Fertilizer Performance of a Digestate from Swine Wastewater as Synthetic Nitrogen Substitute in Maize Cultivation: Physiological Growth and Yield Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Materials
2.2.1. Test Crop
2.2.2. Mineral Fertilizers
2.2.3. Biofertilizer
2.3. Experimental Design and Treatments
2.4. Parameter Measurements
2.4.1. Morphological and Physiological Parameters
2.4.2. Leaf Nutritional Status
2.4.3. Maize Grain Yield
2.5. Statistical Analyses
3. Results and Discussion
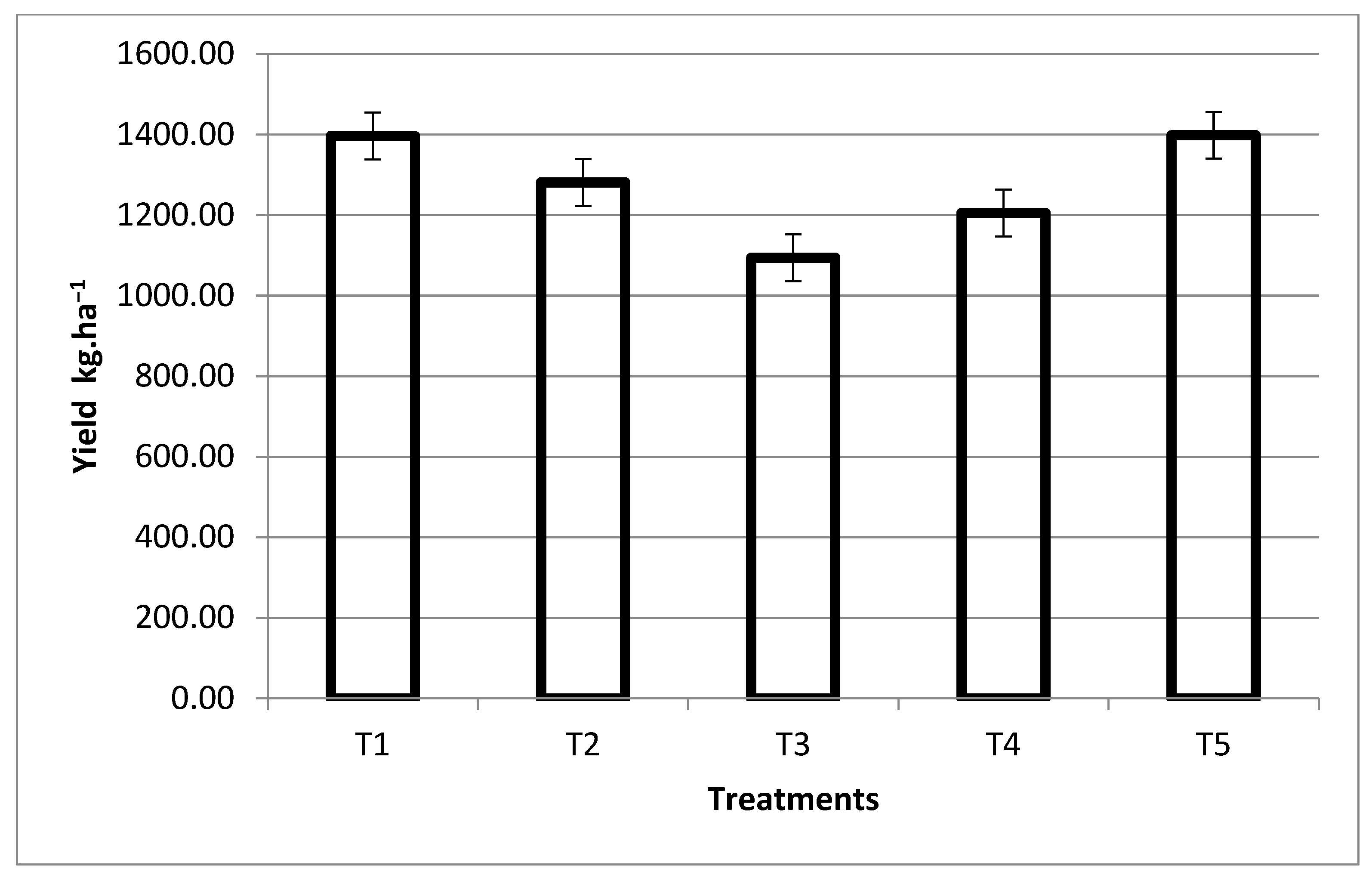
3.1. Effects of the Biofertilizer on Maize Grain Yield
3.2. Effects of the Biofertilizer on the nutritional Value of Maize Leaves
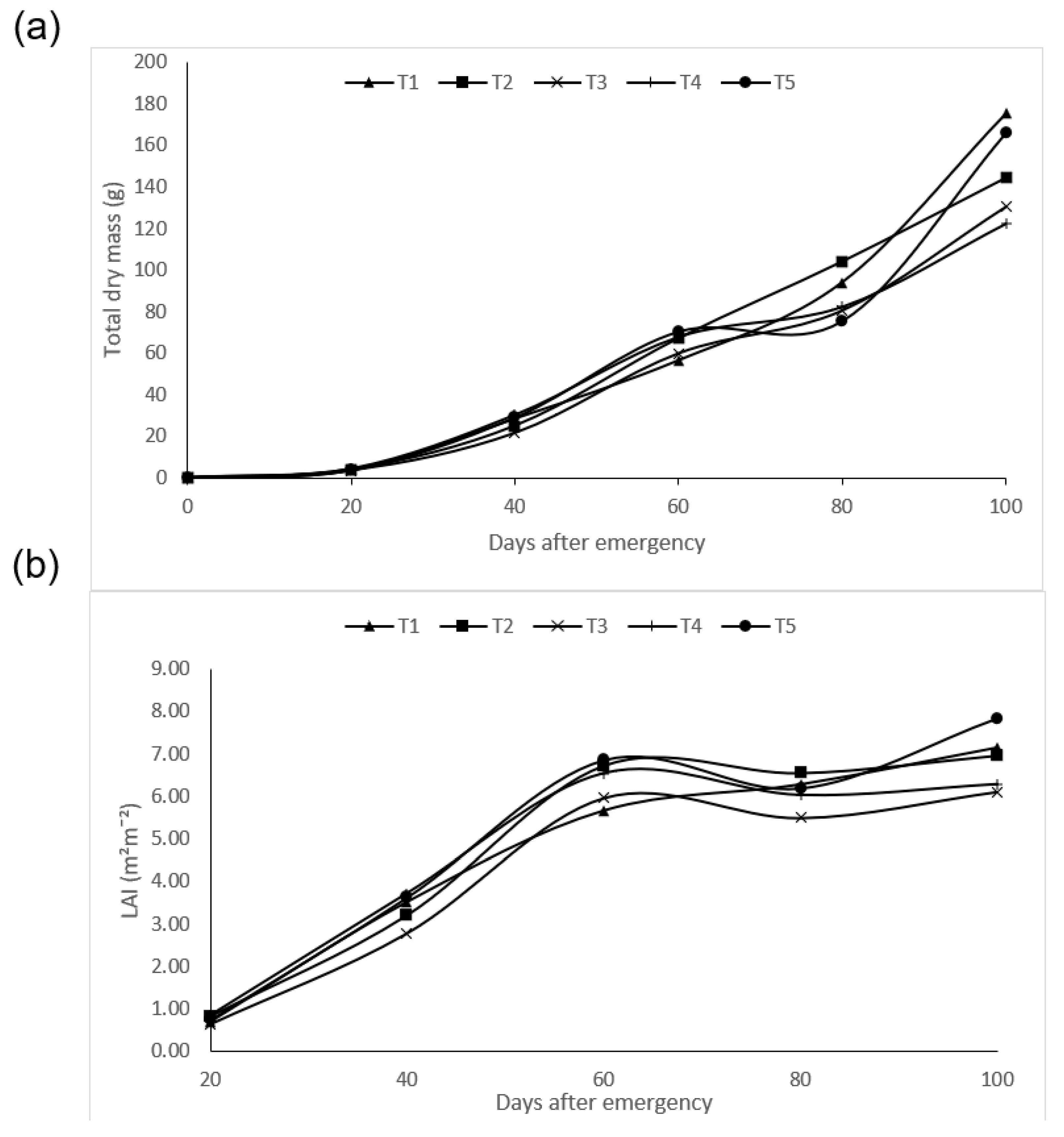
3.3. Effects of the Biofertilizer on the Growth Parameters of Maize
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aragão, A.; Contini, E.O. Agriculture in Brazil and in the World: A Synthesis of the 2000–2020 Period, 1st ed.; SIRE/EMBRAPA: Brasília, Brazil, 2021; pp. 1–68. Available online: https://www.embrapa.br/documents/10180/62618376/O+AGRO+NO+BRASIL+E+NO+MUNDO.pdf/41e20155-5cd9-f4ad-7119-945e147396cb (accessed on 8 December 2022).
- Asencios, Y.J.O. The Importance of hydrogen for Brazil: A source of clean energy and a path to the production of nitrogen fertilizers. SciELO Prepr. 2022, 4408. [Google Scholar] [CrossRef]
- Fioroni, B. Deposits and Bioinputs Minimize the Lack of Foreign Fertilizer; FAEP: Curitiba, Brazil, 2022; Volume 1558, pp. 18–27. Available online: https://www.sistemafaep.org.br/wp-content/uploads/2022/03/BI_1558_web.pdf (accessed on 8 December 2022).
- Costa, M.S.S.M.; Lorin, H.E.F.; Costa, L.A.M.; Cestanaro, T.; Pereira, D.C.; Bernardi, F.H. Performance of four stabilization bioprocesses of beef cattle feedlot manure. J. Environ. Manag. 2016, 181, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.T.; Costa, M.S.S.M.; Costa, L.A.M.; Trindade, H.; Tonial, L.M.S.; Lorin, H.E.F.; Goufo, P. Spectroscopic and physicochemical characterization of poultry waste-based composts and charcoal–compost mixtures for the prediction of dry matter yield of giant of Italy parsley. Agronomy 2021, 12, 256. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez- Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez- Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Goufo, P.; Fonseca, J.; Pereira, J.; Ferreira, L.; Coutinho, J.; Trindade, H. Effect of lignocellulosic and phenolic compounds on ammonia, nitric oxide and greenhouse gas emissions during composting. J. Clean. Prod. 2018, 171, 548–556. [Google Scholar] [CrossRef]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.S.M.; Trindade, H. Comparison of five agro-industrial waste–based composts as growing media for lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef]
- Novotny, E.H.; Balieiro, F.C.; Auccaise, R.; Benites, V.M.; Coutinho, H.L.C. Spectroscopic investigation on the effects of biochar and soluble phosphorus on grass clipping vermicomposting. Agriculture 2022, 12, 1011. [Google Scholar] [CrossRef]
- Santos, C.; Monte, J.; Vilaça, N.; Fonseca, J.; Trindade, H.; Cortez, I.; Goufo, P. Evaluation of the potential of agro-industrial waste-based composts to control Botrytis gray mold and soilborne fungal diseases in lettuce. Processes 2021, 9, 2231. [Google Scholar] [CrossRef]
- Santos, F.T.; Trindade, H.; Costa, M.S.S.M.; Costa, L.A.M.; Goufo, P. Effects of composts made from broiler chicken residues and blended with biochar on the minerals and phenolic compounds in parsley (Petroselinum crispum Mill.). Agriculture 2021, 11, 1168. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural use of biogas digestate as a replacement fertilizer. Agric. Sci. Proc. 2015, 7, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Locoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: Characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, H.; Michels, E.; Ryckaert, B.; Ghkiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 599–600, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Tsachidou, B.; Scheuren, M.; Gennen, J.; Debbaut, V.; Toussaint, B.; Hissler, C.; George, I.; Delfosse, P. Biogas residues in substitution for chemical fertilizers: A comparative study on a grassland in the Walloon Region. Sci. Total Environ. 2019, 166, 212–225. [Google Scholar] [CrossRef]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Herrera, A.; Tambone, F.; Geromel, G.; Meers, E.; Schoumans, O.; Giordano, A.; Adani, F. Using highly stabilized digestate and digestate-derived ammonium sulphate to replace synthetic fertilizers: The effects on soil, environment, and crop production. Sci. Total Environ. 2022, 815, 152919. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, P.R.; Caramori, P.H.; Ricce, W.S.; Pinto, L.F.D. Climate Atlas of the State of Paraná, 1st ed.; IAPAR: Londrina, Brazil, 2019; pp. 1–210. Available online: https://www.idrparana.pr.gov.br/system/files/publico/agrometeorologia/atlas-climatico/atlas-climatico-do-parana-2019.pdf (accessed on 8 December 2022).
- Pavinato, P.S.; Pauletti, V.; Motta, A.C.V.; Adônis, M. Fertilization and Liming Manual for the State of Paraná in Brazil, 2nd ed.; SBCS/NEPAR: Curitiba, Brazil, 2017; pp. 1–482. Available online: https://issuu.com/editoracubo/docs/issuu-nepar-calagem (accessed on 8 December 2022).
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997; pp. 1–319. Available online: https://repositorio.usp.br/item/001070906 (accessed on 8 December 2022).
- Silva, F.C. Manual of Chemical Analysis of Soils, Plants and Fertilizers, 2nd ed.; EMBRAPA/IT: Brasília, Brazil, 2009; pp. 1–319. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/330496 (accessed on 8 December 2022).
- Guimarães, D.P.; Sans, L.M.A.; Moraes, A.V.C. Estimativa da Área Foliar de Cultivares de Milho. In Proceedings of the XXIV Congresso Nacional de Milho e Sorgo, Florianópolis, Brazil, 1–5 September 2002; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/34888/1/Estimativa-area.pdf (accessed on 8 December 2022).
- Benincasa, M.M.P. Plant Growth Analysis, Basic Notions, 1st ed.; FUNEP: Jaboticabal, Brazil, 1988; pp. 1–42. Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&id=418424&biblioteca=vazio&busca=autoria:"M"&qFacets=autoria:"M"&sort=&paginacao=t&paginaAtual=3 (accessed on 8 December 2022).
- Weismann, M. Fases de desenvolvimento da cultura do milho. In Technology and Production: Off-season Corn and Winter Crops, 2008th ed.; Roscoe, R., Lourenção, A.L.F., Grigolli, J.F.J., Melotto, A.M., Pitol, C., Miranda, R.A.S., Eds.; Fundação MS: Maracaju, Brazil, 2008; pp. 4–7. Available online: https://www.fundacaoms.org.br/tipo_da_publicacao/conteudo-safrinha/ (accessed on 8 December 2022).
- Bull, L.T.; Cantarella, H. Maize Cultivation: Factors Affecting Productivity, 1st ed.; POTAFOS: Piracicaba, Brazil, 1993; pp. 63–145. Available online: https://megaleitores.com.br/livro/estante/11993301-cultura-do-milho-fatores-que-afetam-a-produtividade-leonardo-theodoro-bull-e-heitor-cantarella/59797/4433/50300 (accessed on 8 December 2022).
- Martinez, H.E.P.; Carvalho, J.G.; Souza, R.B. Diagnose foliar. In Recommendations for the Use of Correctives and Fertilizers in Minas Gerais, 5th ed.; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; CFSEMG/UFV: Viçosa, Brazil, 1999; pp. 143–168. Available online: https://www.sbcs.org.br/loja/index.php?route=product/product&product_id=133 (accessed on 8 December 2022).
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Rev. Bras. De Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef] [Green Version]
- CONAB. Boletin da Safa de Grãos 2018/19, 9° Levantamento Junho 2019. In Observatório Agrícola Acompanhamento da Safra Brasileira Grãos, 1st ed.; Santos, C.M.R., Ruas, J.F., Salvador, M.A., Amazonas, L., Guth, T.L.F., Nogueira, B.P., Júnior, S.R.G.S., Soares, F.M.S., Campos, P.M., Eds.; CONAB: Brasília, Brazil, 2019; Volume 6, pp. 1–113. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos?start=4 (accessed on 8 December 2022).
- Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Franco-Andreua, L.; Benítezc, C.; Parrado, J. Use of biofertilizers obtained from sewage sludges on maize yield. Eur. J. Agron. 2016, 78, 3–19. [Google Scholar] [CrossRef]
- Schulten, A.; Krämer, U. Interactions between copper homeostasis and metabolism in plants. Prog. Bot. 2017, 79, 111–146. [Google Scholar] [CrossRef]
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The transcriptional control of iron homeostasis in plants: A tale of bHLH transcription factors? Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Manganese and the evolution of Photosynthesis. Orig. Life Evol. Biosph. 2015, 45, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition—A review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian System of Soil Classification, 5th ed.; EMBRAPA: Brasília, Brazil, 2018; pp. 1–356. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1094003/sistema-brasileiro-de-classificacao-de-solos (accessed on 8 December 2022).
- Ambrosini, V.G.; Rosa, D.J.; Basso, A.; Borghezan, M.; Pescador, R.; Miotto, A.; Melo, G.W.B.; Soares, C.R.F.S.; Comin, J.J.; Brunetto, G. Liming as an ameliorator of copper toxicity in black oat (Avena strigosa Schreb.). J. Plant Nutr. 2017, 40, 404–416. [Google Scholar] [CrossRef]
- Casarin, V. Dinâmica de nutrientes no sistema solo-planta visando BPUFs. In Proceedings of the IX Regional Symposium of the International Plant Nutrition Institute, Paragominas, Brazil, 30–31 August 2016; Available online: https://www.npct.com.br/npctweb/npct.nsf/article/BRS-3424/$File/Palestra%20Valter%20Casarin.pdf (accessed on 8 December 2022).
- Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Parrado, J. Degradation of chlorpyrifos using different biostimulants/biofertilizers: Effects on soil biochemical properties and microbial community. Appl. Soil Ecol. 2014, 84, 158–165. [Google Scholar] [CrossRef]
- Nascimento, R.C. O uso do biofertilizante em solos agrícolas do cerrado da região do Alto Paranaíba (MG). Bol. Goiano Geogr. 2010, 30, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.F.; Peixoto, C.P.; Ledo, C.A.S. Physiological indexes and initial growth of papaya plants (Carica papaya L.) under greenhouse conditions. Ciênc. Agrotec. 2007, 31, 1358–1363. [Google Scholar] [CrossRef] [Green Version]
- Urchei, M.A.; Rodrigues, J.D.; Stone, L.F. Growth analysis of two bean cultivars under irrigation in no tillage and the conventional tillage. Pesqui. Agropecuária Bras. 2000, 35, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Najafi, S.; Nazari Nasi, H.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588. [Google Scholar] [CrossRef]
| Nutrient | Unit | Content | Classification |
|---|---|---|---|
| Ca2+ | cmolc dm−3 | 4.59 | High |
| Mg2+ | cmolc dm−3 | 1.61 | High |
| PO43− | mg dm−3 | 6.38 | Medium |
| K+ | cmolc dm−3 | 0.36 | High |
| Al+3 | cmolc dm−3 | 0.31 | Low |
| H + Al | cmolc dm−3 | 9.01 | High |
| Sum of bases | cmolc dm−3 | 6.56 | High |
| Cation exchange capacity at pH 7.0 | cmolc dm−3 | 15.57 | High |
| Cation exchange capacity efective | cmolc dm−3 | 6.87 | High |
| Carbon | g dm−³ | 25.75 | High |
| Organic matter | g dm−³ | 44.29 | High |
| Aluminum saturation | % | 4.51 | Low |
| Base saturation | % | 42.13 | Low |
| B | mg dm−³ | 0.24 | Low |
| S | mg dm−³ | 4.59 | Low |
| Fe+2 | mg dm−³ | 27.40 | Medium |
| Mn+2 | mg dm−³ | 43.40 | High |
| Cu+2 | mg dm−³ | 5.20 | High |
| Zn+2 | mg dm−³ | 1.80 | Medium |
| pH (CaCl2) | NA | 4.60 | NA |
| Nutrient | N | P2O5 | K2O | Ca | Mg | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|
| Unit | g L−1 | mg L−1 | |||||||
| Content | 2.50 ± 0.11 | 0.45 ± 0.15 | 0.15 ± 0.0 | 77.42 ± 5.8 | 0.08 ± 0.08 | 3.92 ± 0.02 | 7.45 ± 0.11 | 1.36 ± 0.08 | 2.08 ± 0.01 |
| Treatments | Application Mode | Description |
|---|---|---|
| T1 | Basal | Dose of biofertilizer corresponding to 100% of the amount of recommended mineral N + P and K supplementation with synthetic fertilizers |
| T2 | Foliar | Dose of biofertilizer corresponding to 100% of the amount of recommended mineral N + P and K supplementation with synthetic fertilizers |
| T3 | Basal | Dose of biofertilizer corresponding to 50% of the amount of recommended mineral N + N, P, and K supplementation with synthetic fertilizers |
| T4 | Foliar | Dosage of biofertilizer corresponding to 50% of the amount of recommended mineral N + N, P, and K supplementation with synthetic fertilizers |
| T5 | In-row broadcasting | Mineral fertilization as recommended for the maize crop in the Paraná State of Brazil |
| Treatments | Biofertilizer (L) | Nutrient Composition of the Biofertilizer (g) | Synthetic Nutrients Applied Directly to Soil (g) | ||||
|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | N | P2O5 | K2O | ||
| T1 | 122.88 | 307.20 | 56.30 | 19.00 | 0 | 225.30 | 160.20 |
| T2 | 122.88 | 307.20 | 56.30 | 19.00 | 0 | 225.30 | 160.20 |
| T3 | 61.44 | 153.60 | 28.10 | 9.50 | 153.60 | 253.50 | 169.70 |
| T4 | 61.44 | 153.60 | 28.10 | 9.50 | 153.60 | 253.50 | 169.70 |
| T5 | 0 | 0 | 0 | 0 | 307.20 | 281.60 | 179.20 |
| Physiological Indices | Equation | Description of Abbreviations |
|---|---|---|
| Total dry matter (W) | W = WL + WS + WE | WL = leaf dry matter (g) WS = stem dry matter (g) WE = ear dry matter (g) |
| Total leaf area (TLA) | TLA = 0.7458 × LW × LL | LW = leaf width (cm) LL = leaf length (cm) |
| Leaf area index (LAI) | LAI = TLA/S | TLA = total leaf area (cm2) S = soil surface area (cm2) |
| Absolute growth rate (AGR) | AGR = (W2 − W1)/(t2 − t1) | W = total dry matter (g) t = time (d) 1,2 = two successive sampling periods |
| Relative growth rate (RGR) | RGR = (lnW2 − lnW1)/(t2 − t1) | Ln = Naperian logarithm W = total dry matter (g) t = time (d) 1,2 = two successive sampling periods |
| Leaf area relative growth rate (RGRLA) | RGRLA = (lnTLA2 − lnTLA1)/(t2 − t1) | Ln = Naperian logarithm TLA = total leaf area W = total dry matter (g) t = time (d) 1,2 = two successive sampling periods TLA = total leaf area |
| Net assimilation rate (NAR) | NAR = [(W2 − W1)/(t2 − t1)] × [(lnTLA2 − lnTLA1)/(TLA2 − TLA1)] | W = total dry matter (g) t = time (d) 1,2 = two successive sampling periods |
| Leaf area ratio (LAR) | LAR = (TLA/W) | TLA = total leaf area W = total dry matter (g) |
| Specific leaf area (SLA) | SLA = (TLA/WE) | TLA = total leaf area W = total dry matter (g) |
| Treatments | T1 | T2 | T3 | T4 | T5 | CV (%) | Reference |
|---|---|---|---|---|---|---|---|
| N (g kg−1) | 40.60 | 38.60 | 42.00 | 40.50 | 37.80 | 12.40 | 27–35 |
| P (g kg−1) | 0.11 | 0.08 | 0.09 | 0.08 | 0.09 | 32.83 | 1.9–4.0 |
| K (g kg−1) | 11.40 | 12.10 | 8.40 | 10.30 | 9.80 | 18.88 | 17–35 |
| Ca (g kg−1) | 6.80 | 5.80 | 8.50 | 6.30 | 8.50 | 50.53 | 2.3–8.0 |
| Mg (g kg−1) | 0.035 | 0.029 | 0.041 | 0.034 | 0.037 | 30.11 | 1.5–5.0 |
| Cu (mg kg−1) | 140.90 | 68.61 | 69.34 | 85.27 | 172.00 | 66.19 | 6–20 |
| Zn (mg kg−1) | 67.54 | 54.43 | 54.73 | 50.25 | 71.24 | 30.99 | 15–100 |
| Fe (mg kg−1) | 540.68 A | 313.03 AB | 217.66 B | 300.97 AB | 326.55 AB | 41.23 | 30–250 |
| Mn (mg kg−1) | 36.94 | 31.38 | 26.44 | 29.31 | 37.61 | 56.07 | 20–200 |
| Treatments | Total Leaf Area (cm²) | N° of Leaves | Leaf DM (g) | Stem DM (g) | Ear DM (g) | Total DM (g) | Ear DM: Total DM |
|---|---|---|---|---|---|---|---|
| T1 | 5874 AB | 14 | 42.54 AB | 97.27 A | 35.47 | 175.28 A | 0.21 |
| T2 | 5709 AB | 14 | 39.11 AB | 76.22 AB | 29.08 | 144.41 AB | 0.20 |
| T3 | 5006 B | 14 | 34.69 AB | 72.71 AB | 23.00 | 130.40 AB | 0.18 |
| T4 | 5150 B | 14 | 34.09 B | 65.94 B | 22.39 | 122.42 B | 0.18 |
| T5 | 6424 A | 14 | 44.15 A | 86.63 AB | 35.25 | 166.03 AB | 0.22 |
| CV (%) | 13.43 | 7.22 | 16.05 | 22.7 | 36.68 | 21.61 | NA |
| Treatments | DAE 1 | AGR | RGR | RGRLA | NAR | LAR | SLA |
|---|---|---|---|---|---|---|---|
| Unit | (d) | (g d−1) | (g g−1d−1) | (dm2dm−2 d−1) | (g m−2 d−1) | (m2 g−1) | (m2 g−1) |
| T1 | 40 | 1.25 | 0.10 | 0.08 | 8.75 | 1.60 | 0.02 |
| 60 | 1.40 | 0.03 | 0.02 | 4.07 | 1.01 | 0.01 | |
| 80 | 1.88 | 0.03 | 0.00 | 3.87 | 0.86 | 0.01 | |
| 100 | 4.05 AB | 0.03 AB | 0.01 | 7.37 | 0.56 | 0.01 | |
| T2 | 40 | 1.05 | 0.09 | 0.07 | 7.37 | 1.68 | 0.02 |
| 60 | 2.11 | 0.05 | 0.04 | 5.48 | 1.05 | 0.01 | |
| 80 | 1.84 | 0.02 | 0.00 | 3.27 | 0.83 | 0.01 | |
| 100 | 2.02 B | 0.02 B | 0.00 | 3.64 | 0.53 | 0.01 | |
| T3 | 40 | 0.90 | 0.09 | 0.07 | 7.59 | 1.58 | 0.02 |
| 60 | 1.92 | 0.05 | 0.04 | 5.61 | 1.07 | 0.01 | |
| 80 | 1.03 | 0.01 | 0.00 | 2.20 | 0.82 | 0.01 | |
| 100 | 2.50 B | 0.02 B | 0.01 | 5.32 | 0.56 | 0.01 | |
| T4 | 40 | 1.30 | 0.10 | 0.07 | 8.05 | 1.63 | 0.02 |
| 60 | 1.88 | 0.04 | 0.03 | 4.70 | 1.01 | 0.01 | |
| 80 | 0.72 | 0.01 | 0.00 | 1.30 | 0.81 | 0.01 | |
| 100 | 2.00 B | 0.02 B | 0.00 | 4.16 | 0.63 | 0.01 | |
| T5 | 40 | 1.26 | 0.10 | 0.08 | 8.54 | 1.64 | 0.02 |
| 60 | 2.07 | 0.04 | 0.03 | 4.92 | 1.02 | 0.01 | |
| 80 | 0.26 | 0.00 | 0.00 | 0.59 | 0.80 | 0.01 | |
| 100 | 4.53 A | 0.04 A | 0.01 | 8.05 | 0.67 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buligon, E.L.; Costa, L.A.M.; de Lucas, J., Jr.; Santos, F.T.; Goufo, P.; Costa, M.S.S.M. Fertilizer Performance of a Digestate from Swine Wastewater as Synthetic Nitrogen Substitute in Maize Cultivation: Physiological Growth and Yield Responses. Agriculture 2023, 13, 565. https://doi.org/10.3390/agriculture13030565
Buligon EL, Costa LAM, de Lucas J Jr., Santos FT, Goufo P, Costa MSSM. Fertilizer Performance of a Digestate from Swine Wastewater as Synthetic Nitrogen Substitute in Maize Cultivation: Physiological Growth and Yield Responses. Agriculture. 2023; 13(3):565. https://doi.org/10.3390/agriculture13030565
Chicago/Turabian StyleBuligon, Eduardo L., Luiz A. M. Costa, Jorge de Lucas, Jr., Francielly T. Santos, Piebiep Goufo, and Monica S. S. M. Costa. 2023. "Fertilizer Performance of a Digestate from Swine Wastewater as Synthetic Nitrogen Substitute in Maize Cultivation: Physiological Growth and Yield Responses" Agriculture 13, no. 3: 565. https://doi.org/10.3390/agriculture13030565






