water deficiency.
Rehydration of plants regulates the metabolism of stressed plant tissue and is associated with the repair of possible damage caused by water stress [100]. In this study, the recovery observed through the equalization between the +D and −D conditions in most of the analyzed variables evidences the reestablishment of plant metabolism, which indicates that the 20-day rehydration period was sufficient for the recovery of safflower plants physiologically. However, the imposition of water deficiency during flowering for 30 days negatively affected the yield components of the lines, with reduced impact on IMA 04, IMA 14, and IMA 21.
5. Conclusions
In the present research, the safflower lines responded differently to water restriction in the flowering period, indicating genetic variability. IMA 02 was the most affected line by the imposition of water deficiency, with less recovery of the parameters evaluated after rehydration, indicating susceptibility to drought. All lines showed reductions in physiological and yield traits under water deficiency; however, IMA 04, IMA 14, and IMA 21 showed smaller reductions in water potential, relative water content, net CO2 assimilation, stomatal conductance, transpiration rate, and, consequently, in water use efficiency and carboxylation efficiency, in addition to greater efficiency of photochemistry. It showed better recovery after the rehydration period, which evidences the ability to tolerate drought, even if this occurs in the flowering phase, which is one of the most critical to production; therefore, these lines’ superior physiological performance allowed a smaller yield component decrease. Thus, they can be recommended for commercial use and safflower breeding programs to obtain germplasm and for the selection of superior genotypes under water deficiency conditions.
Author Contributions
Conceptualization: M.d.A.S.; methodology: M.d.A.S., D.M.R.S. and J.C.C.d.S.; software: H.L.S.; formal analysis: H.L.S. and L.d.S.F.; investigation: M.d.A.S., D.M.R.S., J.C.C.d.S. and F.P.d.A.P.B.; writing—original draft preparation: M.d.A.S., H.L.S. and L.d.S.F.; writing—review and editing: M.d.A.S., H.L.S. and L.d.S.F.; supervision: M.d.A.S.; project administration: M.d.A.S.; funding acquisition: M.d.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
M.A.S. and F.P.A.P.B. would like to thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the “Productivity in Research” fellowship (Proc. 305952/2018-8) and “Ph.D.” fellowship (Proc. 162258/2015-1), respectively. Furthermore, we would like to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the master’s fellowship for L.S.F., D.M.R.S., and J.C.C.S. (financing code 001), and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for the master’s fellowship for H.L.S. (Proc. 2021/02991-0).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Queiroga, V.P.; Girão, E.G.; Albuquerque, E.M.B. Cártamo (Carthamus tinctorius L.): Tecnologias de plantio e utilização; AREPB: Campina Grande, Brazil, 2021. [Google Scholar]
- Santos, J.C.C.; Silva, D.M.R.; Amorim, D.J.; Sab, M.P.V.; Silva, M.A. Glyphosate hormesis mitigates the effect of water deficit in safflower (Carthamus tinctorius L.). Pest Manag. Sci. 2021, 77, 2029–2044. [Google Scholar] [CrossRef]
- Bortolheiro, F.P.; Silva, M.A. Physiological response and productivity of safflower lines under water deficit and rehydration. An. Acad. Bras. Cien. 2017, 89, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.J. A cultura do Carthamus tinctorius L.: Principais usos e variabilidade genética. Res. Soc. Dev. 2021, 10, 2. [Google Scholar] [CrossRef]
- Silva, D.M.R.; Santos, J.C.C.D.; Sab, M.P.V.; Silva, M.A. Morpho-physiological and nutritional responses of safflower as a function of potassium doses. J. Plant Nutr. 2021, 44, 1903–1915. [Google Scholar] [CrossRef]
- Ramos, A.R.; Bassegio, D.; Nakagawa, J.; Zanotto, M.D. Harvest times and seed germination of three safflower genotypes. Cien. Rural 2021, 51, e20200606. [Google Scholar] [CrossRef]
- Hussain, M.I.; Lyra, D.-A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 2016, 36, 4. [Google Scholar] [CrossRef]
- Lovelli, S.; Perniola, M.; Ferrara, A.; Di Tommaso, T. Yield response factor to water (Ky) and water use efficiency of Carthamus tinctorius L. and Solanum melongena L. Agric. Water Manag. 2007, 92, 73–80. [Google Scholar] [CrossRef]
- Singh, S.; Angadi, S.V.; Grover, K.; Begna, S.; Auld, D. Drought response and yield formation of spring safflower under different water regimes in the semi-arid Southern High Plains. Agric. Water Manag. 2016, 163, 354–362. [Google Scholar] [CrossRef]
- Santos, R.F.; Bassegio, D.; Silva, M.A. Productivity and production components of safflower genotypes affected by irrigation at phenological stages. Agric. Water Manag. 2017, 186, 66–74. [Google Scholar] [CrossRef]
- Shahrokhnia, M.H.; Sepaskhah, A.R. Physiologic and agronomic traits in safflower under various irrigation strategies, planting methods and nitrogen fertilization. Ind. Crops Prod. 2017, 95, 126–139. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghassemi-Golezani, K.; Chaichi, M.R.; Safikhani, S. Seed oil accumulation and yield of safflower affected by water supply and harvest time. Agron. J. 2018, 110, 586–593. [Google Scholar] [CrossRef]
- Yeloojeh, K.A.; Saeidi, G.; Ehsanzadeh, P. Effectiveness of physiological traits in adopting safflower (Carthamus tinctorius L.) genotypes to water deficit condition. Int. J. Plant Prod. 2020, 14, 155–164. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Karas, E. The effect of deficit irrigation applied in different phenological periods on safflower yield and quality. Appl. Ecol. Environ. Res. 2020, 18, 1755–1769. [Google Scholar] [CrossRef]
- Soren, K.R.; Ali, K.; Tyagi, V.; Tyagi, A. Recent advances in molecular breeding of drought tolerance in rice (Oryza sativa L.). Indian J. Biotechnol. 2010, 9, 233–251. Available online: https://hdl.handle.net/123456789/9881 (accessed on 15 December 2022).
- Foster, K.; Lambers, H.; Real, D.; Ramankutty, P.; Cawthray, G.R.; Ryan, M.H. Drought resistance and recovery in mature Bituminaria bituminosa var. albomarginata. Ann. Appl. Biol. 2015, 166, 154–169. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Huang, B. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol. Plant. 2010, 139, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yasuor, H.; Yermiyahu, U.; Zuo, Q.; Ben-Gal, A. Dynamic responses of wheat to drought and nitrogen stresses during re-watering cycles. Agric. Water Manag. 2014, 146, 163–172. [Google Scholar] [CrossRef]
- Oddo, E.; Abbate, L.; Inzerillo, S.; Carimi, F.; Motisi, A.; Sajeva, M.; Nardini, A. Water relations of two Sicilian grapevine cultivars in response to potassium availability and drought stress. Plant Physiol. Biochem. 2020, 148, 282–290. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Servani, M.; Mobasser, H.R.; Ganjali, H.R. Influence of drought stress on photosynthetic, radical oxygen, respiration, assimilate partitioning, activities of enzymes, phytohormones and essential oils in crop plants. Int. J. Biosci. 2014, 5, 223–236. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osrio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Hojati, M.; Modarres-Sanavy, S.; Karimi, M.; Ghanati, F. Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiol. Plant. 2011, 33, 105–112. [Google Scholar] [CrossRef]
- Eslam, B.P. Evaluation of physiological indices for improving water deficit tolerance in spring safflower. J. Agric. Sci. Technol. 2011, 13, 327–338. [Google Scholar]
- Mohammadi, M.; Ghassemi-Golezani, K.; Zehtab-Salmasi, S.; Nasrollahzade, S. Assessment of some physiological traits in spring safflower (Carthamus tinctorius L.) cultivars under water stress. Int. J. Life Sci. 2016, 10, 58–64. [Google Scholar] [CrossRef]
- Nazar, Z.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Glycinebetaine-Induced Alteration in Gaseous Exchange Capacity and Osmoprotective Phenomena in Safflower (Carthamus tinctorius L.) under Water Deficit Conditions. Sustainability 2020, 12, 10649. [Google Scholar] [CrossRef]
- Kazemeini, S.A.; Mohamadi, S.; Pirastehanosheh, H. Growth and photosynthesis responses of safflower cultivars to water stress at two developmental stages. Biol. Forum Int. J. 2015, 7, 923–929. [Google Scholar]
- Amini, H.; Arzani, A.; Bahrami, F. Seed yield and some physiological traits of safflower as affected by water deficit stress. Int. J. Plant Prod. 2013, 7, 597–614. [Google Scholar]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 2020, 259, 108823. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Sriti, J.; Mejri, H.; Limam, F.; Marzouk, B. Effect of drought on safflower natural dyes and their biological activities. EXCLI J. 2014, 13, 1–18. [Google Scholar]
- Aeini, M.; Abad, H.H.S.; Yousefidad, M.; Heravan, E.M.; Madani, H. Effect of seed priming on morphological and biochemical characteristics of safflower (Carthamus tinctorius L.) under drought stress. Crop Res. 2018, 53, 45–52. [Google Scholar] [CrossRef]
- Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Oya, T. Tolerância à seca em plantas: Mecanismos fisiológicos e moleculares. Biotecnolog. Cien. Desenvolv. 2001, 23, 12–18. [Google Scholar]
- Bahrami, F.; Arzani, A.; Karimi, V. Evaluation of yield-based drought tolerance indices for screening safflower genotypes. Agron. J. 2014, 106, 1219–1224. [Google Scholar] [CrossRef]
- Wei, B.; Hou, K.; Zhang, H.; Wang, X.; Wu, W. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L., under drought stress. Ind. Crops Prod. 2020, 151, 112465. [Google Scholar] [CrossRef]
- Nogueira, R.J.; Mansur, C.; Moraes, J.A.P.D.; Burity, H.A.; Bezerra Neto, E. Alterações na resistência à difusão de vapor das folhas e relações hídricas em aceroleiras submetidas a déficit de água. Braz. J. Plant Physiol. 2001, 13, 75–87. [Google Scholar] [CrossRef]
- Masupiemang, M.; Emongor, V.E.; Malambane, G. A review of drought tolerance in Safflower. Int. J. Plant Soil Sci. 2022, 34, 140–149. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.; Oliveira, V.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.; Araujo Filho, J.; Oliveira, J.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Ambrosano, E.J.; Tanaka, R.T.; Mascarenhas, H.A.A. Leguminosas e Oleaginosas. In Recomendações de Calagem e Adubação Para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico de Campinas: São Paulo, Brazil, 1997; pp. 187–204. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Gomes, E.R.; Broetto, F.; Queluz, J.G.T.; Bressan, D.F. Efeito da fertirrigação com potássio sobre o solo e produtividade do morangueiro. Irriga 2015, 1, 107–122. [Google Scholar] [CrossRef][Green Version]
- Jamaux, I.; Steinmetz, A.; Belhassen, E. Looking for molecular and physiological markers of osmotic adjustment in sunflower. New Phytol. 1997, 137, 117–127. [Google Scholar] [CrossRef]
- Campos, P.S.; Thi, A.T.P. Effect of abscisic acid pretreatment on membrane leakage and lipid composition of Vigna unguiculata leaf discs subjected to osmotic stress. Plant Sci. 1997, 130, 11–18. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kramer, D.M.; Avenson, T.J.; Edwards, G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Singh, S.; Angadi, S.V.; Hilaire, R.S.; Grover, K.; Van Leeuwen, D.M. Spring safflower performance under growth stage based irrigation in the Southern High Plains. Crop Sci. 2016, 56, 1878–1889. [Google Scholar] [CrossRef]
- Eslam, B.P. Some Eco-physiological and Agronomic Responses of Several Salinity Tolerant Safflower Varieties to Water Deficit Stress. J. Agric. Sci. Sustain. Prod. 2020, 30, 145–155. [Google Scholar]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of Physiological Indicators Associated with Drought Tolerance in Wheat under Drought and Re-Watering Conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Liu, Q.; Lin, B.; Wang, J. Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant. 2008, 30, 433–440. [Google Scholar] [CrossRef]
- Langaro, A.C.; Nohatto, M.A.; Perboni, L.T.; Tarouco, C.P.; Agostinetto, D. Alterações fisiológicas na cultura do tomateiro devido à deriva simulada de herbicidas. Rev. Bras. Herb. 2014, 13, 40–46. [Google Scholar] [CrossRef]
- Torres Neto, A.; Campostrini, E.; Oliveira, J.G.; Smith, R.E.B. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Reyes, S.M.R.; Hoyos, G.R.; Ferreira Júnior, D.C.; Cecílio Filho, A.B.; Fonseca, L.P.M. Physiological response of Physalis peruviana L. seedlings inoculated with Funneliformis mosseae under drought stress. Rev. Cien. Agrar. 2019, 4, 175–183. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M. Photosynthetic activity and chlorophyll pigment concentration in Medicago x varia T. Martyn leaves treated with the Tytanit growth regulator. Saudi J. Biol. Sci. 2021, 28, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Araújo, S.A.C.; Deminicis, B.B. Fotoinibição da fotossíntese. Rev. Bras. Biocienc. 2009, 7, 463–472. [Google Scholar]
- Hassan, M.A.L.; Fuertes, M.M.; Sanchez, F.J.R.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti. Agrobo. 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the Effects of Water Deficit on Photosynthesis Using Parameters Derived from Measurements of Leaf Gas Exchange and of Chlorophyll a Fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Golkar, P.; Hamzeh, E.; Maibody, S.A.M.; Taghizadeh, M. Safflower’s (Carthamus tinctorius L.) physio-biochemical mechanisms to improve its drought tolerance. Acta Physiol. Plant. 2021, 43, 82. [Google Scholar] [CrossRef]
- Silva, D.M.R.; Santos, J.C.C.; Rosa, V.R.; Santos, A.L.F.; Silva, M.A. Tolerance to water defciency in safflower (Carthamus tinctorius L.) modulated by potassium fertilization. Acta Physiol. Plant. 2022, 44, 99. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khatri, K.; Rathore, M.S.; Jha, B. Introgression of UfCyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco. Mol. Biol. Rep. 2018, 45, 1745–1758. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 2019, 57, 61–74. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Houborg, R.; Fisher, J.B.; Skidmore, A.K. Advances in remote sensing of vegetation function and traits. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Carter, A.G.; Spiering, B.A. Optical properties of intact leaves for estimating chlorophyll concentration. J. Environ. Qual. 2002, 31, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef]
- Carvalho, M.H.C. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhangl, X.S.; Huang, Z.Y. Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J. Plant Ecol. 2010, 3, 79–87. [Google Scholar] [CrossRef]
- Ronchi, C.P.; Araújo, F.C.; Almeida, W.L.; Silva, M.A.A.; Magalhães, C.E.O.; Oliveira, L.B.; Drumond, L.C.D. Respostas ecofisiológicas de cafeeiros submetidos ao déficit hídrico para concentração da florada no Cerrado de Minas Gerais. Pesqui. Agropecu. Bras. 2015, 50, 24–32. [Google Scholar] [CrossRef]
- Picoli-Junior, G.J.; Carbonari, C.A.; Matos, A.K.A.; Rodrigues, L.F.O.S.; Velini, E.D. Influence of glyphosate on susceptible and resistant ryegrass populations to herbicide. Planta Daninha 2017, 35, e017163391. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by afecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef]
- Machado, E.C.; Schmidt, P.T.; Medina, C.L.; Ribeiro, R.V. Respostas da fotossíntese de três espécies de citros a fatores ambientais. Pesqui. Agropecu. Bras. 2005, 40, 1161–1170. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield; leaf gaseous exchange and chlorophyll fuorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 80, 118–125. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.L.; Collins, G.D.; Whitaker, J.; Perry, C.D.; Chastain, D.R. Electron transport through photosystem II is not limited by a wide range of water deficit conditions in field-grown Gossypium hirsutum. J. Agron. Crop Sci. 2014, 200, 77–82. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 2005, 56, 425–433. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Govindjee. On the requirement of minimum number of four versus eight quanta of light for the evolution of one molecule of oxygen in photosynthesis: A historical note. Photosynth. Res. 1999, 59, 249–254. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Govindjee. Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Mota, C.S.; Cano, M.A.O. Respostas fisiológicas de plantas jovens de macaúba a condições de seca cíclica. Pesq. Flor. Bras. 2016, 36, 225–234. [Google Scholar] [CrossRef]
- Falqueto, A.R.; Cassol, D.; Júnior, A.M.M.; Oliveira, A.C.; Bacarin, M.A. Características da fluorescência da clorofila em cultivares de arroz com ciclo precoce, médio e tardio. Rev. Bras. Biocienc. 2007, 5, 579–581. [Google Scholar]
- Kalaji, H.M.; Rastogi, A.; Zivcak, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lofti, R.; Stypinski, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef] [PubMed]
- Veres, S.; Tóth, V.R.; Láposi, R.; Oláh, V.; Lakatos, G.; Mészáros, I. Carotenoid composition and photochemical activity of four sandy grassland species. Photosynthetica 2006, 44, 255–261. [Google Scholar] [CrossRef]
- Pontasch, S.; Fisher, P.L.; Krueger, T.; Dove, S.; Hoegh-Guldberg, O.; Leggat, W.; Davy, S.K. Photoacclimatory and photoprotective responses to cold versus heat stress in high latitude reef corals. J. Phyc. 2017, 53, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.F.; Tatagiba, S.D.; Reis, E.F.; Pezzopane, J.E.M.; Amaral, J.F.T. Limitações fotossintéticas em folhas de cafeeiro arábica promovidas pelo déficit hídrico. Coffee Sci. 2017, 12, 389–399. [Google Scholar] [CrossRef]
- Camas, N.; Çirak, C.; Esendal, E. Seed yield, oil content and fatty acids composition of safflower (Carthamus tinctorius L.) grown in northern Turkey conditions. Anadolu Tarım Bilim. Derg. 2007, 22, 98–104. [Google Scholar]
- Loghmani, S.M.T.T.; Bazrafshan, F.; Alizadeh, O.; Amiri, B.; Bahrani, A. Influence of cut-off irrigation on seed quality and physiological indices of various safflower (Carthamus tinctorius L.) genotypes. Acta Agrobot. 2019, 72, 4. [Google Scholar] [CrossRef]
- Joshan, Y.; Sani, B.; Jabbari, H.; Mozafari, H.; Moaveni, P. The effect of late season drought stress on some morphophysiological characteristics of Iranian safflower varieties in Karaj region. Environ. Stress. Crop Sci. 2020, 13, 1093–1104. [Google Scholar] [CrossRef]
- Movahhedy-Dehnavy, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Ind. Crops Prod. 2009, 30, 82–92. [Google Scholar] [CrossRef]
- Heydarian, M.; Moghadam, H.R.T.; Hassanpour, J.; Zahedi, H. Effect of boron foliar application and irrigation withholding on yield and yield components of safflower. Res. Crops 2012, 13, 166–173. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Chen, X.; Fu, J.; Zhang, Y. Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric. Water Manag. 2016, 172, 62–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).