Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. Capitata) under Water-Stress Conditions
Abstract
1. Introduction
2. Material and Methods
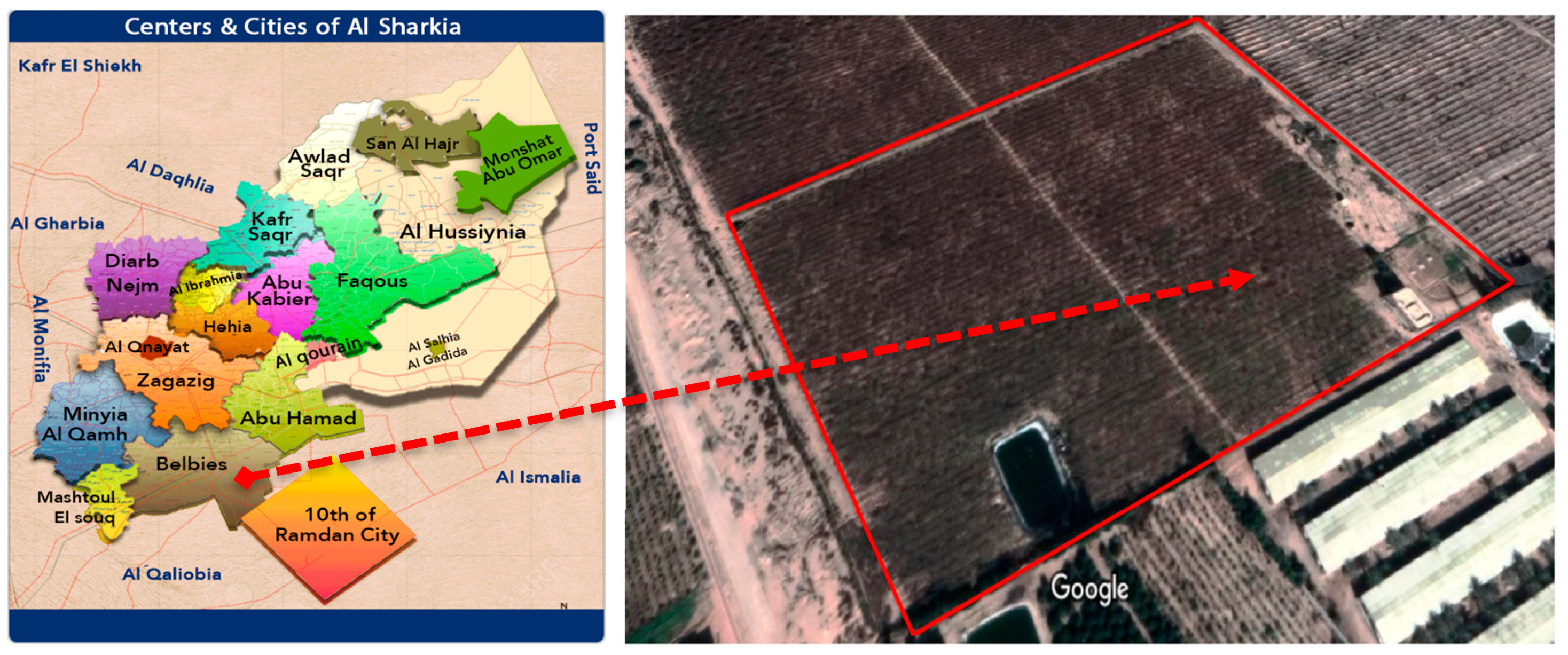
2.1. The Experimental Site and Crop
2.2. Irrigation System and Experimental Description
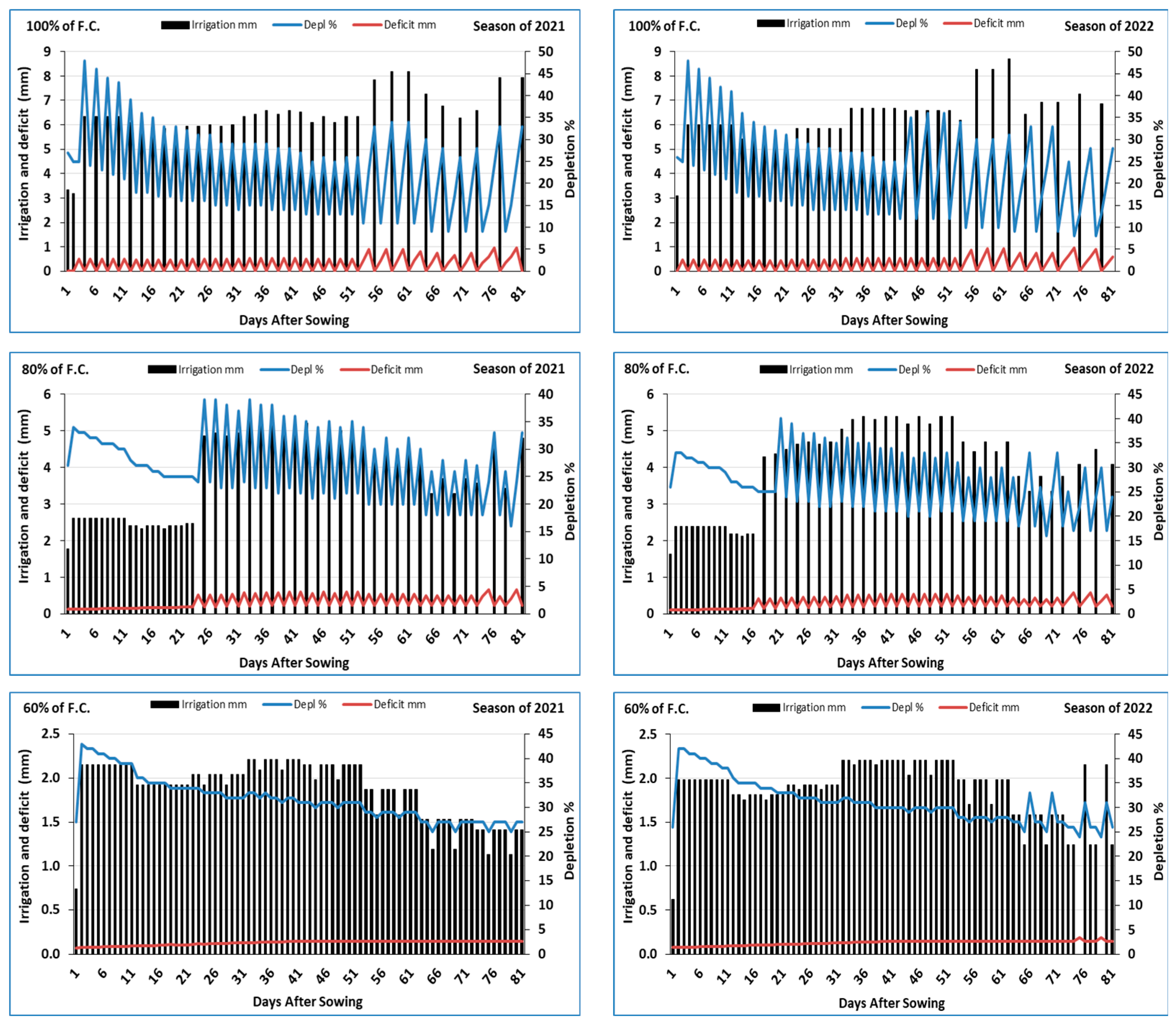
2.3. Moisture Deficit and Depletion % of Irrigation
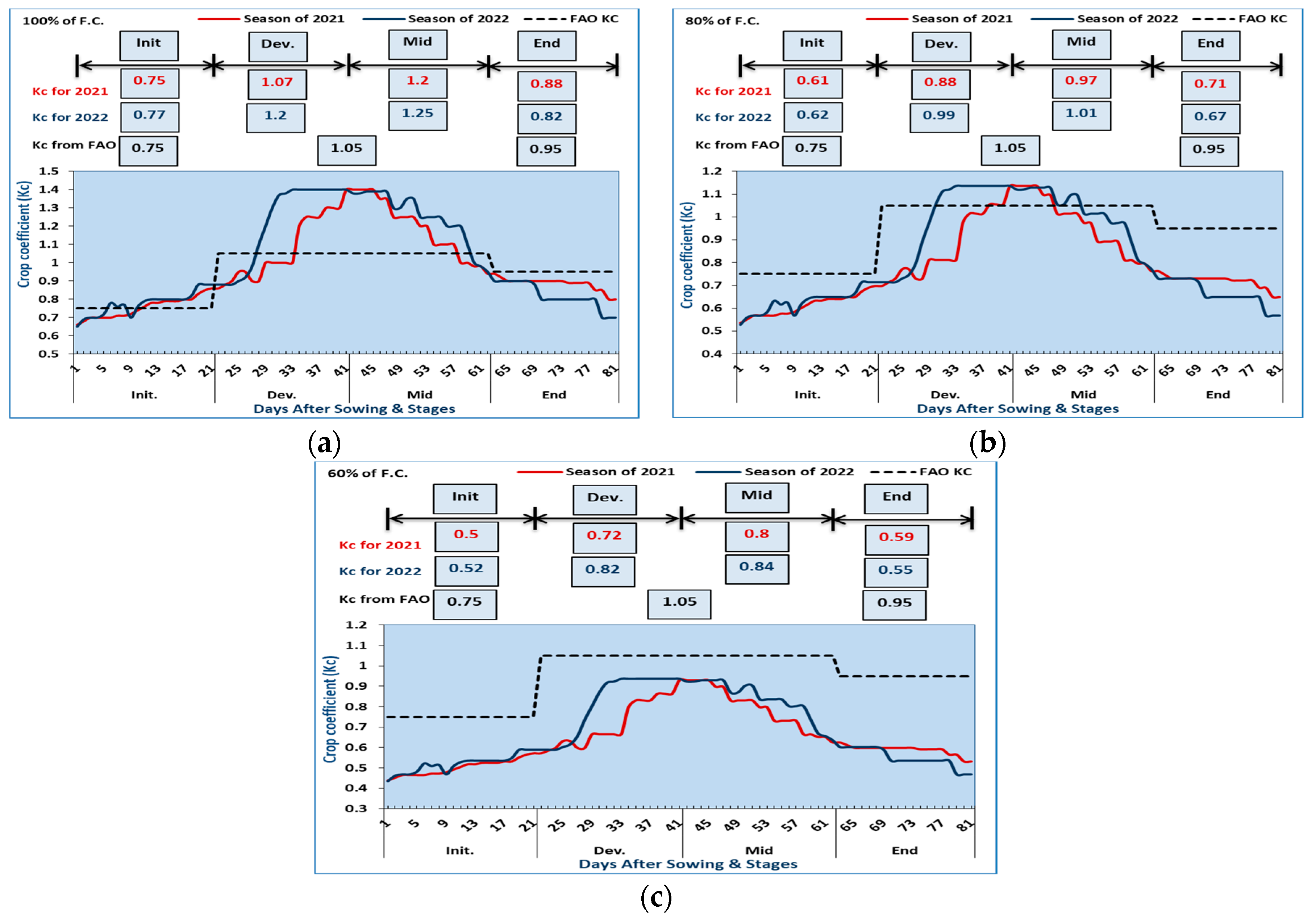
2.4. Estimation of the Crop Coefficient (Kc) for Red Cabbage
2.5. Experimental Design and Layout
2.6. Crop Characteristics
2.6.1. Vegetative Growth Characteristics
2.6.2. Leaf Total Chlorophyll
2.6.3. Leaf TSS Content
2.6.4. Leaf Cell Sap Osmotic Pressure (atm)
2.7. Total Yield of Red Cabbage
2.8. Statistical Analysis
3. Results and Discussion
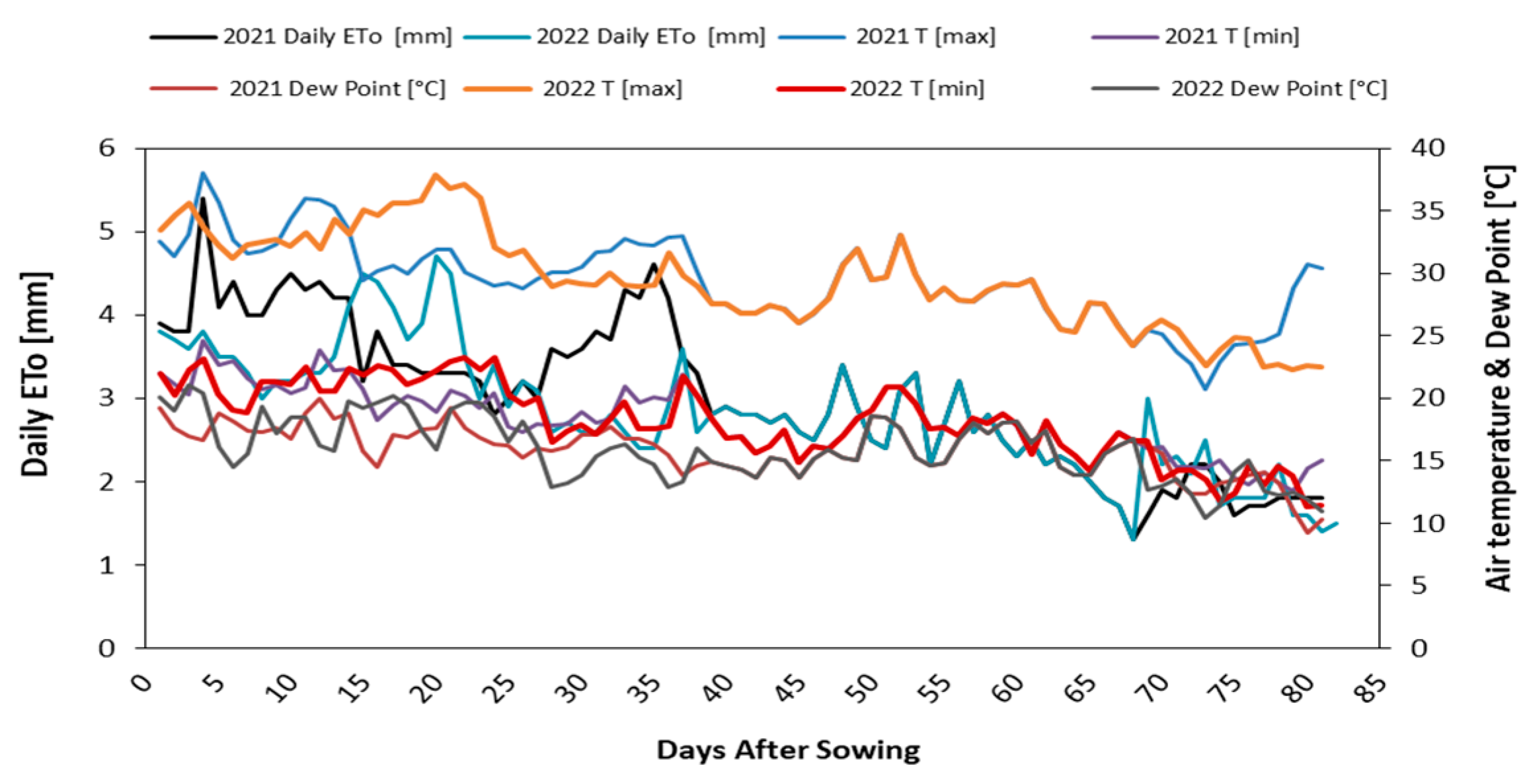
3.1. Moisture Deficit and Depletion % of Irrigation
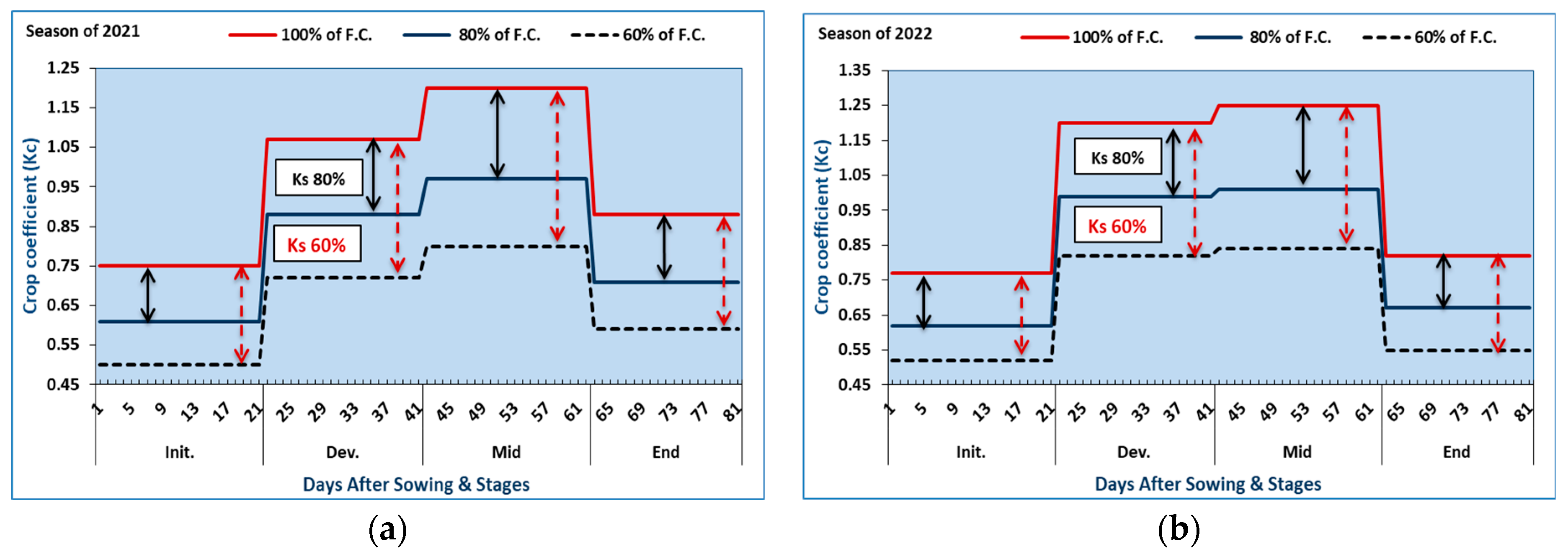
3.2. Estimation of the Crop Coefficient (Kc) for Red Cabbage
3.3. Crop Characteristics
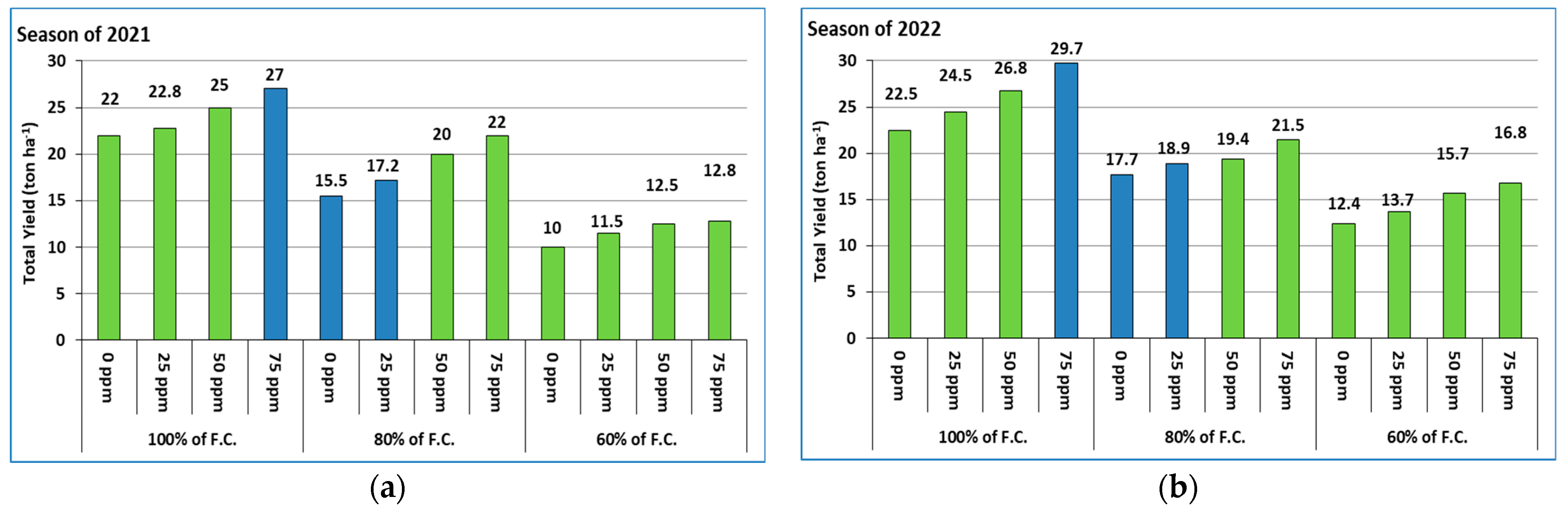
3.4. Total Yield of Red Cabbage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Impacts, adaptation and vulnerability. Part A: Global and sectoral aspects. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., White, L.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 688. [Google Scholar]
- Dewedar, O.; Plauborg, F.; El-Shafie, A.; Marwa, A. Response of potato biomass and tuber yield under future climate change scenarios in Egypt. J. Water Land Dev. 2021, 49, 139–150. [Google Scholar]
- Kumar, M. Impact of climate change on crop yield and role of model for achieving food security. Environ. Monit. Assess. 2016, 188, 465. [Google Scholar] [CrossRef] [PubMed]
- Mall, R.K.; Gupta, A.; Sonkar, G. Effect of climate change on agricultural crops. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–46. [Google Scholar] [CrossRef]
- Lipovac, A.; Mandić, M.V.; Vuković, A.; Stričević, R.; Ćosić, M. Assessment of AquaCrop Model on Potato Water Requirements in Climate Change Conditions. In Proceedings of the 10th Eastern European Young Water Professionals Conference IWA YWP, Zagreb, Croatia, 7–12 May 2018; pp. 70–77. [Google Scholar]
- Hegazi, A.M.; El-Shraiy, A.M. Stimulation of photosynthetic pigments, anthocyanin, antioxidant enzymes in salt stressed red cabbage plants by ascorbic acid and potassium silicate. Middle East J. Agric. 2017, 6, 553–568. [Google Scholar]
- Ministry of Agriculture and Land Reclamation (MALR) Arab Republic of Egypt. Sustainable Agricultural Development Strategy Towards 2030; The Food and Agriculture Organization (FAO): Cairo, Egypt, 2020. [Google Scholar]
- Taha, N.M.; Noura, M.; Shaimaa, S.H.; Elrahman, F.A.; Hashem, F.A. Improving yield and quality of garlic (Allium sativum L.) under water stress conditions. Middle East J. Agric. 2019, 8, 330–346. [Google Scholar]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Ngoc-Thang, V.U.; Jong-Man, P.; Il-Soep, K.; Anh-Tuan, T.; Dong-Cheol, J. Effect of abscisic acid on growth and physiology of arabica coffee seedlings under water deficit condition. Sains Malays 2020, 49, 1499–1508. [Google Scholar]
- Marwa, M.A.; El-Shafie, A.F.; Dewedar, O.M.; Molina-Martinez, J.M.; Ragab, R. Predicting the water requirement, soil moisture distribution, yield, water productivity of peas and impact of climate change using saltmed model. Plant Arch. 2020, 20, 3673–3689. [Google Scholar]
- Allen, R.G.; Luis, S.P.; Dirk, R.; Martin, S. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Pereira, L.S.; Paredes, P.; López-Urrea, R.; Hunsaker, D.J.; Mota, M.; Shad, Z.M. Standard single and basal crop coefficients for vegetable crops, an update of FAO56 crop water requirements approach. Agric. Water Manag. 2021, 243, 106196. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Hunsaker, D.J.; López-Urrea, R.; Shad, Z.M. Standard single and basal crop coefficients for field crops. Updates and advances to the FAO56 crop water requirements method. Agric. Water Manag. 2021, 243, 106466. [Google Scholar] [CrossRef]
- Valiantzas, J.D. Simplified forms for the standardized FAO-56 Penman–Monteith reference evapotranspiration using limited weather data. J. Hydrol. 2013, 505, 13–23. [Google Scholar] [CrossRef]
- Smith, M. CROPWAT A Computer Program for Irrigation Plans and Management and ETo Calculation Using Penman-Montieth Method; FAO Irrigation and Drainage: Rome, Italy, 1992; Volume 46, pp. 112–140. [Google Scholar]
- Savva, A.P.; Frenken, K. Irrigation Manual, Planning, Development Monitoring and Evaluation of Irrigated Agriculture with Farmer Participation; Food and Agriculture Organization of the United Nations (FAO) Sub-Regional Office for East and Southern Africa (SAFR): Harare, Zimbabwe, 2002; Volume IV, Available online: https://www.fao.org/docrep/pdf/010/ai596e/ai596e.pdf (accessed on 10 January 2023).
- Yadava, U.L. Rapid non-destructive methods to determine chlorophyll in intact leaves. Hortic. Sci. 1986, 21, 1449–1450. [Google Scholar]
- Gusev, N.A. Some Methods of Studying the Water Regime of Plants; Publishing House of the USSR Academy of Sciences: Leningrad, Russia, 1960; Volume 61. (In Russian) [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; p. 1191. [Google Scholar]
- Duncan, D.B. Multiple range and multiple “F” test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Youssef, E.A.; Marwa, M.A.; El-Shafie, A.F.; Hussein, M.M. Study the applications of water deficiency levels and ascorbic acid foliar on growth parameters and yield of summer squash plant (Cucurbita pepo L.). Agric. Eng. Int. CIGR J. 2017, 147–158. [Google Scholar]
- Hozayn, M.; Ali, H.M.; Marwa, M.A.; El-Shafie, A.F. Influence of magnetic water on french basil (Ocimum basilicum L. Var Grandvert) plant grown under water Stress conditions. Plant Arch. 2020, 20, 3636–3648. [Google Scholar]
- Rallo, G.; Paço, T.A.; Paredes, P.; Puig-Sirera, À.; Massai, R.; Provenzano, G.; Pereira, L.S. Updated single and dual crop coefficients for tree and vine fruit crops. Agric. Water Manag. 2021, 250, 106645. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; López-Urrea, D.J.; Jovanovic, N. Updates and advances to the FAO56 crop water requirements method. Agric. Water Manag. 2021, 24, 106697. [Google Scholar] [CrossRef]
- Herrera-Puebla, J.; Meneses-Peralta, J.; Duarte-Díaz, C.; González-Robaina, F.; Hervís-Granda, G. Determination of Crop Coefficients for Estimating Evapotranspiration in a Paddy Field in Cuba. Rev. Cienc. Técnicas Agropecu. 2020, 29, 5–20. [Google Scholar]
- Vijay, K.; Singh, V.B.; Priyanka, S.; Shalini, K. Effect of various mulches on soil moisture content, soil properties, growth and yield of Kinnow under rainfed condition. Int. J. Agric. Sci. 2014, 10, 225–229. [Google Scholar]
- Falivene, S.; Giddings, J.; Hardy, S.; Sanderson, G. Managing citrus orchards with less water. N. S. W. DPI Primefact 2006, 427, 1–12. [Google Scholar]
- Youssef, E.A. Increasing Drought Tolerance of Gladiolus Plants through Application of Some Growth Regulators. Master’s Thesis, Faculty Agriculture Zagazig University, Zagazig, Egypt, 2007; p. 130. [Google Scholar]
- Mahmoud, T.A.; Youssef, E.A. Water deficiency and mulching effects on Valencia orange trees. Ann. Agric. Sci. Moshtohor J. 2017, 55, 113–128. [Google Scholar]
- Agarwal, S.; Sairam, R.K.; Srivatava, G.C.; Tyagi, A.; Meena, R.C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 2005, 169, 559–570. [Google Scholar] [CrossRef]
- Anbarasi, G.; Bhagavathi, G.; Vignesh, R.; Srinivasan, M.; Somasundaram, S.T. Effect of exogenous abscisic acid on growth and biochemical changes in the halophyte Suaeda maritima. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 442–447. [Google Scholar]
- Mahmoud, T.A.; Youssef, E.A. Effect of partial root-zone drying drip irrigation system on Valencia orange trees. Ann. Agric. Sci. Moshtohor J. 2017, 55, 101–112. [Google Scholar]
- Mahmoud, T.A.; Youssef, E.A.; El-harouny, S.B.; Abo Eid, A.M. Effect of magnetized water and different levels of water supply on growth and yield of navel orange trees. J. Hortic. Sci. Ornam. Plants 2018, 10, 118–128. [Google Scholar]
- Youssef, E.A.; Hozayen, A.M.A. Study the effect of chitosan on some growth and yield parameters in wheat grown under water stress condition. Plant Arch. 2019, 19, 684–694. [Google Scholar]
- Koshita, Y.; Takahara, T. Effect of water stress on flower-bud formation and plant hormone content of satsuma mandarin (Citrus unshiu Marc). Sci. Hortic. 2004, 99, 301–307. [Google Scholar] [CrossRef]
- Ghosh, S.G.; Asanuma, K.; Kusutani, A.; Toyota, M. Effect of moisture stress at different growth stages on the amount of total nonstructural carbohydrate, nitrate reeducates activity and yield of potato. Jpn. J. Trop. Agric. 2000, 44, 158–166. [Google Scholar]
- Ahmed, M.E.M.; Abd El-Kader, N.I. The influence of magnetic water and water regimes on soil salinity, growth, yield and tubers quality of potato plants. Middle East J. Agric. Res. 2016, 5, 132–143. [Google Scholar]
- Youssef, E.A.; Ahmed, M.A.; Nadia, M.B.; Magda, A.F.S.; Karima, M.G. Alleviation of drought effect on sunflower (Helianthus annus L.) c.v. sakha-53 cultivar by foliar spraying with antioxidant. Middle East J. Agric. 2015, 4, 794–801. [Google Scholar]
- Mahmoud, T.A.; Youssef, E.A.; El-harouny, S.B.; Abo Eid, M.A.M. Study of the effect of salinity stress and magnetized water irrigation on growth of Washington navel orange seedlings budded on sour orange rootstock. Plant Arch. 2019, 19, 711–720. [Google Scholar]
- Youssef, E.A.; Hozayen, A.M.A. The effect of drought stress condition combined with Kaolin spraying application on growth and yield parameters of Maize (Zea mays). Plant Arch. 2019, 19, 674–683. [Google Scholar]
- Li, J.; Wang, X.Q.; Watson, M.B.; Assmann, S.M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 2000, 287, 300–303. [Google Scholar] [CrossRef]
- Mahmoud, T.A.; Youssef, E.A.; El-harouny, S.B.; Abo Eid, M.A.M. Effect of irrigation with magnetic water on nitrogen fertilization efficiency of navel orange trees. Plant Arch. 2019, 19, 966–975. [Google Scholar]
- Vu, N.T.; Kang, H.M.; Kim, Y.S.; Choi, K.Y.; Kim, I.S. Growth, physiology and abiotic stress response to abscisic acid in tomato seedlings. Hortic. Environ. Biotechnol. 2015, 56, 294–304. [Google Scholar] [CrossRef]
- Youssef, E.A.; Taha, S.S. Effect of moisture stress and magnetized water on growth parameters and yield characteristics of onion plants. Int. J. Pharm Technol. Res. 2016, 9, 104–111. [Google Scholar]
- Chen, Z.; Wang, Z.; Yang, Y.; Li, M.; Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- McLAREN, J.S.; Smith, H. The effect of abscisic acid on growth, photosynthetic rate and carbohydrate metabolism in Lemna minor L. New Phytol. 1976, 76, 11–20. [Google Scholar] [CrossRef]
- Dewedar, O.M.; Plauborg, F.; Marwa, M.A.; El-Shafie, A.F.; Ragab, R. Improving water saving, yield, and water productivity of bean under deficit drip irrigation: Field and modelling study using the SALTMED model. Irrig. Drain. 2021, 70, 224–242. [Google Scholar] [CrossRef]
| Depth (cm) | Particle Size Distribution (%) | Moisture Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Coarse Sand | Fine Sand | Silt | Clay | Saturation Point (S.P.) | FC | AW | PWP | |
| 0–30 | 43.2 | 19.5 | 24 | 13.3 | 28.6 | 14.3 | 7.15 | 7.15 |
| 30–60 | 44.1 | 18.5 | 24.2 | 13.2 | 28.6 | 14.5 | 7.4 | 7.1 |
| Treatments | Plant Height (cm) | Head Diameter (cm) | Head Volume (cm3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Season | Second Season | First Season | Second Season | First Season | Second Season | |||||||
| Field Capacity | ||||||||||||
| 100% of FC | 50.97 | A | 47.20 | A | 31.18 | A | 34.18 | A | 354.92 | A | 385.45 | A |
| 80% of FC | 44.20 | B | 41.18 | B | 27.93 | B | 31.02 | B | 315.77 | B | 335.71 | B |
| 60% of FC | 36.07 | C | 33.62 | C | 24.89 | C | 27.15 | C | 283.97 | C | 297.81 | C |
| LSD at 5% | 0.224 | 0.439 | 0.194 | 0.910 | 2.312 | 2.846 | ||||||
| Abscisic Acid | ||||||||||||
| ABA 00 ppm | 46.32 | A | 43.19 | A | 26.83 | D | 29.44 | D | 300.70 | D | 326.94 | D |
| ABA 25 ppm | 44.47 | B | 41.30 | B | 27.61 | C | 30.29 | C | 313.35 | C | 333.44 | C |
| ABA 50 ppm | 42.90 | C | 39.74 | C | 28.34 | B | 31.25 | B | 325.85 | B | 343.71 | B |
| ABA 75 ppm | 41.29 | D | 38.44 | D | 29.23 | A | 32.14 | A | 332.96 | A | 354.52 | A |
| LSD at 5% | 0.372 | 0.373 | 0.217 | 0.177 | 3.281 | 1.996 | ||||||
| Interaction | ||||||||||||
| 100% of FC × ABA 00 ppm | 52.73 | a | 48.96 | a | 30.21 | d | 32.98 | d | 329.51 | d | 372.79 | d |
| 100% of FC × ABA 25 ppm | 51.60 | b | 47.40 | b | 30.87 | c | 33.72 | c | 349.33 | c | 378.02 | c |
| 100% of FC × ABA 50 ppm | 50.53 | c | 46.51 | c | 31.51 | b | 34.55 | b | 367.50 | b | 391.54 | b |
| 100% of FC × ABA 75 ppm | 49.01 | d | 45.95 | d | 32.16 | a | 35.45 | a | 373.32 | a | 399.43 | a |
| 80% of FC × ABA 00 ppm | 46.26 | e | 44.38 | e | 26.35 | h | 30.00 | h | 304.17 | h | 320.63 | h |
| 80% of FC × ABA 25 ppm | 45.34 | f | 42.23 | f | 27.50 | g | 30.66 | g | 310.27 | g | 328.52 | g |
| 80% of FC × ABA 50 ppm | 43.61 | g | 40.04 | g | 28.37 | f | 31.22 | f | 321.31 | f | 339.02 | f |
| 80% of FC × ABA 75 ppm | 41.58 | h | 38.05 | h | 29.49 | e | 32.20 | e | 327.32 | e | 354.67 | e |
| 60% of FC × ABA 00 ppm | 39.97 | i | 36.22 | i | 23.93 | l | 25.33 | l | 268.42 | l | 287.40 | l |
| 60% of FC × ABA 25 ppm | 36.47 | j | 34.26 | j | 24.45 | k | 26.50 | k | 280.44 | k | 293.79 | k |
| 60% of FC × ABA 50 ppm | 34.56 | k | 32.67 | k | 25.16 | j | 27.98 | j | 288.75 | j | 300.57 | j |
| 60% of FC × ABA 75 ppm | 33.29 | l | 31.32 | l | 26.03 | i | 28.78 | i | 298.25 | i | 309.46 | i |
| LSD at 5% | 0.593 | 0.641 | 0.358 | 0.279 | 5.283 | 3.580 | ||||||
| Treatments | Plant Fresh Weight (g) | Head Fresh Weight (g) | Root Fresh Weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Season | Second Season | First Season | Second Season | First Season | Second Season | |||||||
| Field Capacity | ||||||||||||
| 100% of FC | 572.69 | A | 622.13 | A | 507.97 | A | 542.12 | A | 29.71 | A | 34.05 | A |
| 80% of FC | 443.32 | B | 467.09 | B | 390.15 | B | 403.71 | B | 25.81 | B | 26.54 | B |
| 60% of FC | 283.18 | C | 352.82 | C | 244.79 | C | 306.08 | C | 18.66 | C | 21.03 | C |
| LSD at 5% | 12.081 | 12.566 | 11.644 | 12.207 | 0.680 | 0.587 | ||||||
| Abscisic Acid | ||||||||||||
| ABA 00 ppm | 391.14 | D | 437.23 | D | 333.83 | D | 367.37 | D | 26.87 | A | 30.10 | A |
| ABA 25 ppm | 416.54 | C | 462.95 | C | 361.69 | C | 396.92 | C | 25.94 | B | 28.55 | B |
| ABA 50 ppm | 448.49 | B | 494.23 | B | 398.54 | B | 432.46 | B | 24.07 | C | 26.25 | C |
| ABA 75 ppm | 476.08 | A | 528.31 | A | 429.83 | A | 472.45 | A | 22.04 | D | 23.93 | D |
| LSD at 5% | 9.951 | 6.521 | 10.136 | 6.358 | 0.515 | 0.525 | ||||||
| Interaction | ||||||||||||
| 100% of FC × ABA 00 ppm | 535.06 | d | 558.58 | d | 465.44 | d | 471.40 | d | 30.96 | a | 37.99 | a |
| 100% of FC × ABA 25 ppm | 545.91 | c | 595.01 | c | 479.53 | c | 512.31 | c | 30.11 | b | 35.50 | b |
| 100% of FC × ABA 50 ppm | 582.83 | b | 640.41 | b | 520.86 | b | 562.69 | b | 29.04 | c | 32.67 | c |
| 100% of FC × ABA 75 ppm | 626.98 | a | 694.50 | a | 566.04 | a | 622.06 | a | 28.73 | d | 30.04 | d |
| 80% of FC × ABA 00 ppm | 385.98 | h | 441.34 | h | 326.42 | h | 371.25 | h | 28.39 | e | 28.78 | e |
| 80% of FC × ABA 25 ppm | 419.94 | g | 457.37 | g | 363.13 | g | 391.61 | g | 27.54 | f | 27.71 | f |
| 80% of FC. × ABA 50 ppm | 467.85 | f | 467.62 | f | 416.98 | f | 406.27 | f | 25.08 | g | 25.55 | g |
| 80% of FC × ABA 75 ppm | 499.50 | e | 502.06 | e | 454.07 | e | 445.72 | e | 22.24 | h | 24.13 | h |
| 60% of FC × ABA 00 ppm | 252.37 | l | 311.78 | l | 209.61 | l | 259.46 | l | 21.27 | i | 23.55 | i |
| 60% of FC × ABA 25 ppm | 283.78 | k | 336.48 | k | 242.41 | k | 286.85 | k | 20.16 | j | 22.45 | j |
| 60% of FC × ABA 50 ppm | 294.79 | j | 374.65 | j | 257.78 | j | 328.42 | j | 18.09 | k | 20.52 | k |
| 60% of FC × ABA 75 ppm | 301.77 | i | 388.36 | i | 269.36 | i | 349.57 | i | 15.13 | l | 17.61 | l |
| LSD at 5% | 17.205 | 12.912 | 17.341 | 12.536 | 0.907 | 0.894 | ||||||
| Treatments | No. of Green Leaves Per Plant | No. of Red Leaves Per Plant | No. of Total Leaves Per Plant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Season | Second Season | First Season | Second Season | First Season | Second Season | |||||||
| Field Capacity | ||||||||||||
| 100% of FC | 6.69 | A | 10.45 | A | 17.32 | A | 20.46 | A | 24.01 | A | 30.91 | A |
| 80% of FC | 6.19 | B | 9.33 | B | 15.99 | B | 17.36 | B | 22.18 | B | 26.70 | B |
| 60% of FC | 5.53 | C | 8.42 | C | 13.67 | C | 15.17 | C | 19.21 | C | 23.59 | C |
| LSD at 5% | 0.026 | 0.045 | 0.149 | 0.194 | 0.162 | 0.156 | ||||||
| Abscisic Acid | ||||||||||||
| ABA 00 ppm | 6.37 | A | 9.75 | A | 16.45 | A | 18.59 | A | 22.82 | A | 28.34 | A |
| ABA 25 ppm | 6.23 | B | 9.50 | B | 15.81 | B | 18.00 | B | 22.04 | B | 27.50 | B |
| ABA 50 ppm | 6.07 | C | 9.25 | C | 15.42 | C | 17.42 | C | 21.49 | C | 26.67 | C |
| ABA 75 ppm | 5.88 | D | 9.11 | D | 14.96 | D | 16.65 | D | 20.84 | D | 25.76 | D |
| LSD at 5% | 0.515 | 0.062 | 0.119 | 0.170 | 0.157 | 0.166 | ||||||
| Interaction | ||||||||||||
| 100% of FC × ABA 00 ppm | 6.94 | a | 10.81 | A | 17.80 | a | 21.82 | a | 24.75 | a | 32.62 | a |
| 100% of FC × ABA 25 ppm | 6.78 | b | 10.48 | B | 17.41 | b | 20.93 | b | 24.20 | b | 31.41 | b |
| 100% of FC × ABA 50 ppm | 6.59 | c | 10.33 | C | 17.16 | c | 20.07 | c | 23.75 | c | 30.40 | c |
| 100% of FC × ABA 75 ppm | 6.44 | d | 10.19 | D | 16.91 | d | 19.04 | d | 23.34 | d | 29.23 | d |
| 80% of FC × ABA 00 ppm | 6.34 | e | 9.66 | E | 16.60 | e | 18.25 | e | 22.94 | e | 27.90 | e |
| 80% of FC × ABA 25 ppm | 6.22 | f | 9.51 | F | 16.15 | f | 17.60 | f | 22.38 | f | 27.10 | f |
| 80% of FC × ABA 50 ppm | 6.15 | g | 9.14 | G | 15.76 | g | 17.11 | g | 21.91 | g | 26.25 | g |
| 80% of FC × ABA 75 ppm | 6.05 | h | 9.03 | H | 15.43 | h | 16.49 | h | 21.49 | h | 25.52 | h |
| 60% of FC × ABA 00 ppm | 5.83 | i | 8.78 | I | 14.93 | i | 15.72 | i | 20.76 | i | 24.50 | i |
| 60% of FC × ABA 25 ppm | 5.69 | j | 8.51 | J | 13.87 | j | 15.47 | j | 19.55 | j | 23.98 | j |
| 60% of FC × ABA 50 ppm | 5.47 | k | 8.28 | K | 13.35 | k | 15.08 | k | 18.82 | k | 23.37 | k |
| 60% of FC × ABA 75 ppm | 5.16 | l | 8.10 | L | 12.54 | l | 14.42 | l | 17.70 | l | 22.52 | l |
| LSD at 5% | 0.081 | 0.099 | 0.208 | 0.291 | 0.264 | 0.276 | ||||||
| Treatments | Total Chlorophyll SPAD | Leaf Cell Sap TSS | Leaf Cell Sap Osmotic Pressure (atm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Season | Second Season | First Season | Second Season | First Season | Second Season | |||||||
| Field Capacity | ||||||||||||
| 100% of FC | 89.29 | A | 85.67 | A | 4.63 | C | 4.77 | C | 3.58 | C | 3.69 | C |
| 80% of FC | 75.81 | B | 80.68 | B | 5.54 | B | 5.63 | B | 4.28 | B | 4.35 | B |
| 60% of FC | 66.59 | C | 71.75 | C | 6.50 | A | 6.39 | A | 5.04 | A | 4.95 | A |
| LSD at 5% | 3.096 | 0.789 | 0.189 | 0.094 | 0.151 | 0.067 | ||||||
| Abscisic Acid | ||||||||||||
| ABA 00 ppm | 82.63 | A | 82.73 | A | 5.00 | D | 5.20 | D | 3.86 | D | 4.02 | D |
| ABA 25 ppm | 78.24 | B | 80.50 | B | 5.28 | C | 5.50 | C | 4.08 | C | 4.25 | C |
| ABA 50 ppm | 75.69 | C | 78.30 | C | 5.94 | B | 5.74 | B | 4.59 | B | 4.44 | B |
| ABA 75 ppm | 72.36 | D | 75.94 | D | 6.00 | A | 5.94 | A | 4.64 | A | 4.59 | A |
| LSD at 5% | 3.200 | 0.581 | 0.185 | 0.048 | 0.147 | 0.068 | ||||||
| Interaction | ||||||||||||
| 100% of FC × ABA 00 ppm | 98.15 | a | 88.35 | A | 4.00 | g | 4.00 | k | 3.12 | g | 3.12 | k |
| 100% of FC × ABA 25 ppm | 89.37 | b | 86.13 | B | 4.50 | f | 4.60 | j | 3.49 | f | 3.56 | j |
| 100% of FC × ABA 50 ppm | 87.33 | c | 84.63 | C | 5.00 | e | 5.13 | i | 3.86 | e | 3.97 | i |
| 100% of FC × ABA 75 ppm | 82.30 | d | 83.57 | D | 5.00 | e | 5.33 | h | 3.86 | e | 4.12 | h |
| 80% of FC × ABA 00 ppm | 80.00 | e | 82.87 | E | 5.00 | e | 5.50 | g | 3.86 | e | 4.25 | g |
| 80% of FC × ABA 25 ppm | 77.60 | f | 81.47 | F | 5.33 | d | 5.50 | g | 4.12 | d | 4.25 | g |
| 80% of FC × ABA 50 ppm | 74.67 | g | 80.37 | G | 5.83 | c | 5.60 | f | 4.51 | c | 4.32 | f |
| 80% of FC × ABA 75 ppm | 70.97 | h | 78.03 | H | 6.00 | b | 5.93 | e | 4.64 | b | 4.59 | e |
| 60% of FC × ABA 00 ppm | 69.73 | i | 76.97 | I | 6.00 | b | 6.10 | d | 4.64 | b | 4.72 | d |
| 60% of FC × ABA 25 ppm | 67.77 | j | 73.90 | J | 6.00 | b | 6.40 | c | 4.64 | b | 4.96 | c |
| 60% of FC × ABA 50 ppm | 65.07 | k | 69.90 | K | 7.00 | a | 6.50 | b | 5.45 | a | 5.04 | b |
| 60% of FC × ABA 75 ppm | 63.80 | l | 66.23 | L | 7.00 | a | 6.57 | a | 5.45 | a | 5.09 | a |
| LSD at 5% | 5.327 | 1.029 | 0.309 | 0.143 | 0.247 | 0.116 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, E.A.; Abdelbaset, M.M.; Dewedar, O.M.; Molina-Martínez, J.M.; El-Shafie, A.F. Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. Capitata) under Water-Stress Conditions. Agriculture 2023, 13, 610. https://doi.org/10.3390/agriculture13030610
Youssef EA, Abdelbaset MM, Dewedar OM, Molina-Martínez JM, El-Shafie AF. Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. Capitata) under Water-Stress Conditions. Agriculture. 2023; 13(3):610. https://doi.org/10.3390/agriculture13030610
Chicago/Turabian StyleYoussef, Ebtessam A., Marwa M. Abdelbaset, Osama M. Dewedar, José Miguel Molina-Martínez, and Ahmed F. El-Shafie. 2023. "Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. Capitata) under Water-Stress Conditions" Agriculture 13, no. 3: 610. https://doi.org/10.3390/agriculture13030610
APA StyleYoussef, E. A., Abdelbaset, M. M., Dewedar, O. M., Molina-Martínez, J. M., & El-Shafie, A. F. (2023). Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. Capitata) under Water-Stress Conditions. Agriculture, 13(3), 610. https://doi.org/10.3390/agriculture13030610








